Amphipathic Janus Nanofibers Aerogel for Efficient Solar Steam Generation
Abstract
Solar steam generation is a promising water purification technology due to its low-cost and environmentally friendly applications in water purification and desalination. However, hydrophilic or hydrophobic materials alone are insufficient in achieving necessary characteristics for constructing high-quality solar steam generators with good comprehensive properties. Herein, novel hydrophile/hydrophobe amphipathic Janus nanofibers aerogel is designed and used as a host material for preparing solar steam generators. The product consists of an internal cubic aerogel and an external layer of photothermal materials. The internal aerogel is composed of electrospun amphipathic Janus nanofibers. Owing to the unique composition and structure, the prepared solar steam generator integrates the features of high water evaporation rate (2.944 kg m−2 h−1 under 1 kW m−2 irradiation), self-floating, salt-resisting, and fast performance recovery after flipping. Moreover, the product also exhibits excellent properties on desalination and removal of organic pollutants. Compared with traditional hydrophilic aerogel host material, the amphipathic Janus nanofibers aerogel brings much higher water evaporation rate and salt resistance.
1 Introduction
Solar steam generation (SSG) is an emerging water purification technology and has become a research hotspot in the field of water treatment in recent years.[1-3] Its working principle is to utilize solar energy to convert water from waste water or sea water into water steam which is then collected and condensed into fresh water. This technique can separate water from contaminants and other impurities in a process similar to the water cycle in the natural environment. Hence, it is an eco-friendly and low-cost water purification strategy that does not require extra energy.
However, due to the low light absorption and photothermal conversion efficiency of water, an efficient SSG process requires placing the materials with the function of photothermal conversion on the surface of water to realize interfacial heating, and these materials are usually called solar steam generators or solar absorbers.[4-6] To date, many types of solar steam generators, such as photothermal aerogels,[7] hydrogels,[8] foams,[9] and membranes,[10] have been fabricated. Macroscopically, solar steam generators can be classified into one-dimensional (1D), 2D, and 3D materials. Among them, 3D solar steam generators are considered to be the most promising materials owing to their high water evaporation rates and versatility.[11-13] In general, 3D solar steam generators have large thicknesses, which is conductive to reduce thermal dissipation from evaporation surface to bulk water and also enhance the energy efficiency by allowing multiple reflections of light inside the 3D solar steam generators. Moreover, the 3D structure provides more feasibility for structural design. By far, many different shapes of 3D solar steam generators have been prepared to endow the materials with the features, such as self-floating,[14] salt-resisting,[15] self-cleaning,[16] and cold evaporation.[17]
The solar steam generators based on 3D aerogels are hotspot of current researches.[18-20] As a commonly used method for preparing 3D aerogels, electrospinning combined with freeze-drying has been developed for several years.[21-24] In addition to their applications in SSG, electrospun 3D aerogels also exhibit other application prospects in thermal insulation,[25] tissue engineering,[26] photocatalysis,[27] oil/water separation,[28] and etc. However, in the existing reports, without taking into account the post-processing procedures, the aerogels prepared by direct electrospinning are either completely hydrophilic or hydrophobic, which causes some issues in the application of SSG. Although hydrophilic aerogels can pump water to the evaporation surface, the excessive water inside the aerogels acts as a “thermal bridge” which results in serious thermal dissipation from evaporation surface to bulk water. For another, because hydrophilic aerogels cannot float on the water surface, additional supporting materials are usually necessary. By contrast, completely hydrophobic aerogels are hardly suitable for preparing solar steam generators due to the incapability of pumping water to the evaporation surface. Therefore, it is rational that proper combination of hydrophilic and hydrophobic materials should be an ideal solution for constructing solar steam generators. To date, there have been many reports on macroscopic combination of hydrophilic and hydrophobic materials for SSG,[29-32] that is, hydrophilic and hydrophobic materials are separated in different regions of the reported solar steam generators, and some reported solar steam generators possess Janus structure.[32-37] The term “Janus structure” in these works refers to bilayer structure at the macro level, where the hydrophilic materials act as a water pump, while the hydrophobic materials enable the solar steam generators to float on water surface. However, no research on microscopic combination of hydrophilic and hydrophobic materials can be found. Therefore, it is meaningful to construct a solar steam generator composed of microscopically bonded hydrophilic and hydrophobic materials and discover the advantages of this design philosophy.
Herein, novel hydrophile/hydrophobe amphipathic Janus nanofibers aerogel is first proposed and used as a host material for constructing the solar steam generator. Every amphipathic Janus nanofiber in the aerogel is composed of a hydrophilic cellulose acetate (CA) side and a hydrophobic polyvinyl butyral (PVB) side, forming a “microscopically” Janus structure, as depicted in Figure 1a. Such peculiar architecture and composition endow the aerogel with water-pumping, self-floating, heat-insulating, salt-resisting properties, and excellent continuous working stability which are crucial characteristics for solar steam generators. To obtain the solar steam generator, the amphipathic Janus nanofibers aerogel is coated by photothermal materials consisted of carbon nanotubes (CNTs), silicon dioxide nanoparticles (SiO2 NPs), and polydopamine (PDA), in which CNTs play a prominent role in photothermal conversion, SiO2 NPs can regulate the water state, and PDA is the adhesive and contributes to photothermal conversion as well. The prepared solar steam generator exhibits excellent water evaporation, desalination, and removal of organic pollutants performances. Another advantage of the solar steam generator is that its water evaporation performance can quickly recover after it is flipped on the water surface, which is a meaningful feature for dealing with dynamic water environment.
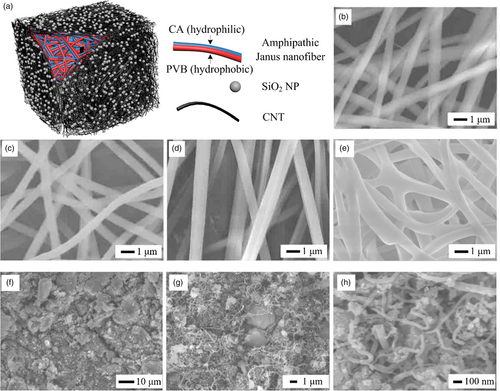
2 Results and Discussion
2.1 Structural Characterization
The microstructures of CA nanofibers, PVB nanofibers, CA//PVB Janus nanofibers, CA//PVB Janus nanofibers aerogel, and the surface of the solar steam generator are determined by SEM observation. As shown in Figure 1b,c, the prepared CA nanofibers and PVB nanofibers have similar morphologies, and their diameters are about 600 nm. Figure 1d reveals that every CA//PVB Janus nanofiber is composed of two tightly adjacent nanofibers whose diameters are both about 600 nm, indicating a typical Janus nanofiber structure. Although it is hard to determine the difference in the chemical compositions of the two adjacent nanofibers by existing material characterization techniques because CA and PVB both consist of elemental C, H and O, it is still safe to conclude that the two adjacent nanofibers are respectively comprised of CA and PVB according to the existing reports on fabrications of Janus nanofibers via parallel electrospinning. In these reports, it has been proved that the two adjacent nanofibers of a Janus nanofiber are respectively derived from two spinning solutions for parallel electrospinning.[38-40] Figure 1e manifests that the Janus nanofibers in the aerogel are successfully cross-linked, which reinforces the shape stability of the aerogel. The cross-linked Janus nanofibers have slightly larger sizes than those before cross-linking, which can be due to the swelling of polymer nanofibers in the solution containing cross-linking agent and volume expansion during thermal cross-linking. Furthermore, it can be noticed that micron-sized irregular channels exist among the Janus nanofibers and can allow water transfer. Figure 1f–h are SEM imagery of the surface of solar steam generator at different levels of magnification. The CNTs, with the diameters of ~40 nm, are evenly distributed on the product surface, and SiO2 NPs are attached to the CNTs. Hierarchical pores are formed among the CNTs and SiO2 NPs, which can facilitate water transfer and steam escape.
2.2 Hydrophilicity
The hydrophilicities of CA nanofibers, PVB nanofibers, CA//PVB Janus nanofibers, and photothermal materials are measured, as presented in Figure S3, Supporting Information. CA nanofibers can completely absorb the water droplet within 0.5 s, manifesting their very high hydrophilicity. On the contrary, the water droplet shows a stable contact angle of about 130° on hydrophobic PVB nanofibers, which indicates that PVB nanofibers alone are not a suitable host material for constructing solar steam generators because water cannot permeate through PVB nanofibers. As for the CA//PVB Janus nanofibers, the water droplet is slowly absorbed, which brings an important benefit: the water supply rate is not too high in SSG process. Previous studies have found that too fast water supply, normally caused by too high hydrophilicity of host materials, results in decreased temperature of evaporation surface and reduced water evaporation rate.[41] Additionally, the hydrophile/hydrophobe amphipathic CA//PVB Janus nanofibers can float on water surface, whereas CA nanofibers cannot. Therefore, from the perspective of hydrophilicity, CA//PVB Janus nanofibers should be more applicable for constructing solar steam generators compared with CA nanofibers and PVB nanofibers. As seen from Figure S3d, Supporting Information, the water droplet is quickly absorbed into photothermal materials, which is because the used CNTs, SiO2 NPs, and PDA are hydrophilic. Hence, in the prepared solar steam generator, the water pumped through the CA//PVB Janus nanofibers aerogel can quickly and uniformly spread all over the outer photothermal materials layer owing to the high hydrophilicity of the photothermal materials, which is conducive to water evaporation.
2.3 Solar Steam Generation Performance
Firstly, the performance of SSG using pure water as analyte is evaluated to optimize the product composition. The impacts of aerogel density, loading amount, and composition of the photothermal materials are respectively studied.
The density of an aerogel mainly affects saturation water content of the solar steam generator. As seen from Figure 2a, the density of solar steam generator becomes larger with increased mass ratio of Janus nanofibers to dispersion medium in freeze-drying process, while the saturation water content is decreased. Figure 2b shows the water evaporation performances of the solar steam generators. As the density increases, the solar steam generators display increased first and then decreased water evaporation rates. The increase in water evaporation rates can be attributed to alleviative thermal dissipation from evaporation surface to bulk water caused by decreased water contents in the solar steam generators. The thermal conductivities of CA and PVB are determined to be 0.17 and 0.23 W m−2 K−2, respectively, which are substantially lower than that of water (0.59 W m−2 K−2). Hence, decreased water content in the solar steam generator is beneficial to reduce heat dissipation and increase the temperature of evaporation surface. As seen in Figure 2c, the temperature (after 1-h irradiation) of evaporation surface increases with increased density of the solar steam generator, whereas that of the bulk water is reduced. However, the increased density of solar steam generator simultaneously results in increased resistance of water transfer, which is negative to water evaporation. Therefore, appropriate aerogel density is an important basis to ensure high-efficient water evaporation.
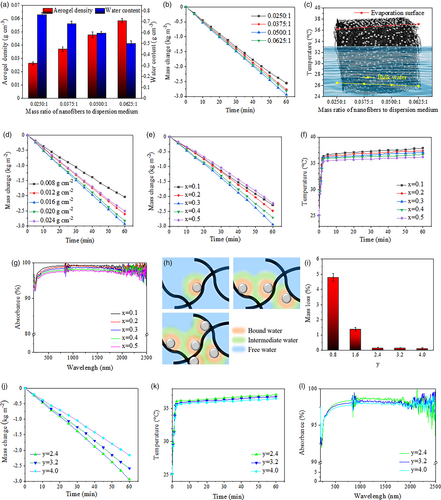
Secondly, optimized loading amount of photothermal materials is explored, as shown in Figure 2d. It can be seen that the solar steam generator with the photothermal materials of 0.016 g cm−2 exhibits the best performance. Insufficient photothermal materials cannot produce enough heat to sustain rapid water evaporation. On the contrary, because the pores in the photothermal materials are small (at the nanoscale), excessive photothermal materials leads to too high resistance of water transfer and thus decreases water evaporation rate.
Thirdly, the dosages of raw materials for preparing photothermal materials are optimized. To start with, the concentration of dopamine is fixed (y = 2.4), while the dosage of TEOS is varied (x = 0.1, 0.2, 0.3, 0.4, 0.5), and relevant SSG results are shown in Figure 2e. With the increased dosage of TEOS (i.e., enlarged content of SiO2 NPs), the water evaporation rates reveal an increasing trend at the beginning and then decrease when x > 0.3. To explain this phenomenon, at first, the surface temperatures of the solar steam generators are measured. As seen from Figure 2f, with the gradual increase of SiO2 NPs content, the surface temperatures of the solar steam generators show a falling trend, which can be ascribed to the fact that the SiO2 NPs, although have been modified with dark-colored PDA, possess relatively lower photothermal conversion performance than CNTs. The light absorption spectra of the solar steam generators shown in Figure 2g can prove the degradation of light absorption performance with increased SiO2 NPs content. Therefore, from the perspective of surface temperature, the performance enhancement of the products within x = 0.1–0.3 cannot be well explained. It has been known that there are three kinds of water in a material: bound water (BW), intermediate water (IW), and free water (FW).[42] Among them, BW is the water molecule in direct contact with the hydrophilic materials. Because strong chemical bonds are formed between BW and the materials, BW is difficult to evaporate. The FW is relatively far from the adsorption materials, and its property is consistent with the bulk water. The IW is sandwiched between the BW and FW. Due to the interaction between the adjacent BW and adsorption materials, the hydrogen bonds between IW and BW are weakened, leading to the lowest energy required for the evaporation of IW. That is, IW has the lowest evaporation enthalpy among the three kinds of water.[43] In this study, SiO2 NPs can fill in the gaps among CNTs and thus regulate the water state in the photothermal materials. Figure 2h visually depicts the increased proportion of IW and reduced proportion of FW when more SiO2 NPs are introduced. In addition, the variation of BW can be ignored because BW is in monomolecular type and its proportion is very low among the three kinds of water. It has been reported that the ratio of IW to FW in a material can be quantitatively measured by Raman spectroscopy, and Gaussian function can be used to fit the peaks at 3233, 3401, 3514 and 3630 cm−1.[44] The peaks at 3233 and 3401 cm−1 correspond to the in-phase and out-of-phase vibration modes of O–H bonds in water that forms four hydrogen bonds with surrounding water molecules, which represent the existence of FW. The peaks at 3514 and 3630 cm−1 are assigned to the symmetric and asymmetric stretching of O–H bonds in the weakened hydrogen bonds, which are the characteristic peaks of IW that is relatively weakly affected by hydrogen bond. The ratio of the fitted peak areas of the two kinds of water is the molar ratio of the two kinds of water. The Raman spectra of the hydrated photothermal materials are given in Figure S4, Supporting Information. According to the calculation of fitted peak areas, the molar ratios of IW to BW in these samples (x = 0.1, 0.2, 0.3, 0.4, 0.5) are 0.38, 0.52, 0.67, 0.71, 0.73, respectively, which further demonstrates increased proportion of IW when more SiO2 NPs are introduced. However, excessive SiO2 NPs (x = 0.4 and 0.5) cannot substantially increase the proportion of IW, but make the light absorption performance of the photothermal materials continuously degrade, as revealed in Figure 2g, resulting in the decreased water evaporation rate.
The impact of PDA is evaluated while the ratio of CNTs and SiO2 NPs is fixed (x = 0.3). Here, the PDA mainly acts as an adhesive in the photothermal materials, and also contributes to photothermal conversion due to its dark color. In order to study the adhesive performance of the PDA, the solar steam generators with different PDA contents were completely immersed in water for 1 h and then naturally dried. The mass changes of the samples before and after soaking are given in Figure 2i. When PDA is not enough (y = 0.8 and 1.6), some photothermal materials fall off the solar steam generators and disperse into the water, and obvious mass losses of the samples are found. When sufficient PDA is introduced (y ≥ 2.4), the photothermal materials can be stable on the solar steam generators. In spite of this, excess PDA content is still detrimental because the light absorption performance is impaired and the water evaporation rate is decreased, as indicated in Figure 2i–l.
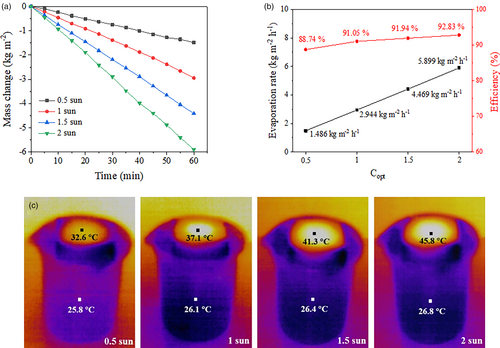
Based on the above, the optimized parameters for preparing the solar steam generator are as follows: the mass ratio of nanofibers to water is 0.0500:1 in the dispersion medium for freeze-drying; the dosages of TEOS (x) and dopamine (y) are respectively 0.3 and 2.4 for preparing the photothermal materials; the loading amounts of the photothermal materials is 0.016 g cm−2. The optimized solar steam generator can provide a state-of-the-art water evaporation rate (2.944 kg m−2 h−1) under 1 sun irradiation. Next, the impact of light irradiation intensity on water evaporation rate and energy efficiency is investigated. Light irradiation intensity is one of the important factors affecting the water evaporation rate. To further explore the water evaporation performance of the optimized solar steam generator under different light irradiation intensities, 0.5, 1, 1.5, and 2 suns are respectively adopted. As shown in Figure 3a,b, water evaporation rates of 1.486, 2.944, 4.469 and 5.899 kg m−2 h−1 are achieved when the irradiation intensities are 0.5, 1, 1.5, and 2 suns, while the energy efficiencies are 88.74%, 91.05%, 91.94% and 92.83%, respectively. In most reports on solar steam generators, energy efficiency generally increases with the increase of irradiation intensity.[10, 32, 45] Of the solar steam generator prepared in this work, the evaporation surface temperature significantly rises with increased irradiation intensity, whereas the temperature of bulk water varies little, which indicates excellent thermal insulation performance of the prepared solar steam generator. The experimental results show that the product possesses superior SSG performance under different irradiation intensities, suggesting its application potential in the natural environment where sunlight intensity changes frequently and the conditions with concentrated sunlight by using optical concentrators such as convex lens and heliostats.
The stability of solar steam generators is also a key factor affecting their practical application. Hence, the real-time water content, continuous working stability and reusability of the prepared solar steam generator are studied. The test method of real-time water content is as following: the solar steam generator is taken out from bulk water every 20 min during SSG process, the water drops on the surface of solar steam generator are wiped off by using filter papers, and then the weight of the waterlogged solar steam generator is measured and compared with that of the dry solar steam generator. Continuous working stability is evaluated by 10-h uninterrupted irradiation, and the mass changes of water are recorded per hour to calculate hourly water evaporation rates. The reusability is studied by 20-cycle SSG processes. Continuous 10-h irradiation is employed for each cycle, and the solar steam generator is dried after each cycle.
The test results are shown in Figure 4a–c. The water content of the solar steam generator is basically constant in SSG process, indicating continuous and stable water pumping. The water evaporation rates remain steady within both 10-h continuous SSG process and 20-cycle reusing. These results manifest that the product possesses excellent stability and has great application potential.
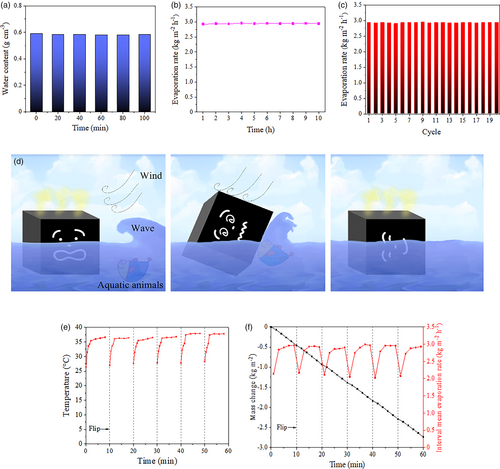
An unavoidable problem with solar steam generators in practical outdoor applications is the flip of the generators due to the effects of wind, water wave, and etc. To accommodate this situation, the prepared cubic solar steam generator is fully covered with photothermal materials on its six surfaces, so that its all surfaces can be used for water evaporation, as depicted in Figure 4d. The performance recovery of the solar steam generator after flipping is investigated, and the specific experimental methods are as follows: in a 60-min SSG experiment, the solar steam generator is randomly flipped every 10 min to switch the evaporation surface. The temperature of evaporation surface (Figure 4e) and water mass loss are recorded after flipping. To meticulously investigate the effect of flipping on water evaporation rate, the concept of “interval mean evaporation rate” is proposed. As seen from the red dots in Figure 4f, every dot is also calculated by Formula S1, Supporting Information, and the irradiation time (T) and initial mass of water are reset at the initial point of each interval. The results reveal that temperature of evaporation surface can quickly rise to more than 90% of the maximum temperature within 2 min, and concurrently, the interval mean evaporation rate is recovered to near maximum. The black line in Figure 4f presents the water mass change (2.740 kg m−2 h−1 in total) over the course of 60-min SSG experiment with quintuplicate flipping of the solar steam generator. It can be seen that the flipping of the solar steam generator does not have a great influence on the total water evaporation rate, meaning that the prepared solar steam generator is adequate for dealing with dynamic water environment.
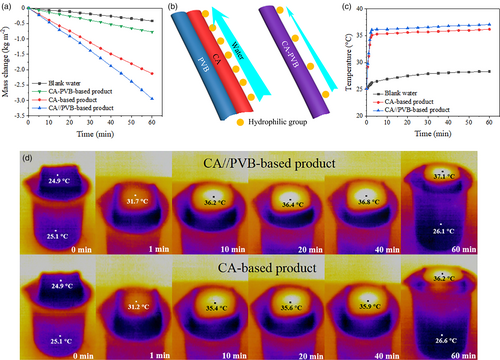
To clarify the superiority of using hydrophile/hydrophobe amphipathic Janus nanofibers aerogels (CA//PVB-based product for short) as host materials for solar steam generators, completely hydrophilic CA nanofibers aerogel (CA-based product), hydrophobic PVB nanofibers aerogel (PVB-based product), and CA-PVB-blended nanofibers aerogel (CA-PVB-based product) are also respectively fabricated and coated with photothermal materials through the same freeze-drying and air spraying processes as those for preparing amphipathic Janus nanofibers aerogel. The PVB-based product cannot be used as a solar steam generator at all due to its inability to pump water. The CA-based product can pump water but cannot float on water surface. Therefore, a scaffold has to be used to immobilize the CA-based product on the water surface. CA-PVB-based product also can float on water surface by itself. The water evaporation performances of CA//PVB-based product, CA-PVB-based product, and CA-based product as well as the water without using any solar steam generators (blank water), are compared. The results of water evaporation rates are shown in Figure 5a. The CA//PVB-based product exhibits much higher water evaporation rate than CA-PVB-based product and CA-based product (2.944 kg m−2 h−1 vs 0.771 kg m−2 h−1 and 2.125 kg m−2 h−1), and the evaporation rate of blank water is only 0.414 kg m−2 h−1. Compared with CA//PVB-based product, CA-PVB-based product shows a much lower water evaporation performance, even though they have the same composition in raw materials. The poor water evaporation performance of CA-PVB-based product can be due to the very low vertical water transportation along CA-PVB blended nanofibers aerogel. The vertical water transportation along an aerogel is measured according to the reference.[46] The transportation time for water through three types of aerogels, namely, CA/PVB Janus nanofibers aerogel, CA-PVB blended nanofibers aerogel, and CA nanofibers aerogel, each with a thickness of 3 cm, and fully covering their upper surfaces, is 47, 169, and 45 s, respectively. As illustrated in Figure 5b, the CA components of CA//PVB Janus nanofibers facilitate rapid upward pumping of water molecules due to the closely situated hydrophilic groups on CA. As for CA-PVB blended nanofibers, the presence of PVB molecular chains results in an increased distance between hydrophilic groups, leading to a much weaker water-pumping ability. Thus, a simple blending of hydrophilic and hydrophobic materials is not applicable for fabricating solar steam generators. The relatively lower water evaporation performance of the CA-based product can be attributed to its excessive internal water content (~0.95 g cm−3), which aggravates heat dissipation, as revealed in Figure 5c,d. The thermal conductivities of the wet CA//PVB-based product and CA-based product are 0.31 and 0.47 W m−2 K−2, respectively. In addition, it is also found that the CA-based product exhibits poor reusability, as shown in Figure S5, Supporting Information. To explain this result, the physical photos of CA//PVB Janus nanofibers aerogel, CA nanofibers aerogel, CA//PVB-based product, and CA-based product before and after water soaking are provided in Figure S6, Supporting Information. After 10 cycles, the photothermal materials are seriously peeled off the CA-based product. This phenomenon is attributed to the swelling of CA nanofibers aerogel in water. After water soaking, CA-based product experiences a noticeable expansion in volume, which creates internal stress between photothermal materials and the swollen CA nanofibers aerogel and thus leads to the detachment of photothermal materials. As for the CA//PVB-based product, the hydrophobic PVB components suppress the volume expansion of the CA components, and the entire aerogel retains almost the same morphology before and after being placed in water. Thus, photothermal materials can be stable on CA//PVB Janus nanofibers aerogel during SSG process, thereby achieving excellent reusability. From the above results, it is obvious that the hydrophile/hydrophobe amphipathic Janus nanofibers aerogels are superior for preparing solar steam generators in view of their high performance, reusability and ease of use.
2.4 Desalination and Pollutants Removal Performances
Desalination and pollutants removal performances are important application aspects of solar steam generators. Fast evaporation rate of saline water and effective inhibition on salt crystallization are crucial for desalination applications. The water evaporation performances and salt-resisting properties of CA//PVB-based product and CA-based product are comparatively studied. The 3.5% NaCl aqueous solution, which is close to the average salinity of seawater, is adopted as a model substance. As seen from Figure 6a, in the first-hour SSG experiment, the water evaporation rates with CA//PVB-based product and CA-based product are 2.909 and 1.916 kg m−2 h−1, respectively. Compared with the evaporation rates of pure water when using the two kinds of solar steam generators, the evaporation rates of 3.5% NaCl solution decrease 1.19% and 9.84%, respectively. From careful observation on the red curve in Figure 6a, the slope of the curve decreases with the extension of evaporation time, which means CA-based product occurs obvious performance attenuation during the process of treating saline water. To amplify the effect of saline water evaporation on the two solar steam generators, 5-h evaporation experiments are carried out, and the photographs of the two post-use solar steam generators are provided in Figure 6b,c. It is obviously seen that a lot of salt crystals deposit on CA-based product, whereas the situation of CA//PVB-based product is much better, demonstrating excellent salt-resisting property of CA//PVB Janus nanofibers aerogel. On the one hand, salt crystals block water transfer channels, and on the other hand, they reflect light. Both of these effects negatively impact on water evaporation. According to the references,[32, 47] it has been proved that hydrophobic materials can inhibit the shuttle of salt ions. Therefore, the excellent salt-resisting property of CA//PVB Janus nanofibers aerogel can be owing to the hydrophobic PVB components. To sum up, in terms of desalination property, the amphipathic Janus nanofibers aerogel also shows its superiority over the hydrophilic substrate.
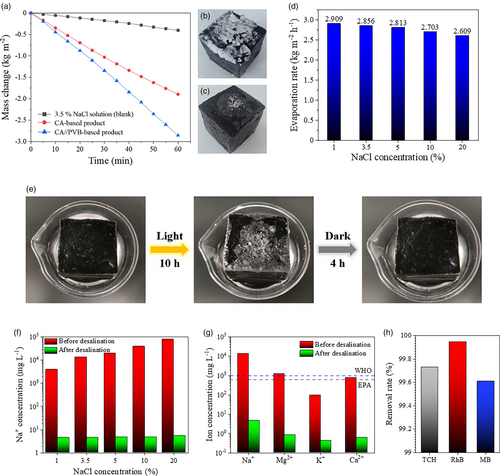
The evaporation rates of saline water with different salinities when using the CA//PVB-based product are also studied. The NaCl solutions with different concentrations (1%, 3.5%, 5%, 10%, and 20%) are prepared as analytes, and the results are shown in Figure 6d. The evaporation rate decreases with the increase of salinity, but the difference is not great, which proves that the product can be able to effectively treat high-salinity water. The desalination performances of the CA//PVB-based product on saline water with different salinities are revealed in Figure 6f, and the desalination results on actual seawater (sampled from Bohai Sea, geological coordinate of sampling site: 40°53′23.90″N, 121°12′26.72″E) are given in Figure 6g. As revealed in Figure S7, Supporting Information, the SSG process is performed in a lab-made acrylic tank (18 cm × 9 cm × 12 cm) in which the water vapor is condensed and collected at a rate of 2.227 kg m−2 h−1 (this value is obtained when pure water is treated with the amphipathic Janus nanofibers aerogel under 1 sun). The results demonstrate that the concentrations of salt ions in the collected liquids greatly decrease compared with those of the original saline water and actual seawater and are much lower than the standard of the World Health Organization (WHO) and the United States Environmental Protection Agency (EPA) for salt ion concentration in drinking water. Moreover, it is also found that the prepared solar steam generator is self-cleaning in the dark. As seen from Figure 6e, after 10-h light irradiation (close to the total energy of sunlight in a day), some visible salt crystals deposit on the surface of product, and these salt crystals disappear automatically within 4 h in the dark. This character may be owing to the large pores in the aerogel that are conducive to the rapid downward diffusion of salt ions under the driving of concentration gradient between evaporation surface and bulk water. This self-cleaning property is beneficial for performance recovery of the product with day/night changes in outdoor applications. In addition, a faster way to eliminate the influence of salt deposition is to flip the solar steam generator. That is, after the evaporation surface is altered, the water evaporation rate can be recovered quickly.
The treatment on wastewater containing antibiotics or organic dyes is another essential application of SSG. To evaluate the related performance of CA//PVB-based product, TCH, RhB, and MB solutions, with the concentrations of 0.01 g L−1, are selected as model substances. As is seen from Figure 6h and Figure S8, Supporting Information, the removal rates of these organic contaminants are all above 99% in the condensed water.
3 Conclusion
In summary, hydrophile/hydrophobe amphipathic Janus nanofibers aerogel is fabricated by the combination of parallel electrospinning technology and freeze-drying. Photothermal materials are covered on the six surfaces of the cubic aerogel to obtain a 3D self-floating solar steam generator. The light absorption rate of the product is above 95% in the full spectrum range. Under light irradiation, the surface temperature of the product rapidly rises to meet the requirement of fast water evaporation within 2 min. The water evaporation rate can reach up to 2.944 kg m−2 h−1 with the energy efficiency of 91.05% under 1 sun irradiation, and after the product is flipped, the water evaporation rate can be quickly recovered. Owing to the salt-resisting property, the formation of salt deposition can be effectively slowed down, so that the performance attenuation in the evaporation of saline water is suppressed. The product possesses excellent ability of solar desalination and removing organic pollutants to acquire drinkable water.
4 Experimental Section
See Supporting Information for the Experimental Section.
Acknowledgements
This work was financially supported by Natural Science Foundation of Chongqing, China (cstc2021jcyj-msxmX0898), Natural Science Foundation of Jilin Province (20210101080JC), National Natural Science Foundation of China (51803012, 51573023).
Conflict of Interest
The authors declare no conflict of interest.




