New Strategy for Boosting Cathodic Performance of Protonic Ceramic Fuel Cells Through Incorporating a Superior Hydronation Second Phase
Abstract
For protonic ceramic fuel cells, it is key to develop material with high intrinsic activity for oxygen activation and bulk proton conductivity enabling water formation at entire electrode surface. However, a higher water content which benefitting for the increasing proton conductivity will not only dilute the oxygen in the gas, but also suppress the O2 adsorption on the electrode surface. Herein, a new electrode design concept is proposed, that may overcome this dilemma. By introducing a second phase with high-hydrating capability into a conventional cobalt-free perovskite to form a unique nanocomposite electrode, high proton conductivity/concentration can be reached at low water content in atmosphere. In addition, the hydronation creates additional fast proton transport channel along the two-phase interface. As a result, high protonic conductivity is reached, leading to a new breakthrough in performance for proton ceramic fuel cells and electrolysis cells devices among available air electrodes.
1 Introduction
With increasing concerns from the public about sustainable energy supply, fuel cells have received considerable attention during the past several decades because of their advantageous features of high energy conversion efficiency, low emission, quiet operation, and size flexibility for on-demand electric power production.[1-11] Most research efforts to-date have focused on either low-temperature polymer electrolyte membrane fuel cells (PEMFCs) operating at room temperature to 80 °C, or solid oxide fuel cells (SOFCs) operating at 650–1000 °C. However, it is generally believed that the advantages of both high-temperature SOFCs (fuel flexibility, non-precious electrode electrocatalysts, and high efficiency) and PEMFCs (quick start-up and easy sealing) can be achieved while their respective disadvantages are avoided if fuel cells can be created that operate at intermediate temperatures (e.g. 300–650 °C).[8-20] Since SOFCs do not involve any liquid cell component, reduced-temperature SOFCs are more promising for practical application. However, due to high activation energy associated with oxygen ion diffusion in a solid electrolyte, a lot of SOFCs based on an oxygen-ion conducting electrolyte (O2−-SOFCs) are inefficient at low temperatures. As an alternative to these efforts, protonic ceramic fuel cells (PCFCs) have emerged as a subcategory of SOFCs that may realize operation at intermediate temperatures, and thereby extract many of the advantages of both SOFCs and PEMFCs.[21-25]
Similar to that for O2−-SOFCs, state-of-art cathode materials for PCFCs are mixed oxygen ion and electronic conducting cobalt-rich perovskites or perovskite-based composites considering their high activity for ORR, such as BaCo0.4Fe0.4Zr0.1Y0.1O3−δ (BCFZY),[14] and Ba0.5Sr0.5Co0.8Fe0.2O3−δ,[26] which however demonstrate several drawbacks, including inadequate compatibility with the electrolyte, poor structural and thermal stability, and high thermal expansion coefficients (TECs).[27] In particular, as reported by Maier et al., cobalt on the perovskite B-site can lead to a decrease of the proton uptake, so cobalt-rich perovskites usually show a higher energy barrier for the proton transfer from the electrolyte to the cathode.[28] To maximize the proton conductivity in perovskites, enhancing the degree of oxygen vacancies hydration is required, thus high-water content in the surrounding atmosphere is preferred, which however, will increase the competitive adsorption of water over electrode as well reduce the oxygen partial pressure in the surrounding atmosphere, thus causing negative effect on ORR activity. It is thus unlikely to develop a “perfect” single-phase cathode for PCFC simply based on structure and defect tuning.
Different from prior efforts to optimize single-phase PCFC cathodes, here we showed how the introduction of a second phase with super hydrating capability into a cobalt-free perovskite dominant phase with high ORR activity can effectively overcome the challenge for realizing simultaneously high activity for ORR and proton conductivity, and thus achieving superior performance for PCFC applications. By rational design of a precursor material with the nominal composition of NaxSr1−xTi0.1Fe0.9O3−δ, we created a self-assembling two-phase composite cathode consisting of an electrocatalytically active NaySrzTiuFe1−uO3−δ (NSTF) perovskite main phase and nanosized β-NaFeO2 (NF) domains with superior proton affinity and water adsorption capability. A synergy is created between the two phases that facilitates proton diffusion inside the electrode even at low water vapor partial pressures. Different from most cathode materials that show a negative dependence on -a sign that hydration/water adsorption competitively interferes with ORR activity, the NSTF/NF nanocomposite cathode developed here showed a positive dependence in PCFC application, which we ascribed to enhanced proton uptake and the potential synergistic development of a hydronium-ion (H3O+) transport pathway at the interlayer between the NSTF and NF phases.
A cell with the composite cathode yields a peak power density (PPD) of 0.807 W cm−2 at 600 °C, a higher value than most PCFCs ever reported with cobalt-free electrodes, and even overperforming lots of PCFCs with cobalt-based electrodes. Infiltrating the cathode with SrCoO3−δ leads to further enhancement in power output, with the PPD reaching 0.966 W cm−2 at 600 °C. The nanocomposite also shows a reduced TEC compared with cobalt-based alternatives, making it more compatible with the electrolyte, and demonstrates stable operation under constant polarization for hundreds of hours without degradation. In addition, the high proton/water affinity of the nanocomposite electrode also makes it a superior steam electrode for proton ceramic electrolysis cells (PCECs). A record-breaking polarization current of 1.42 A cm−2 at a cell voltage of 1.28 V at 600 °C is achieved at the water content of 80 vol.% under optimized conditions.
2 Results and Discussion
2.1 Nanocomposite Formation and Morphology
We have previously demonstrated that SrTi0.1Fe0.9O3−δ (STF) perovskite is a favorable cobalt-free cathode for O2−-SOFCs with high ORR activity at intermediate temperatures.[29] However, it shows poor hydronation capability at low water content in atmosphere, thus although high activity in O2−-SOFC it did not produce favorable performance in PCFC. As showed in Figure 1a, a PPD of 600 mW cm−2 was achieved in a thin-film oxygen-ion conducting SDC (∼20 μm) electrolyte fuel cell at 600 °C, while it reduced to only 361 mW cm−2 in a PCFC with BZCYYb electrolyte of similar conductivity to SDC and the same thickness (∼20 μm) at the same temperature using the same 3% humidified air as the cathode atmosphere. Based on EIS on symmetric cell test, much larger electrode polarization resistance was observed when STF was applied in protonic electrolyte when compared with that on oxygen-ion conducting SDC electrolyte (Figure 1b). The poor proton conductivity of STF should be the major resistance for its poor performance on BZCYYb electrolyte.[30]
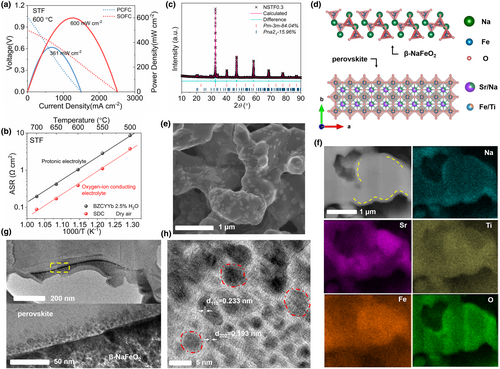
β-NaFeO2 was reported to be a superior material for water storage and regenerated at the temperatures above 450 °C even have absorbed CO2 in the presence of water vapor.[31] By introducing NF into STF, the high-water content as stored by NF may facilitate the hydronation of STF to reduce the concentration of oxygen vacancies and increase the proton concentration, as a result increased protonic conductivity to enhance performance in PCFC could be reached even at low water content in atmosphere. Clearly, the formation of NF/STF nanocomposite will increase the interface contact area between the two phases to facilitate the hydronation of STF, consequently maximizing the proton conductivity. Here, we applied a facile way for the synthesis of such nanocomposite by thermal-induced phase decomposition of perovskite precursors with the nominal compositions of STF and NaxSr1−xTi0.1Fe0.9O3−δ (NSTFx, x = 0.1, 0.2, 0.3, and 0.4, Figure 1c,d and Figures S1–S7, Supporting Information).
As shown by the scanning electron microscope (SEM) images of NSTF0.3 as a representative example, the composite showed the morphology of nanosized NF sheets evenly distributed within a perovskite oxide matrix (Figure 1e). To further identify the morphology of highly efficient network of surfaces and interfaces in NSTF0.3, SEM was used to characterize STF and Na-doping samples with the typical images shown in Figure S8, Supporting Information. Focused ion beam (FIB)-SEM and energy-dispersive X-ray (EDX) analysis (Figure 1f) demonstrated that the Na element distribution was inhomogeneous due to the surface lamellar characteristic of the decorating NF phase. Two typical high-resolution transmission electron microscope TEM (HR-TEM) images of the NSTF0.3 nanocomposite microstructure were obtained (Figure S9, Supporting Information), in which well-defined crystalline fringes with lattice spacings of 0.279 and 0.249 nm were clearly observed, corresponding to the (011) crystal plane of the perovskite phase and the (201) crystal plane of the NF phase, respectively. The phase distribution in the porous composite NSTF0.3 electrode was further investigated by TEM (Figure 1g,h, selected areas of increasing magnification extracted from the cross-section of the NSTF0.3 electrode from the sample with FIB-SEM). Darker colored crystallites of NF with diffraction fringe distance of 0.193 and 0.233 nm, corresponding to (202) and (112) crystal planes can be seen imbedded within a perovskite bulk phase matrix. In high surface area materials, significant proton transport can also occur via H2O–H3O+ species adsorbed on surfaces and interfaces at the low temperatures.[32] The interfacial protonic conduction could be realized potentially while the nanograins (smaller than 100 nm) materials uniformly disperse on the perovskite oxide surfaces connecting with the long-length interfacial hydrated layers. Thus, the large interface contact in NSTF0.3 may enable the interfacial proton conduction in PCFC field.
2.2 Enhanced Proton Conductivity via Grotthuss Mechanism
Proton conduction in perovskite oxide bulk is believed to occur via a Grotthuss mechanism, involving first proton adsorption (uptake) associated with an oxygen ion, followed by rotation and hopping processes that allow transfer of the proton to adjacent oxygen ions.[33, 34] In humid and oxidizing environments (as in PCFC cathodes), protonic carriers [] are formed by the dissociative incorporation of water into oxygen vacancies (acid–based reaction: ).[28] Thus, the fundamental prerequisite for proton formation and subsequent proton migration in Grotthuss mechanism in the perovskite lattice in humid and oxidizing environments is the presence of oxygen vacancies. However, typically only a small fraction of the available oxygen vacancies in perovskite oxides can be hydrated, and the hydration process may also compete with oxygen reduction for oxygen vacancies.[28] This charge transfer within oxide bulk along with the formation of proton carriers usually associates with the change in electronic structure, while X-ray absorption spectroscopy (XAS) is a powerful technique to investigate the oxidation state of transition metals with element-selective character.[35-38] We carried out XAS measurements to investigate the local electronic and geometric structure of STF and NSTFx with the increasing Na amount (Figures S11–S13, Supporting Information). Here, to further probe the proton affinity of the NSTF0.3 nanocomposite under realistic operating conditions, we performed operando hard XAS measurements to directly monitor the electronic structure and geometric structure changes of the cathodes.
The XAS measurements of STF and NSTF0.3 materials were carried out in the electrolyte supported cells (NSTF0.3/STF|BZCYYb|Ag) under mimetic electrochemical conditions (in dry and wet air under a constant load current ∼25 mA cm−2) (Field equipment diagram: Figure S14, Supporting Information). In previous works, the Fe K-edge data has been used to investigate the valence state of Fe in Fe-based perovskite oxides.[39, 40] The XAS results are shown in Figure 2a,b. Both STF and NSTF0.3 showed a clear shift of the Fe K-edge absorption edge, namely X-ray absorption near edge structure (XANES), towards lower energy upon heating from room temperature to the operating temperature of 450 °C, indicating a decrease of the Fe oxidation state.[39] This expected change is due to the loss of lattice oxygen with increasing temperature, which is accompanied by reduction of the Fe ion. After the introduction of water (3 vol.%) at 450 °C, however, the Fe oxidation state gradually increases in both samples, as the acid–base reaction fills some of the oxygen vacancies and creates protonic carriers. The Fe-K absorption edges under 450 °C wet air for both cathodes did not shift fully back to the original state, namely the room-temperature state, suggesting their incomplete hydration of the oxygen vacancies. However, compared with STF, NSTF0.3 showed a relatively larger Fe valence recovery behavior, indicating a stronger hydration reaction for NSTF0.3 cathode (the formation of proton defect causes oxygen vacancy to be occupied and leads to the change of Fe valence state).
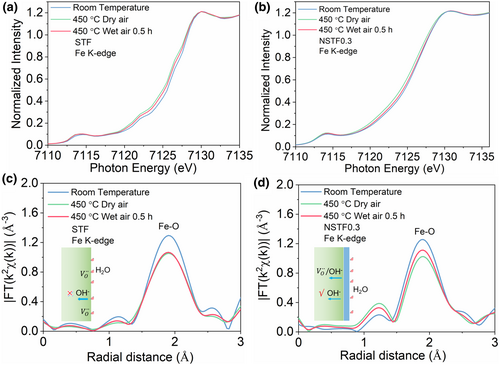
In addition to the analysis of Fe valence state for the two materials under mimetic electrochemical conditions, changes in the local environment of the STF and NSTF0.3 electrodes were also probed by extended X-ray Absorption Fine Structure (EXAFS) measurement (Figures S15 and S16, Supporting Information and Fourier-transformed (FT) k2-weighted Fe K-edge EXAFS in Figure 2c,d). Upon first heating to the operating temperature in dry air, the intensity of the scattering peak corresponding to the Fe–O shell decreases notably for both electrodes, consistent with the formation of oxygen vacancies as discussed above. Then under water vapor at 450 °C, the intensity of the Fe–O coordinated peak partially recovers more significantly for NSTF0.3 cathode, again verifying the partial hydration of the oxygen vacancies by the acid–base reaction in humid air. In contrast, the Fe–O peak intensity recovers just slightly in the STF cathode, suggesting limited hydration in this material under these conditions. Together, these operando XANES and FT-EXAFS results support the presence of protonic carriers in the NSTF0.3 nanocomposite electrode formed by acid–base reaction of water with lattice oxygen vacancies under conditions consistent with the operating environment of this cathode electrode. We also compared the hydration capacity of STF and NSTF0.3 using TG measurement while the samples treated under 20 vol.% H2O in air at 250 °C for 3 h (Figure S17, Supporting Information). The more weight loss under the water desorption process of NSTF0.3 shows that the superior water storage capacity. A beneficial proton uptake of NSTF0.3 showed that the NF phase is helpful to carry the water from the humid air to the interface between the NF phase and the perovskite phase.
2.3 Creation of Interfacial Conductivity via Vehicle Mechanism
In addition to Grotthuss mechanism, proton conduction could also be proceeded via vehicle mechanism, in particular for oxide with rich grain boundaries at lower temperatures. Considering the rich NF-STF two phase boundaries and the high-water storage capability of NF, the potential proton conduction in NSTF0.3 at grain boundary (vehicle mechanism) was also preliminarily investigated by using a “sandwich-type” three-layer membrane conductivity test (Figure 3a). Specifically, we sandwiched a layer of the oxide (either STF, NF, or NSTF0.3) between two layers of Nafion 211, each of which was contacted on the outside by a standard PEMFC Pt/C catalyst-coated membrane (CCM) gas diffusion layer (GDL) electrode. It is well known the proton conductivity in Nafion membrane is via the way of H3O+. Thus, the measured conductivity in the oxides mostly reflects the one via vehicle mechanism since the Grotthuss mechanism involves no H2O vehicle. The significant (∼5–10×) increase in the high-frequency real-impedance intercept for the STF and NF-based sandwich cells compared with the Nafion control indicates poor proton conductivity via the H3O+ way, while the NSTF0.3 cell showed a much smaller increase in impedance, suggesting that the NSTF0.3 composite accommodated significant proton conductivity via the vehicle mechanism (H3O+) under these conditions (Figure 3b). The calculated proton (H3O+) conductivities (according to equation σ (S cm−1) = (h (cm))/(R (ohm) × S (cm2)), h is the oxide layer thickness, R is the resistance, and S is the cross-sectional area across where the H3O+ migrate) for STF, NF, and NSTF0.3, based on the measured thickness of each oxide layer (obtained from the SEM images in Figures S18–S20, Supporting Information), are summarized in Figure 3c.[41-43] The proton (H3O+) conductivity of the NSTF0.3 nanocomposite (0.022 S cm−1) is more than 15 time higher than the single-phase perovskite oxide STF (0.00135 S cm−1) and the NF phase (0.00158 S cm−1). Because neither NF nor STF show appreciable proton conductivity on their own, we thus attributed the significantly higher conduction behavior of the NSTF0.3 composite to H3O+-based transport along the network of surfaces and interfaces between the perovskite and the NF phases. We also measured fuel cell polarization curves for all four sandwich cells, in accordance with the EIS results, the STF and NF-based cells showed negligible cell performance with peak power densities (PPD) of 5 and 13.5 mW cm−2, respectively (Figures S21 and S22, Supporting Information), while the NSTF0.3-based cell attained a PPD of 126 mW cm−2 (Figure 2d). Finally, the contrast cell (double layers of Nafion without an intervening oxide layer) attained a PPD of 291 mW cm−2 (Figure S23, Supporting Information). The detailed XRD pattern of NSTF0.3 after the measurement in PEMFC mode is displayed in Figure S24, Supporting Information. The XRD results of the existing NF and NSTF phases justifies the reliability of the PEMFC measurement.
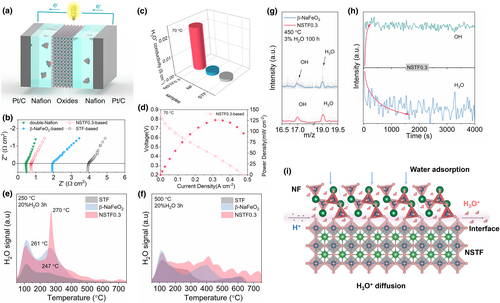
The above results suggest the potential presence of surface/interface-based proton/H3O+ transport in the NSTF0.3 nanocomposite at low temperatures, further evidence is required to establish whether this transport pathway also persists at higher temperatures. We then carried out H2O-TPD for all three oxides after treatment under 20 vol.% H2O for 3 h at 250 °C and at 500 °C. By quenching from high temperature to room temperature, the proton adsorption state of the samples at the high temperatures can be preserved. After treatment at 250 °C followed by a quench to room temperature, the subsequent TPD curves for all three samples showed two desorption peaks, at around 120 and 260 °C (Figure 3e). The water absorption capacities of these three oxides were estimated from the desorption peak areas, yielding values of 57.52 × 10−9 A s mg−1, 193.6 × 10−9 A s mg−1 and 202.2 × 10−9 A s mg−1, for STF, NF and NSTF0.3, respectively. The two desorption peaks are related to surface chemically adsorbed water and water uptake within the bulk of oxide, respectively. The NF phase shows a higher H2O desorption temperature and peak intensity than that of the single perovskite phase STF, suggesting its higher surface chemical adsorption and bulk water storage capability. Interestingly, the NSTF0.3 composite still exhibited higher H2O desorption temperature and capacity than either of the component oxides, suggesting the creation of synergy between the perovskite and NF phases in the nanocomposite. Also, the sharp shape of the desorption peak for the NSTF0.3 sample from 250 to 500 °C suggests the fast diffusion of water molecules between the perovskite layer and the NF layer.
The H2O-TPDs were re-measured to investigate the water storage capacity at realistic cathode operating temperatures (500 °C). As shown in Figure 2f, the NF and NSTF0.3 samples had significant retention of water up to approximately 500–600 °C. The NSTF0.3 nanocomposite sample again showed the largest water storage capacity and the highest dehydration cut-off temperature (>600 °C, the water storage on the interface or in the bulk of the oxide will not all be lost before 600 °C, which ensures the necessary carrier for the transport of H2O–H3O+), suggesting that the nanocomposite can retain a large number of water molecules between the perovskite and NF layers even at typical PCFC cathode operating temperatures. Herein, we also firstly discuss the intermediate-temperature proton transport behavior via vehicle mechanism (H2O–H3O+) within the nanocomposite PCFC electrode material on the basis of the experiment of time-to-flight secondary ion mass spectrometry (TOF-SIMS). The NF and NSTF0.3 samples were treated in the form of discs by dry pressing under 3 vol.% H2O for 100 h at 450 °C, then quenched from high temperature to room temperature to hold its phase structure and oxidation state. As shown in Figure 3g, the OH* (m/z ≈ 17) and H3O+ (m/z ≈ 19) signals are successfully detected from the surface of NF and NSTF0.3 discs. This result indicates that a fraction of protons carriers introduced in the NF and NSTF0.3 sample at the high temperature at 450 °C does exist in the form of H3O+. From the depth profile of the TOF-SIMS for NSTF0.3, the H3O+ signal weakens with the increasing depth, while the OH* signal shows the opposite in order to maintain the overall electrical neutrality (Figure 3h). We assume that the proton carrier may be mainly in form of H3O+ on the surface of the electrode. While in the bulk phase, the protons transported with the H2O carriers were held by oxygen species in form of OH−.
In summary, based on the results of PEMFC sandwich cell, H2O-TPD, XANES, EXAFS, and TOF-SIMS studies, the introduction of NF into STF with the formation nanocomposite, both hydration and interfacial protonic conduction was facilitated due to the extensive interfacial area network created between the perovskite matrix and the NF phases. We then further proposed the transport pathway of H3O+ in NSTF0.3, as shown in Figure 3i. Exerting the strong water vapor absorption capacity of the NF phase, the proton combines with the water molecule at the two-phase interface to form a H3O+, which is transported through the interface for a long distance from the TPB to the upper cathode. The NF phase provides high proton affinity and water storage capability, thereby ensuring that high proton conductivity and improved proton concentration may still be reached even with a low water concentration in the surrounding atmosphere. Meanwhile, the perovskite matrix phase provides the requisite electronic conductivity in concert with mobile oxygen vacancies. Improved cathode performance is therefore expected since both high proton conductivity and high oxygen reaction activity may be reached simultaneously.
2.4 Cell Performance
An ideal PCFC cathode should have high intrinsic activity for the reduction of oxygen to oxygen ions as well as high proton conductivity to increase the active reaction sites for water formation.[32, 41, 44, 45] In our previous work, we have proved that the effect of hydration reaction on ORR in PCFCs can be negative due to the competitive adsorption between water and oxygen species over the cathode for active sites, and the decrease of oxygen partial pressure in the atmosphere by water dilution.[46-48] Therefore, we evaluated the electrocatalytic activities of single-phase STF and a series of nanocomposite NSTFx electrodes (x = 0.1, 0.2, 0.3, and 0.4) using proton-conducting BZCYYb and oxygen-ion conducting Sm0.2Ce0.8O2 (SDC)-supported symmetric cells.
Because water adsorbates can competitively interfere with the ORR, the effect of water content on the electrochemical performance of the STF and NSTFx electrodes on the SDC electrolyte was evaluated as a function of increasing water partial pressure at 600 °C (Figures S25–S28, Supporting Information). Figure 4a shows the relative changes in ASR as a function of the water vapor content for the series of NSTFx electrodes (ASR (H2O content)/ASR (Dry air)). ASR increases with increasing water vapor content, and the relative ASR increase is generally between 1.5 and 2.5 times for most electrode compositions. However, the NSTF0.3 electrode shows a notably smaller increase in ASR at low water vapor content, with a relative increase of just 1.17 times in 2.5 vol.% H2O and 1.23 times in 5 vol.% H2O. This suggests that the NSTF0.3 electrode may offer the best balance between ORR-activity provided by the NSTF phase and water-adsorption capacity provided by the NF phases so as to minimize competition between oxygen and water adsorbates under low conditions. To gain further insight into the effects of H2O content on O2 adsorption behavior in the NSTFx electrodes, we also performed O2-TPD on samples of STF, NF, and NSTF0.3 after treatment in humid air (20 vol.% for 3 h) at 250 °C (Figure 4b). We observe small O2 desorption peaks at temperatures below 200 °C in all three samples that can be attributed to the release of dissolved oxygen from surface/interfacial water phases. The NF phase shows no further O2 desorption at higher temperatures, consistent with its lack of ORR activity. Interestingly, if the STF sample is treated in dry air rather than humid air, this broad O2 desorption peak shifts to slightly lower temperature (Figure S29, Supporting Information), suggesting that H2O co-adsorbed on the STF surface can indeed inhibit the oxygen desorption. We see this same broad O2 desorption peak (albeit lower in magnitude and shifted slightly to higher temperature) for the NSTF0.3 nanocomposite sample, but a new, sharply distinct O2 desorption peak at 271 °C also appears which is remarkably similar to the sharp H2O desorption peak observed at 270 °C for the NSTF0.3 sample during H2O-TPD (Figure 3e). These results suggest that the NSTF0.3 electrode provides two distinct sites for oxygen adsorption/desorption: lattice-based O2 adsorption/desorption from the surface of the parent oxide phase and interfacial O2 adsorption/desorption from the network of NF/NSTF interfaces and grain boundaries. We hypothesize that the additional O2 adsorption sites provided by the NSTF0.3 nanocomposite, in concert with its enhanced water uptake capacity enable this electrode to maintain high ORR activity even under moderate humidity (<5 vol.% H2O) conditions.
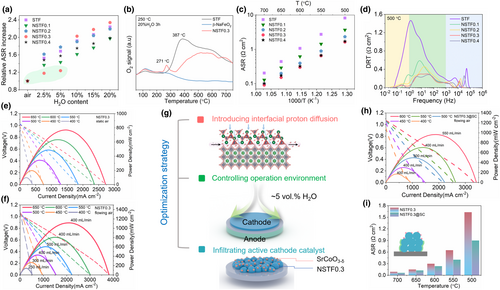
We then probed the ORR activity of the various electrodes under PCFC operation by measuring the electrode polarization ASRs in humid air on proton conducting BCZYYb electrolyte-based symmetric cells and carrying out the distribution of relaxation times (DRT) analysis to deconvolve the impedance response into a series of frequency-resolved peaks (Figure 4c,d and Figures S30–S33, Supporting Information). In this case, both the ORR and water reaction processes occur in concert and protonic carriers now play a role in the electrochemistry. The NSTF0.3 electrode shows the lowest ASR (and hence should have the highest performance) across all temperatures and water contents. The second phase NF with its superior proton affinity and water adsorption capability makes it possible for the NSTF0.3 to maximize both the ORR and water reaction activity, at least under moderate (<5–10 vol.% H2O) water vapor pressure conditions. Furthermore, the DRT response shows that the NF phase successfully minimizes competitive interference between oxygen and water adsorption as long as the water partial pressure is not too high. This suggests that adequate control of the humidity level during PCFC cathode operation is likely crucial for optimizing performance, a point that will be further addressed when we discuss the full fuel cell results below.
Since the NSTF0.3 electrode showed the lowest ASR over proton-conducting electrolyte in symmetrical cell configuration, we fabricated complete anode-supported button cells by applying it as the cathode composition (configuration: NSTF0.3|BZCYYb (22.5 μm)|Ni + BZCYYb) to test performance in real single cell (Figure 4e,f and Figures S34–S39, Supporting Information). The NSTF0.3-based fuel cell attains a PPD of 770 mW cm−2 at 650 °C under static air, with the PPD climbing to 1116 mW cm−2 under 400 mL min−1 flowing air. A similar effect under static versus flowing air is also observed at other temperatures. The performance is reduced under static air conditions due to poor mass transport and catalytic inhibition/interference caused by the local accumulation of high water vapor concentration over the cathode surface. In fact, using a thermohygrometer (testo 635-1), we measured the local absolute humidity in the cathode as a function of the cell operating conditions (Figure S35, Supporting Information), which revealed that in all cases the optimum PPD is achieved under air flow and temperature conditions that lead locally to ∼4–5 vol.% H2O in the cathode electrode. This “optimal” humidity level corresponds almost exactly to the optimal humidity level of 5 vol.% H2O observed in the symmetric cell ASR tests discussed previously, reinforcing our hypothesis that cathode performance is maximized at moderate humidity levels which ensures adequate proton transport in the cathode while still minimizing competitive interference between oxygen and water adsorbates. To investigate the durability of the NSTF0.3-based cathode under real single cell operation condition, we monitored the stability of the terminal voltage of a single cell under load in both static and flowing dry air for 200 h (Figure S36, Supporting Information). In all cases, a stable and consistent response is observed with little degradation or loss in performance. Backscatter electron (BSE) FIB-SEM images of the NSTF0.3-based single cell before and after the long-term stability test were also collected (Figures S38 and S39, Supporting Information). In both the pre- and post-mortem images, the lighter NF phase can be seen to extensively decorate the surface/interfacial regions of the primary NSTF perovskite phase, suggesting a percolating network of the NF phase that provides sites for water adsorption and pathways for facile H3O+ transport throughout the electrode. In addition, we calculated the theoretical TEC values of the perovskite and NF phases from their lattice parameters at high temperatures (Figure S42, Supporting Information). Compared the measured TECs of the individual phases, the composite cathodes demonstrate markedly lower TECs, suggesting interactions between the two phases (as well as the presence of porosity) reduces the effective thermal expansion of the composite system. This TEC suppression effect increases with increasing NF phase fraction.
Because PCFC cathode design requires maximizing active sites for both oxygen reduction and water formation as well as facilitating both oxygen ion and proton conductivity, we hypothesize that infiltrating an additional ORR-active nanoparticulate catalyst onto the porous cathode surface could further enhance performance (Figure 4g and Figure S40, Supporting Information). We therefore infiltrated SrCoO3−δ (SC) into the NSTF0.3 cathode, forming a triple-phase cathode (NSTF0.3@SC) that remarkably leads to even higher fuel cell performance (Figure 4h). The NSTF0.3@SC single cell achieves a PPD of 966 mW cm−2 at 600 °C with an optimum air flow rate of 550 mL min−1, when compared with a PPD of 807 mW cm−2 for the standard NSTF0.3 cell at an optimum air flow rate of 400 mL min−1. Power density performance of NSTF0.3@SC-based single cell reported here is also superior to the other recently reported high performance cathodes-based PCFC (Table S2, Supporting Information). Such superior performance highlights the potential of triple-conducting cathode to obtain high performance low temperature (below 600 °C) PCFCs. The higher optimum air velocity tolerated by the SC-infiltrated cell also suggests that the triple-phase cathode has a better water absorption capacity than the dual-phase NSTF0.3. EIS-derived ASR values for the NSTF0.3@SC cell both in wet air (5 vol.% H2O) on BZCYYb electrolyte and in dry air on SDC electrolyte reveal the remarkable enhancement in ORR activity provided by the infiltration of the SC catalyst (Figure 4i and Figure S41, Supporting Information).
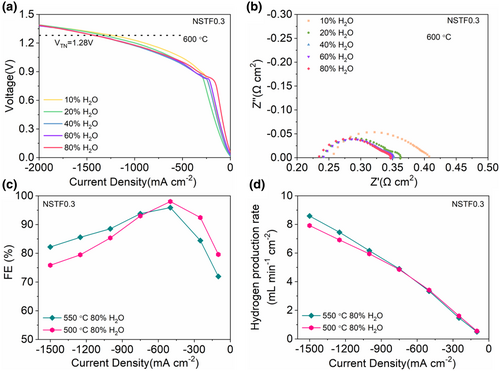
In addition, the high-water affinity and proton conductivity of the NSTF0.3 nanocomposite cathode suggest that it may also work well as the anode (water-splitting electrode) of a PCEC. To investigate this possibility, we operated a BCZYYb-based button cell in electrolysis mode with various water vapor pressures in air supplied to the NSTF0.3 anode. Electrolysis current densities ranging from −1.22 to −1.42 A cm−2 were achieved at an applied voltage of 1.28 V as the water vapor content increased from 10 vol.% to 80 vol.% at 600 °C (Figure 5a). Performance increases slightly with increasing water vapor pressure due to the decrease in both the ohmic and electrode polarization resistances under the increasing humidity (Figure 5b). As reported by O'Hayre et al., electrolysis cells using BZCYYb electrolyte can achieve high faradaic efficiency (90–98%), especially at lower temperatures and higher steam contents.[49] We therefore monitored H2 production from our cell to obtain the faradaic efficiency (FE) at 550 and 500 °C under 80 vol.% water vapor in air. FE increases rapidly with increasing load current before gradually falling at still higher current densities, consistent with prior reports.[49] The FE reaches 98% at an electrolysis current density of −0.5 A cm−2 (Figure 5c) with a corresponding H2 production rate >3.3 mL min−1 cm−2 (standard temperature and pressure) (Figure 5d), underscoring the potential of NSTF0.3 as a superior water-splitting electrode for PCECs.
3 Conclusion
This work demonstrates the potential of introducing a super hydrating phase into a mixed conducting perovskite phase with the development of a new nanocomposite electrode for PCFC with outstanding performance, which can be formed by the facile thermally-induced phase decomposition of Na-doped SrTi0.1Fe0.9O3−δ. Thermal decomposition leads to the formation of a water-absorbing NF phase which decorates the ORR-active and electrically conductive NSTF parent phase, thereby producing a new quadruple-conducting (H3O+/H+/O2−/e−) cathode that achieves high power density and stable PCFC performance. The NF phase facilitates dispersion of the active sites for surface water vapor and oxygen and enhances water adsorption within the cathode to ensure the interfacial proton transport. Infiltration of SC into the NSTF0.3 cathode leads to a triple-phase electrode with even higher performance. The influence of local cathode humidity on cell performance was revealed by controlling the air velocity in cathode chamber, leading to further optimization of fuel cell performance. Finally, the NSTF0.3 nanocomposite electrode also demonstrated excellent performance as a water-splitting electrode, establishing the potential of this quadruple-conducing nanocomposite electrode approach for both fuel cell and electrolysis applications.
4 Experimental Section
Preparation of the materials
NaxSr1−xTi0.1Fe0.9O3−δ and β-NaFeO2 were fabricated by the widely used sol–gel method. As prepared, NaNO3, Sr(NO3)2, Fe(NO3)3∙9H2O and C16H36O4Ti as the metal ionic source, were dissolved into water. Ethylenediaminetetraacetic acid (EDTA) and citric acid (CA) were added to act as complexing agents at a molar ratio of 1:1:2 for total metal ions, EDTA and CA. NH3·H2O was used to adjust the pH value of the aqueous solution to around 7. An atropurpureus gel was obtained after the removal of water in the solution by evaporation, which was pre-decomposed at 250 °C to obtain the precursors. Then, the STF and NSTFx oxide powders were calcined at 1000 °C for 5 h. BZCYYb was fabricated using the conventional solid-state reaction method. Stoichiometric amounts of BaCO3, ZrO2, CeO2, Y2O3, and Yb2O3 were mixed in ethanol and ball milled for 12 h. The BZCYYb precursor powder was obtained after drying under the sodium lamp and pre-sintering at 1000 °C for 5 h. All reagents were purchased from Sinopharm and were certified analytically pure.
Symmetrical and single cells fabrications
Symmetrical button cells NSTFx|BZCYYb|NSTFx and full button-cell fuel cells (NSTFx|BZCYYb|Ni + BZCYYb) were prepared for the measurement of electrochemical impedance spectroscopy (EIS) and current versus potential (I–V) polarization, respectively. The half-cells were fabricated with a dry-pressing and co-sintering method at 1475 °C for 10 h. For the symmetrical cell, the colloidal suspension of the appropriate cathode composition (NSTFx, x = 0, 0.1, 0.2, 0.3, 0.4) was deposited by spray deposition onto the opposing sides of a sintered BZCYYb disk (for a single cell, the cathode was deposited on the BZCYYb of the half-cell) with a defined circular active area of 0.45 cm2, which was then further calcined in air for 2 h at 1000 °C. Silver paste was finally painted on the surface of the porous cathode layer to serve as a current collector.
PEMFC cells fabrications and test
The membrane electrode assemblies (MEAs) in this study were fabricated by following the traditional catalyst-coated membrane (CCM) method. Briefly, the MEA was prepared using a commercial Pt/C ink (JM, 20%) for both the anode and the cathode, and Pt loading was 0.1 mg cm−2. The I-C (Ionomer to Carbon) ratio of the catalyst ink was 0.45:1 with isopropanol as the solvent and the solid content of the ink was around 1.0 wt.%. The solid oxide suspensions were prepared with 0.5 g powders and 10 mL isopropanol as the solvent. A Siansonic UC 320 was used for spray deposition and the spray process followed the standard protocol for this equipment with a drying temperature of 70 °C to spray on the Nafion 211 membrane (DuPont, USA, 4 cm2) (structure: Pt/C|Nafion|oxide|Nafion|Pt/C). Teflon gaskets were used for sealing. Carbon paper electrodes with a gas diffusion layer (HCP120, Shanghai Hesen) were applied to both sides of the CCM for electrodes. A hot-pressing temperature of 100 °C for 90 s was used to bond the electrodes to the CCM. Single-cell performance was tested in a fuel cell system. For the H2–O2 single fuel cell test, high-purity hydrogen (99.999%, 300 mL min−1) and 21% O2/79% N2 (99.999%, 500 mL min−1) were delivered to the anode and cathode chambers. Both the cell temperature and gas channel temperature were set at 70 °C. The back pressure of both the oxygen side and hydrogen side was 0.15 MPa. Before the test, the MEA was activated for 1 h at 0.3 V. The I–V performance curve was obtained by the scanning voltage method, sweeping from 0.9 to 0.3 V and taking points at 0.05 V intervals of 30 s. The EIS was obtained at the open circuit voltage.
Characterizations
Powder X-ray diffraction (XRD, Rigaku Smart Lab) was applied to identify the crystal structure of STF and the NSTFx (x = 0.1, 0.2, 0.3, 0.4) powders. Structural refinements were performed via the TOPAS-4.2 software packages. The prepared focused ion beam transmission electron microscopy (FIB-TEM) samples and microstructure images of the materials were obtained with a focused ion beam transmission electron microscope (FIB-SEM, Zeiss crossbeam 540). High-resolution transmission electron microscopy (HR-TEM) was performed on a JEOL JEM-2100 instrument at a 200-kV accelerating voltage. The presence of protons was analyzed by TOF-SIMS (Time of Flight Secondary Ion Mass Spectrometry).
Symmetrical cells incorporating the perovskite cathode samples were subjected to electrochemical impedance spectroscopy (EIS) measurements using a potentiostat (Solartron 1287) and galvanostat combined with a frequency response analyzer (FRA, Solartron 1260A). EIS measurements were performed in air at a frequency range of 1 MHz to 0.01 Hz with a 10 mV AC bias amplitude at an open circuit voltage (OCV) condition. Single cells were subjected to the current versus potential (I–V) polarization tests using a digital source meter (Keithley 2420) and EIS tests under an OCV condition. The electrical conductivities of the perovskite samples were executed by a four-probe direct current (DC) method with the Keithley 2420 source meter as the current source and the voltage indicator in air from 400 to 850 °C. Data acquisition and instrumentation were achieved using a home-developed LabVIEW (National Instruments) program.
Operando hard XAS measurement
The hard XAS (XANES and EXFAS) was performed at the TPS 44A beamline of the NSRRC in Taiwan and at the BL14W beamline in Shanghai Synchrotron Radiation Facility (SSRF). The operando hard XAS measurements were performed with STF/NSTF0.3 as the cathode on an electrolyte-supported cell (STF/NSTF0.3|BZCYYb|Ag) using a home-made electrochemical cell setup for operando X-ray investigations. The load current was conducted on a computer-controlled electrochemical analyzer. The acquired hard XAS data were processed according to the standard procedures using the ATHENA module implemented in the IFEFFIT software packages. The k2-weighted EXAFS spectra were obtained by subtracting the post-edge background from the overall absorption and then normalizing with respect to the edge-jump step. Subsequently, k2-weighted w(k) data of Co K-edge and Fe K-edge were Fourier transformed to real (R) space using a Hanning window (dk = 1.0 Å−1) to separate the EXAFS contributions from different coordination shells.
Acknowledgements
This work was supported from the National Key R&D Program of China (No. 2022YFB4002502), National Natural Science Foundation of China under (No. 22278203, 22279057), the Jiangsu Funding Program for Excellent Postdoctoral Talent and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We acknowledge support from the Max Planck-POSTECH-Hsinchu Center for Complex Phase Materials. R.O. acknowledges support from the Fulbright Foundation Global Scholars Program and the U.S. Army Research Office under grant number W911NF-17-540 1-0051.
Conflict of Interest
The authors declare no conflict of interest.




