Surface Molecular Encapsulation with Cyclodextrin in Promoting the Activity and Stability of Fe Single-Atom Catalyst for Oxygen Reduction Reaction
Abstract
Fe single-atom catalysts (Fe-SACs) have been extensively studied as a highly efficient electrocatalyst toward the oxygen reduction reaction (ORR). Nonetheless, they suffer from stability issue induced by dissolution of Fe metal center and the OH− blocking. Herein, a surface molecular engineering strategy is developed by using β-cyclodextrins (CDs) as a localized molecular encapsulation. The CD-modified Fe-SAC (Fe-SNC-β-CD) shows obviously improved activity toward the ORR with 0.90 V, 4.10 and 4.09 mA cm−2 for E1/2, J0 and Jk0.9, respectively. Meanwhile, the Fe-SNC-β-CD shows the excellent long-term stability against aggressive stress and the poisoning. It is confirmed through electrochemical investigation that modification of β-CD can, on one hand, regulate the atomic Fe coordination chemistry through the interaction between the CD and FeNx moiety, while on the other mitigate the strong adsorption of OH− and function as protective barrier against the poisoning molecules leading to enhanced ORR activity and stability for the Fe-SACs. The molecular encapsulation strategy demonstrates the uniqueness of post-pyrolysis surface molecular engineering for the design of single-atom catalyst.
1 Introduction
Fe-SACs have been extensively studied as the most potential alternatives to Pt-based catalysts due to their high electrocatalytic ORR activity.[1-10] However, it has been widely discovered that the Fe-SACs, as the ORR electrocatalysts, show high activity but suffers from the stability issue that limits their practical applications.[11-13] Some fundamental processes have been reported and identified as the main reasons for their instability issue, among which the dissolution of Fe atoms and the strong adsorption of OH- on the Fe sites are considered as the two factors that cannot be ignored.[14, 15] The demetallation of FeNx sites causes the collapse of the catalytic active sites, eventually leading to the deactivation of catalysts. Maillard et al., through a systematic investigation on the degradation of the Fe-N-C structure, revealed that the O2, along with the reactive oxygen species, could be responsible for the loss of a significant fraction of the atomically dispersed FeNx sites and the formation of Fe oxides during the load cycling.[16] The adsorption of excess OH− can block the metal center and suppress the adsorption of oxygen onto the Fe sites. Xie et al. reported that the -OH ligands could coordinate to the metal center and promote the selectivity into H2O2, leading to the weakening of the 4-electron ORR processes.[17] Additionally, the Fe sites also are easily susceptible to adsorption of small molecules or ions (such as, SCN−, ) and could eventually lose their catalytic activity through the poisoning.[18-20] Therefore, enhancing the structural rigidity of metal active sites and prevention of “spectator ligands” that de-activate the metal center are of high importance to the fabrication of highly stable catalytic centers for the design of Fe-SACs.
Surface molecular engineering has gained wide attention to the optimization of the structural design of the catalytic center for metal-based nanocatalysts,[21-25] through the modulation of the electrolyte/catalyst interface,[26] the suppressed generation of surface oxide species (e.g. -OH)[27] et al. For instance, Yamazaki et al. utilized porphyrin-based organic molecules as surface adsorbed species to optimize the ORR performance through the perturbation of the H2O network and hence the de-stabilization of OHad species.[28-30] Markovic et al. demonstrated that a calix[4] arene-modified Pt electrode, by promoting the formation of O2− tolerant Pt sites that are active for the adsorption of H2 and consequent H-H bond breaking, can enhance the hydrogen oxidation reaction (HOR) while at the same time suppress the ORR.[31] Yu’s groups reported a dual polysulfide confinement strategy accomplished by confinement of sulfur in polydopamine-coated MXene nanosheets, which can suppress the polysulfide shuttle while maintaining a high sulfur loading mass.[32] Flake and coworkers designed a 4-pyridinylethanemercaptan functionalized Au electrodes for selective electrocatalytic reduction of CO2 into formate.[33] They proposed that the improved selectivity and activity could be attributed to the proton-induced desorption facilitated by the adsorption of 4-pyridinylethanemercaptan. As for the SACs, molecular engineering has also been adopted as a strategy toward the design of highly active catalytic center.[34, 35] Zhang et al. designed a “ligand-mediated” method for the synthesis of metal SACs with high metal loading, whereby the porphyrin-like moiety can prevent the aggregation of the metal atoms into clusters or nanoparticles.[34] However, for SACs, majority efforts in molecular engineering are hitherto focused mainly on the precursor materials prior to the pyrolysis, which can be difficult to be directly translated to the structure regulation of metal center due to the aggressive graphitization process.[4, 36]
Based on the above analysis, aiming to improve the ORR activity and stability of Fe-SACs, the surface molecular engineering strategy is adopted to modify metal center for the Fe-SACs in this work, specifically targeting the post-pyrolysis material. The cyclodextrin, featuring a cyclic structure with hydrophobic and hydrophilic exterior moieties commonly employed as ion-channel compound, shows a structural uniqueness in protecting the metal center and regulating OHad adsorption/desorption.[37, 38] We have successfully optimized the ORR performance of the prepared single-atom catalysts Fe-SNC through the modifying of β-CD. As-prepared Fe-SNC-β-CD shows the improvement in both activity and stability toward the ORR. The β-CD regulates the coordination chemistry of Fe active sites by binding to the adjacent N/S atoms. Electrochemical results indicate that the modification by β-CD can inhibit the blocking of OH- on atomic Fe sites, which contributes to the improved activity and stability. Additionally, Fe-SNC-β-CD also shows the excellent resistance to KSCN poison, which can be ascribed to the protection of the Fe sites by surface β-CD. This work provides a simple but effective method to regulate the atomic Fe microenvironment by forming a single-site protective barrier against the poisoning species to optimize the ORR activity and stability of Fe-SACs.
2 Results and Discussion
2.1 Characterization of Fe-SNC
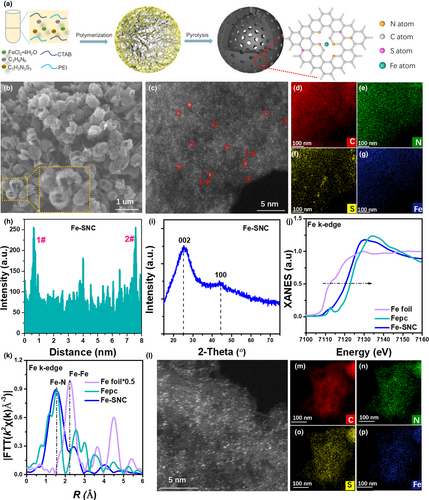
The preparation of the single-atom Fe-SNC catalyst have been described with detail in Supporting Information (SI). It is briefly described as follows. Nanospheres anchored with Fe were synthesized using the self-assembly of melamine and thiocyanuric acid by employing hexadecyl trimethyl ammonium bromide (CTAB) and poly(ethylene imine) (PEI) as the soft templates. The nanospheres were then pyrolyzed in N2 atmosphere to achieve the N, S-co-doping in the Fe-SNC catalysts (Figure 1a). The scanning electron microscopy (SEM, Figure 1b) indicates that the Fe-SNC shows morphology with half- opening hollow nanosphere of 500–600 nm in diameter. The dispersion state of Fe species at the atomic scale was investigated by aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images. In Figure 1c, isolated bright dots associated with single Fe atoms (marked by red circles) can be clearly distinguished. Energy-dispersive spectroscopy (EDS) mappings of Fe-SNC reveal the distributions of C, N, S and Fe elements (Figure 1d–g). The elemental contents, measured by X-ray photoelectron spectroscopy (XPS), are determined to be 1.49, 62.24, 17.75, 16.12 and 2.70 wt% for S, C, N, O and Fe, respectively (Table S2), indicating the abundance of N species on the surface or near surface. The content of Fe in the powder was determined to be 1.08 wt% by inductively coupled plasma optical emission spectrometry (ICP-OES). The higher Fe content determined by XPS indicates that a large number of atomic Fe active sites are located on the surface of the carbon, revealing the advantage of the synthetic protocol. The dispersion of Fe species in Fe-SNC can be further confirmed by the intensity profile analysis (Figure 1h). As shown in Figure 1i, X-ray diffraction (XRD) pattern of Fe-SNC exhibits two broad peaks at approximately 25.4 and 43.9° (2θ) indexed to the (002) and (101) crystallographic plane of graphitic carbon material, excluding the existence of large quantity of Fe or FeOx nanoparticles. In addition, the coordination environment and chemical state of the Fe atoms were investigated by X-ray absorption fine structure (XAFS) spectroscopy. Figure 1j shows the Fe k-edge X-ray absorption near-edge structure (XANES) spectra of Fe-SNC, compared with the reference spectra of FePc (with Fe[4+]) and Fe foil (with Fe[0]). The absorption edge positions of Fe-SNC was found to be located between 0 and +4, which is closer to that of FePc than to Fe foil, indicating that the valence of Fe atoms in Fe-SNC might be close to +3 (Figure S1). Notably, as for the peak at ~7112.5 eV, the difference between Fe-SNC and the FePc can be attributed to the doping of S atoms. The Fourier-transformed (FT) extended XAFS curves are shown in Figure 1k. The main peak for both Fe-SNC and FePc at 1.54 Å is ascribed to the Fe-N/Fe-S coordination peak. Meanwhile, the absence of the Fe-Fe path at 2.23 Å confirms that the Fe atoms in Fe-SNC are dispersed as single-atomic sites. After the modification by the β-CD, the atomic and elemental distribution of Fe-SNC-β-CD are characterized by HAADF-STEM. As shown in Figure 1l–p, no obvious change can be observed in Fe-SNC-β-CD, indicating that Fe sites are still remained as single-stomic sites. In addition, the C, N, S and Fe elements are uniformly distributed in Fe-SNC-β-CD (Figure 1o,p). The Fe content of the Fe-SNC-β-CD tested by ICP-OES is 0.81 wt%, which is close to that of the Fe-SNC. The slight difference in Fe contents of both Fe-SNC-β-CD and Fe-SNC can be attributed to the weight of the β-CD and reasonable experimental error.
2.2 Surface modification of β-CD on Fe-SNC
The XPS was used to investigate the chemical states, coordination states and surface electronic structure of the Fe-SNC and Fe-SNC-β-CD. All the XPS data are calibrated using the C1s reference peak (284.8 eV) (Figure S2). As shown in Figure 2a, Figure S3 and Table S2, the sharp increase of the O contents for Fe-SNC-β-CD suggests the successful modification of β-CD on the catalyst surface by dramatically increasing the surface oxygen content (Figure S4–S6). In addition, the XPS data (Table S2) shows that the Fe content in Fe-SNC-β-CD (4.22 wt%) is obviously higher than that of Fe-SNC (2.70 wt%), suggesting that the modification of β-CD can facilitate the exposure of Fe atoms that could be buried beneath the surface or migrating from the bulk to the surface. It is consistent with what has seen in the HADDF-STM image, wherein denser and isolated bright dots representing the single Fe atoms are observed on the surface of Fe-SNC-β-CD (Figure 1l). Compared to that of Fe-SNC, the core level XPS peak of Fe2p for Fe-SNC-β-CD shifts to higher binding energy whereas the N1s and S2p peaks shift to the lower binding energy. The high-resolution N1s spectra, in Figure 2c,d, shows that fair amounts of oxidized-N and N-O structures can be detected, while the content of graphitic-N decreases (Figure 2f, compared with that of Fe-SNC), indicating that the -OH groups in β-CD may bond to the graphitic-N. Based on the XPS analyses, it is reasonable to speculate that there could be interaction in electronic structure that donates electrons from the Fe atoms to S/N atoms via Fe-N/S-OH structure. In addition, the modification of the β-CD can promote the formation of the pyridinic-N, which is believed to have the dominating contribution to ORR activity.[39] The Fourier-transform infrared spectroscopy (FTIR) was also employed to gain an insight on the structural properties of Fe-SNC and Fe-SNC-β-CD, as shown in Figure S7. Compared with Fe-SNC, three new peaks of Fe-SNC-β-CD appear at ~1100, 1014, and 607 cm−1 correspond to stretching vibration of the C-O, which is attributed to the surface encapsulation with β-CD.[40] In addition, the peak intensity of the Fe-SNC-β-CD is lower than that of β-CD owing to the low concentration of β-CD in the surface. The broad peak around ~3300 cm−1 is assigned to the stretching vibration of -OH from the intermolecular hydrogen bonds of β-CD. The bonding effect of the Fe-SNC and β-CD prevents the formation of hydrogen bonds between β-CD molecules, resulting in the disappearance of the broad peak.
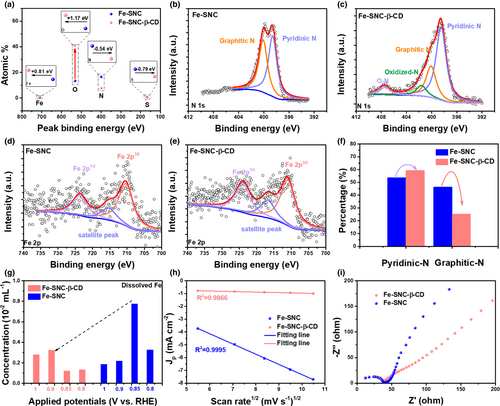
The fundamental electrochemical properties of the as-designed materials were evaluated by diversified electrochemical techniques. The dissolution of metallic atoms is considered as a critical issue limiting the stability of the Fe-based SACs. As shown in Figure 2g, the Fe-SNC-β-CD shows the lower concentrations (normalized by the content of the Fe) of dissolved Fe than that of Fe-SNC at various applied potentials, which indicates that the β-CD modification can significantly reduce the dissolution of Fe atoms. Interestingly, the potential resulting in the most severe Fe dissolution corresponds to the half-wave potential (E1/2) of both Fe-SNC (0.85 V vs. RHE, the same criterion is applied below) and Fe-SNC-β-CD (0.9 V), respectively (Figure 3a). The maximum Fe dissolution of the catalysts appear at the half-wave potential probably because as the potential keeps on increasing, the Fe-OH derived from the adsorption of OH− turns into the stable FeOx anchored on carbon support, which helps to prevent the Fe from dissolution. The linear dependence of the current densities of oxygen reduction peak on the square root of the scan rate (Figure 2h, derived from Figures S8 and S9) reveals that diffusion could be the rate-determining step, whereby the unmodified catalyst showed a heavier dependence. The similar Rct values of Fe-SNC and Fe-SNC-β-CD determined from electrochemical impedance spectroscopy (EIS) reveal that the modification of β-CD does not affect the electron transfer ability (Figure 2i).
2.3 Electrochemical characterization
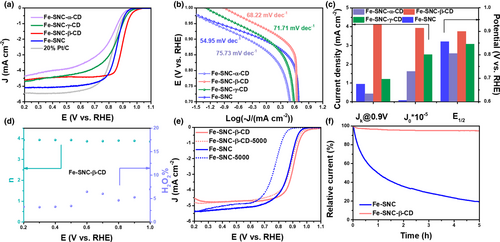
The catalytic ORR performance was then evaluated using a rotating disk electrode (RDE) in 0.1 m KOH. The performance of the Fe-based SACs was optimized by evaluating catalysts synthesized through various conditions. As for the synthetic protocol, it has been discovered that the sequence of adding different precursors, especially the FeCl2·4H2O, may affect the coordination chemistry in the precursor materials and hence the formation of active site in the graphitized material. Figure S10 shows that the adding sequence of FeCl2·4H2O, added before or after the PEI, shows great influence on their ORR activity, owing to the coordination between Fe and N atoms that affects the formation of the stable Fe-N structures. It has also been found that the catalysts pyrolyzed at temperatures of 800 or 900 °C show very similar ORR activity (Figure S11). Therefore, the Fe-SNC-800 (i.e., Fe-SNC) is used as the baseline material for further investigation unless been noted in this work. To ascertain the effect of the size of the CD, α-, β-, and γ-CD were used for the modification and compared in performance. The linear sweep voltammetry (LSV) curves in Figure 3a reveal that the Fe-SNC-β-CD exhibits higher ORR activity than Fe-SNC and other two catalysts modified by α- and γ-CD, with an onset potential (Eonset) at 1.01 V and E1/2 at 0.90 V, which is higher than that of 20% Pt/C. In Figure 3b, the low Tafel slop of 68.22 mV dec−1 for Fe-SNC-β-CD suggests the fast ORR kinetics occurring on the Fe-SNC-β-CD surface. In addition, the superb intrinsic activity of Fe-SNC-β-CD was validated by the calculated high kinetic current density. In Figure 3c, the kinetic current densities at 0.90 V are determined to be 4.10, 1.21, 0.94, 0.43 mA cm−2 for Fe-SNC-β-CD, Fe-SNC-γ-CD, Fe-SNC and Fe-SNC-α-CD, respectively. These electrochemical measurements strongly confirm the effectiveness of the β-CD modification on the Fe-SNC catalyst to improve the ORR activity. The exchange current density (J0), the current density at equilibrium potential, can reflect the intrinsic catalytic activity of the materials. The highest J0 of Fe-SNC-β-CD (4.10 × 10−5 mA cm−2) indicates its most favorable catalytic kinetics, as shown in Figure 3c. The improved ORR performance of Fe-SNC-β-CD may be attributed to the proper cavity diameter of the β-CD. As shown in Table S4, the 6 Å of the cavity diameter for α-CD is smaller than that of the sum of O2 and OH-, which hardly guarantees efficient mass transfer. In addition, the 10 Å of the cavity diameter for γ-CD is far larger than that of the sum of O2 and OH-, which cannot prevent the excess OH- from covering the Fe sites. The Koutecký-Levich (K-L) plots (Figure S12b) converted from LSV curves (Figure S12a) recorded at various rotating rates reveal a 4-electron pathway occurring on Fe-SNC-β-CD electrode, similar to that of Fe-SNC (Figure S13). The rotating ring-disk electrode (RRDE) measurement indicates that the yield of H2O2 is only 5% on the Fe-SNC-β-CD (Figure 3d). Meanwhile, the electron transfer number confirms a four-electron pathway, which is consistent with the K-L plot results. Furthermore, in Figure 3e, the Fe-SNC-β-CD seems to exhibit excellent stability with only 19 mV negative shift for E1/2 after 5000 cycling potential scans between 0.3 and 1.0 V.
It is known that the Fe metallic site could be strongly adsorbed by some impurity ions or small molecules, such as SCN- and et al., and hence be poisoned with inhibited catalytic activities.[18-20] The poison-stability was evaluated by chronoamperometry (CA) test, as shown in Figure 3f. Surprisingly, Fe-SNC-β-CD exhibits high stability with 94% current retention after a 5 h test against the KSCN regarded as poisoning species, while the unmodified Fe-SNC shows only 19% retention in current. It indicates that the surface modification with β-CD not only improves the intrinsic activity and stability of Fe-SNC, but can also prevent the Fe sites from the small poisoning molecules. The effect of the β-CD concentration on the activity during the modification was studied as shown in Figure S14, the optimized concentration seems to be around 0.5 × 10−4 m. Obviously, excessive β-CD could be detrimental to ORR process due to the overwhelming existence of β-CD adsorbing on the catalyst surface, which would fully cover the active sites.
2.4 Catalytic mechanism
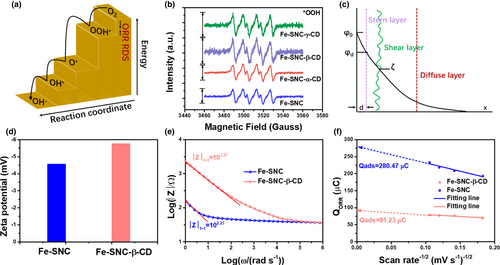
The adsorbed oxygen on the catalyst surface, which can influence the ORR process, can also be considered as the evidence of the above hypothesis. As shown in Figures S19–S21, the background corrected CV curves are used to determine the charge associated to the reduction of adsorbed oxygen (Qads).[54, 55] The high Qads value of Fe-SNC suggests a strong adsorption of OH- on atomic Fe sites (Figure 4f). It can be noticed that the modification of β-CD can reduce the oxygen adsorption, mitigating the blocking of OH- to the atomic Fe sites, which facilitates high activity and stability.
3 Conclusion
In summary, we have successfully optimized the ORR performance of the prepared Fe-SNC SAC through the modification of cyclodextrin. The β-CD can effectively regulate the coordination environment of Fe active sites by binding to the adjacent N/S atoms, while at the same time reduce the dissolution of Fe atoms. Furthermore, the modification with β-CD can mitigate the electric field at the surface that could drives the inward migration of oxygen species, and hence reduce the oxygen adsorption, inhibiting the blocking of OH- on atomic Fe sites, which ensures a high activity and stability. Through the modification of the β-CD, the as-designed Fe-SNC-β-CD shows not only a significantly increased catalytic activity toward the ORR, but also excellent stability and poisoning resistivity. This works provides a simple and practical method to regulate the microenvironment of the catalyst surface for optimizing electrocatalytic performance toward the ORR.
Acknowledgments
C.C. and Y.L. acknowledge Prof. Liqiang Zhang from Yanshan University for the helpful discussion on TEM characterizations. Y.L. acknowledges the National Natural Science Foundation of China (52171199) for the financial support.
Conflict of Interest
The authors declare no conflict of interest.




