Recycling Spent LiCoO2 Battery as a High-efficient Lithium-doped Graphitic Carbon Nitride/Co3O4 Composite Photocatalyst and Its Synergistic Photocatalytic Mechanism
Abstract
The ever-increasing quantity of spent lithium-ion batteries (LIBs) is both a potential environmental pollutant and a valuable resource. The spent LIBs recycling mainly aimed at the separation of valuable elements. Some issues still exist in these processes such as high energy consumption and complex separation procedures. This study avoided element separation and proposed a facile approach to transform spent LiCoO2 electrode into a lithium (Li)-doped graphitic carbon nitride (g-C3N4)/Co3O4 composite photocatalyst through one-pot in situ thermal reduction. During the thermal process, melamine served as the reductant for LiCoO2 decomposition and the raw material for g-C3N4 production. Li was in situ doped in g-C3N4 and the generated Co3O4 was in situ integrated, forming a Li-doped g-C3N4/Co3O4 composite photocatalyst. This special composite exhibited an enhanced photocatalytic performance, and its photocatalytic H2 production and RhB degradation rates were 8.7 and 6.8 times higher than those of g-C3N4. The experiments combined with DFT calculation revealed that such enhanced photocatalytic efficiency was ascribed to the synergy effect of Li doping and Co3O4 integrating, which extended the visible light absorption (450–900 nm) and facilitated the charge transfer and separation. This study transforms waste into a high-efficient catalyst, realizing high-valued utilization of waste and environmental protection.
1 Introduction
Lithium-ion batteries (LIBs) have been widely applied in storage devices and electric vehicles because the lifetimes of LIBs for portable electronic products and electric vehicles only keep 3 and 5–8 years, respectively. As a result, a huge amount of these batteries will be decommissioned in the next 3–6 years.[1, 2] It is estimated that about 500 000 metric tons of spent LIBs were produced from China alone, and more than 11 million tons will be generated by 2030 worldwide.[3] Spent LIBs have been one of the most fast-growing and largest quantity of solid waste in the world. The dispose of this large volume of spent LIBs is a major environmental challenge to be settled urgently since they were considered as a hazardous waste containing organic substances such as electrolytes.[2, 3] However, spent LIBs are also considered as a valuable resource. The content of metals in spent LIBs such as lithium and cobalt could, respectively, reaches 7 and 15 wt%.[4] Thus, the recycling and utilization of spent LIBs are of great significance to environmental protection, resource utilization, and economic benefit.
Currently, pyrometallurgy, hydrometallurgy, and biometallurgy are used to recycle the metallic elements (Li, Co, Ni, etc.) from spent LIBs.[5, 6] Among these, hydrometallurgy is widely studied due to the high purity and recovery rate of metals. This process usually involves multi-step leaching and extraction, which suffer from high consumption of chemicals even caustic acids (HCl and H2SO4), expensive extractants, long processing times, and secondary waste generation (waste acid and sludge).[6] Pyrometallurgy was another dominant process for spent LIBs recycling; however, the traditional process usually performed at high temperature (above 1400 °C) and consumed large amount of energy. Besides, the Li in residual mixed slag was difficult to separate. Very recently, to lower the processing temperature and improve the metal separation rate, some advanced pyrometallurgy such as carbon reduction,[7, 8] chlorinating,[9-11] and sulfide roasting[12, 13] was proposed. These interesting processes also achieved to selectively separate Li from spent LIBs, and the other metals (such as Co or Mn) could be recycled as metal oxides after water leaching.[2] Unfortunately, the obtained products still required a second separation.[7, 8]
Recently, the preparation of advanced functional materials from spent LIBs has attracted much attentions. Due to the transition metal elements (Co, Mn, and Ni) in spent LIBs, the as-prepared materials could be used as supercapacitors, oxygen evolution catalyst, adsorption, and photocatalysts.[3, 14-18] For instance, Natarajan et al.[19] obtained a MnCo2O4 for electrocatalytic oxygen evolution from spent LIBs by chemical leaching, precipitation, hydrothermal conditioning, and calcination. Mei et al.[16] prepared Fe2O3/CoPi photoanodes from spent LiFePO4 and LiCoO2 for water oxidation by leaching, electrochemical deposition, sintering, and photoelectrochemical deposition. Mao et al.[17] synthesized Co3O4 hollow microspheres for supercapacitors using the leaching solution of the spent LIBs as cobalt source by a solvothermal method followed by a calcination process. Assefi et al.[18] prepared a NiO@Co3O4 core–shell photocatalyst using spent LIBs and Ni–Cd battery by NaOH and H2SO4 leaching and then sintering at 500 °C. Though the as-prepared functional materials using spent LIBs displayed the satisfying properties, the synthesis process usually involved in complex extraction, expensive purification, precipitating, and sintering, which are still exhibit some potential environmental risks.
Herein, this study transforms spent LiCoO2 into a Li-doped g-C3N4/Co3O4 composite photocatalyst through one-pot in situ melamine thermal reduction, avoiding element separation. As is known, g-C3N4, exhibiting a narrower bandgap of 2.7 eV, is one of the most star visible light photocatalysts. Furthermore, g-C3N4 can be obtained through a simple sintering of melamine. It has been attracted much attention for pollutant degradation and H2 production.[20-22] Unfortunately, the practical applications of g-C3N4 face a challenge because of its low specific surface area, poor solar light utilization efficiency, and high recombination rate of photo-generated electron and hole. It is still needed to further improve the photocatalytic activity.[20, 21] Based on the waste utilization and photocatalytic activity enhancement, we attempted to directly transform spent LIBs into a g-C3N4-based photocatalyst. We found that melamine could not only be used as the precursor for g-C3N4 but also the reductant for spent LiCoO2 decomposition. During the one-pot reduction process (550 °C), LiCoO2 was decomposed and Li was in situ doped in the g-C3N4, and simultaneously, the generated Co3O4 was in situ integrated with g-C3N4, forming a Li-doped g-C3N4/Co3O4 composite photocatalyst. The effect of spent LiCoO2 adding amount on the microstructure, optical, and photocatalytic property was studied. Interestingly, compared with the pristine g-C3N4, the photocatalytic activity of the special composite photocatalyst on H2 evolution and RhB degradation was significantly enhanced. Furthermore, the synergistic mechanism of photocatalytic activity enhancement was revealed by experiment combined with density functional theory (DFT) calculation. The current spent LIBs recycling is mainly to recover valuable resources and prepare functional materials. These recycling processes usually involve complex separation and extraction of elements. This study recycled the spent LiCoO2 electrode as a high-efficient Li-doped g-C3N4/Co3O4 photocatalyst through one-pot sintering, which realizes waste utilization and environmental protection. Our study enlightens an idea of “in situ utilization instead of separation of elements” for waste treatment and provides a reference for the recycling of other types of spent LIBs.
2 Results and Discussion
2.1 Characterization of the Photocatalysts
To examine the phase structure of the photocatalysts, XRD analysis was performed. As displayed in Figure 1a, the pristine g-C3N4 exhibited two characteristic peaks at 13.1° and 27.5°, corresponding to the (100) and (002) planes, respectively (JCPDS 87-1526).[23] After adding LiCoO2, some new characteristic peaks appear at 19°, 31.3°, 36.8°, 44.8°, 59.4°, and 65.2°, which can index to the cubic Co3O4 (JCPDS 43-1003). With increasing the LiCoO2 adding amount, the peak intensity of g-C3N4 gradually weakened, while Co3O4 peaks gradually enhanced. The reduced intensity was due to the decreased crystallinity of g-C3N4 when adding the Co-based compounds.[24, 25] The coexisting g-C3N4 and Co3O4 XRD peaks demonstrated the successful preparation of g-C3N4/Co3O4 composite photocatalyst through the melamine thermal reduction of spent LiCoO2. In addition, no peaks related to Li were observed, which could be due to the Li doping in g-C3N4.[26, 27] The structure and functional groups of samples were investigated using FT-IR spectroscopy, as shown in Figure 1b. For the pristine g-C3N4, the peak at 806 cm−1 characterized the triazine units and the peaks between 1242 and 1570 cm−1 corresponded to the aromatic C-N stretching vibrations. The peaks at 1635 and 2173 cm−1 were related to the C=N and -C≡N vibration modes, respectively.[28, 29] As for the composite photocatalyst, the peaks of g-C3N4 all existed, and two new peaks were observed at 582 and 669 cm−1. The two peaks could be attributed to the Co (II)-O and Co (II)-O vibrations, respectively.[30] The FT-IR results further suggested the co-existence of g-C3N4 and Co3O4 in the composite photocatalyst. In addition, the solid-state 13C NMR spectra of g-C3N4 and 5Li-C3N4/Co3O4 are presented in Figure S1, Supporting Information. The g-C3N4 exhibited two distinct peaks. The peak at 164.6 ppm was related to the carbon atoms (Cα) connected with -NH2 groups, while the peak at 156.5 ppm was attributed to the carbon species (Cβ) linked with bridging N atoms.[31, 32] Compared with the g-C3N4, the 5Li-C3N4/Co3O4 displayed the same peak positions and no new peaks were observed. The C/N molar ratios of the samples were also measured by element analysis, as listed in Table S1, Supporting Information. The C/N molar ratio of 5Li-C3N4/Co3O4 was almost the same as that of g-C3N4. The NMR and element analysis results suggested that the Li doping and Co3O4 integrating had little influence on the atomic structure of g-C3N4.
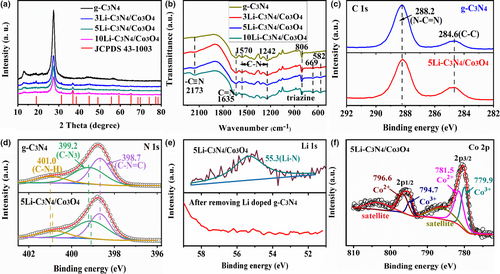
XPS was conducted to further study the chemical states. As seen from the C1s spectra of g-C3N4 (Figure 1c), the peaks at 284.6 and 288.2 eV were due to the surface adventitious carbon and the sp2-bonded carbon (N–C=N), respectively.[33] The N 1s spectra of g-C3N4 (Figure 1d) could be divided into three peaks. The peak at 398.7 eV was due to the sp2-bonded N (C-N=C). The peaks at 399.2 and 401.0 eV were ascribed to the bridging tertiary nitrogen (N-C3) and nitrogen atoms in amino (C-N-H) groups, respectively.[34] For the 5Li-C3N4/Co3O4, the C1s spectra were almost unchanged compared with those of pristine g-C3N4. In contrast, the N 1s peaks of 5Li-C3N4/Co3O4 moved toward lower binding energies, suggesting a strong interaction between g-C3N4 and Co3O4. Similar results were also reported in other g-C3N4-based composite photocatalysts.[35, 36] Such intense interaction was beneficial to the charge transfer between the interface. Figure 1e presents the Li 1s spectrum for 5Li-C3N4/Co3O4. The Li 1s peak at 55.1 eV was assigned to the Li–N bond.[26, 27] It was reported that the Li could stabilize in the interstitial position of g-C3N4 by Li–N coordination bond.[26, 27] In addition, the Li 1s spectrum of the 5Li-C3N4/Co3O4 after removing the Li-doped g-C3N4 (sintered at 700 °C followed by washed with water and dried) is presented in Figure 1e. No Li 1s peak was observed, suggesting that the sample after removing the Li-doped g-C3N4 did not contain Li element (or residual LiCoO2). Moreover, the sample was analyzed by ICP-AES, which further confirmed the same result. Therefore, combined with the XRD result (Figure 1a), it indicated that LiCoO2 was completely reduced to Co3O4 upon melamine thermal reduction. Figure 1f shows the Co 2p spectrum of 5Li-C3N4/Co3O4. The peaks at 779.9 and 794.7 eV corresponding to Co 2p3/2 and Co 2p1/2 were assigned to the Co3+, while the peaks at 781.5 and 796.6 eV were attributed to the Co2+ of Co3O4.[37, 38]
The morphology and microstructure of the samples were characterized by SEM and TEM. As shown in Figure 2a, the g-C3N4 displayed a flake-like morphology. The 5Li-C3N4/Co3O4, as shown in Figure 2b, exhibited similar morphology to g-C3N4. The Co3O4 was not observed due to the very small particle size. We further performed TEM to investigate the microstructure of 5Li-C3N4/Co3O4. As shown in Figure 2c, the TEM and HAADF images clearly show that Co3O4 particles about 5 nm loaded on the flake-like g-C3N4. Unfortunately, the agglomeration of Co3O4 particles was observed, which may hinder the photocatalytic improvement. To improve the distribution of Co3O4 particles on g-C3N4, the ball milling can be used as a pretreatment to make the more uniform mixing of melamine and spent LiCoO2 powder. The selective electron diffraction of the particles (Figure 2c, inset) demonstrated some diffraction rings, among which corresponded to the (110) lattice plane of Co3O4. Figure 2e–h shows the EDS mapping of C, N, Co, and O distribution, which further demonstrated the formation of g-C3N4/Co3O4 composites. Due to the limit of Li EDS detected, the time-of-flight secondary ion mass spectrometry (TOF-SIMS) was used to observe the Li doping distribution. Figure 2i shows the randomly selected area. Figure 2j,k show the uniform Li distribution on X-Y and X-Z faces, demonstrating that Li was uniformly doped in g-C3N4, instead of the surface. In addition, the mass spectra of Li (Figure 2l) clearly specify the Li in the sample when performing the TOF-SIMS. The X-Y and X-Z faces of Co distribution corresponding to the Co mass spectra (Figure 2m–o) suggested that the Co was mainly distributed on the surface, demonstrating the Co3O4 loading on the Li-doped g-C3N4 surface. We also examined the porosity and surface area of the Li-C3N4/Co3O4 sample, as shown in Figure 2p. The SBET and pore volume of 5Li-C3N4/Co3O4 were higher than that of g-C3N4. Such enlarged SBET could offer more reaction sites for photocatalytic reaction.

2.2 Photocatalytic Properties
The optical property of the photocatalyst was examined by UV–vis absorption spectra. As presented in Figure 3a, the g-C3N4 displayed an obvious absorption edge around 450 nm. After forming the Li-doped g-C3N4/Co3O4 composite, a red shift of absorption edge and an enhanced broader visible light absorption (450–900 nm) were observed. Such a red shift of absorption edge could be attributed to the Li doping in g-C3N4,[26] and the enhanced visible light absorption (two absorption bands around 650 and 900 nm) was due to the Co3O4 integrating.[39] Furthermore, with the increase in LiCoO2 adding amount (Li doping and Co3O4 loading), the red shift and visible light absorption gradually enhanced, which accorded with the color of the samples gradually changing from yellow to gray. Such enhanced light absorption can facilitate the production of more photo-generated charge carriers for the photocatalytic reaction. The bandgaps of the Li-doped g-C3N4/Co3O4 composite are shown in Figure S2, Supporting Information. The Li doping decreased the bandgaps of g-C3N4 from 2.85 eV to 2.74–2.76 eV. The 1.40 eV was attributed to the bandgap of Co3O4. It was reported that the p-type Co3O4 had two bandgaps of 1.45 and 2.07 eV, and the latter was the fundamental optical bandgap energy of the interband transition.[39]
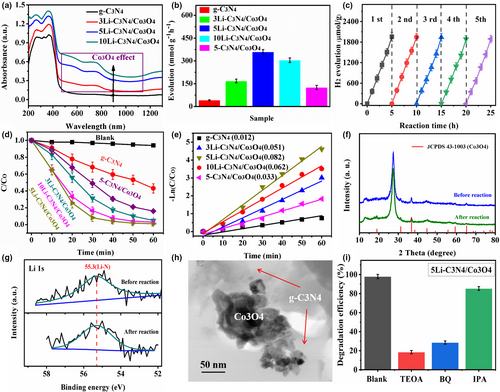
To investigate the photocatalytic performance of the Li-doped g-C3N4/Co3O4 photocatalyst, we firstly examined the photocatalytic H2 evolution under the simulated sunlight. For comparison, the 5-C3N4/Co3O4 without Li doping was also examined. As presented in Figure 3b, the g-C3N4 displayed a low photocatalytic H2 evolution rate of 41.4 μmol g−1 h−1 because of the fast recombination of photocatalytic electron and hole pairs. When integrated with Co3O4, the H2 evolution rate of g-C3N4 increased to μmol g−1 h−1. After the introduction of Li doping and Co3O4 integrating, the photocatalytic H2 evolution of g-C3N4 was further improved, which was due to the synergistic effect of Li doping and Co3O4 integrating. With the increase in LiCoO2 adding amount from 3 to 5 wt%, the photocatalytic H2 evolution rate increased from 168.2 to 359.5 μmol g−1 h−1. The optimal xphotocatalytic H2 evolution rate of the 5Li-C3N4/Co3O4 sample was 8.7 times higher than that of g-C3N4. However, when further increasing the LiCoO2 adding amount to 10 wt%, the photocatalytic H2 evolution rate decreased, which may be attributed to that the Li excess doping decreased the crystallinity and the Co3O4 overloading reduced the light absorption in g-C3N4.[26, 40] The stability of Li-doped g-C3N4/Co3O4 photocatalysts was evaluated by the cycling H2 evolution experiments. As shown in Figure 3c, the photocatalytic H2 evolution of 5Li-C3N4/Co3O4 did not obviously decrease, suggesting a stable photocatalytic performance.
The photocatalytic activity for RhB degradation was also examined under the simulated sunlight. As shown in Figure 3d, the RhB degradation without any catalyst is very little due to the almost unchanged RhB concentration. When the g-C3N4 and Li-doped g-C3N4/Co3O4 photocatalysts were added, an obvious photocatalytic degradation of RhB was observed. The g-C3N4 showed a relatively low RhB degradation efficiency of 57% within 1 h irradiation, while the Li-doped g-C3N4/Co3O4 photocatalysts exhibited much higher RhB degradation efficiency (89–98%) under the same test condition. The RhB degradation process follows the pseudo-first-order model. The apparent rate constant (k) was calculated and presented in Figure 3e. The k of 5Li-C3N4/Co3O4 was calculated to 0.082 min−1, which was 6.8 times higher than that of pristine g-C3N4 (0.012 min−1). The k of other Li-C3N4/Co3O4 samples decreased compared with 5Li-C3N4/Co3O4, further indicated that the Li doping and Co3O4 loading amount could prominently affect the photocatalytic activity of g-C3N4. The k of 5-C3N4/Co3O4 without Li doping was lower than those of Li-C3N4/Co3O4 samples (Figure 3e), suggesting the positive effect of Li on photocatalytic performance. In addition, the recyclable photocatalytic activity for RhB degradation (Figure S3, Supporting Information) also suggested stable photocatalytic performance. The XRD, XPS-Li 1s, and TEM of 5Li-C3N4/Co3O4 after photocatalytic reaction are presented in Figure 3f–h, which shows the stable Li-doped C3N4/Co3O4 composite structure. Such stable composite structure was attributed to the Li–N bond formed in the Li-doped g-C3N4 as well as the strong interaction between g-C3N4 and Co3O4. The results further clarified the above stable photocatalytic activity of this special photocatalyst.
To investigate the main reactive species for RhB photodegradation, the radical trapping experiments were conducted with isopropyl alcohol (IPA), triethanolamine (TEOA), and 1,4-benzoquinone (BQ) and to capture •OH, H+, and •O2−, respectively. As displayed in Figure 3i, the introduction of TEOA obviously decreased the RhB photodegradation efficiency, indicating that H+ was the main reactive species during the photocatalytic reaction. The addition of BQ also decreased the photodegradation efficiency of RhB, whereas the IPA had a little effect, which suggested that •O2− was also an indispensable activated species but •OH almost had a little effect on RhB photodegradation.
Moreover, we made a comparison with recent studies about the preparation process of g-C3N4-based photocatalysts and their H2 evolution and RhB degradation, as listed in Table S2 and Table S3, Supporting Information. It demonstrated that the one-pot sintering preparation of Li-doped g-C3N4/Co3O4 composite photocatalyst using spent LIBs in our study exhibited a simple and low-cost process and the photocatalytic activity could be competitive with the previous studies.
2.3 Enhanced Photocatalytic Mechanism
Photoluminescence (PL) spectra analysis was performed to investigate the transfer and separation efficiency of photogenerated charge carriers. As shown in Figure 4a, the main PL emission peak of g-C3N4 was located at 460 nm, corresponding to the radiative recombination of self-trapped excitation on g-C3N4 surface.[23] After introducing the Li doping and Co3O4 integrating, the emission intensity of the Li-C3N4/Co3O4 composite photocatalysts weakened at the same peak position compared with that of g-C3N4, and the 5Li-C3N4/Co3O4 exhibited the weakest intensity. The results suggested that the Li doping and Co3O4 integration could suppress the recombination of charge carrier, and 5Li-C3N4/Co3O4 possessed the highest charge separation efficiency. When the spent LiCoO2 adding amount further increased to 10 wt%, however, the PL intensity increased. It was attributed that the excess Li doping in g-C3N4 served as the charge recombination center.
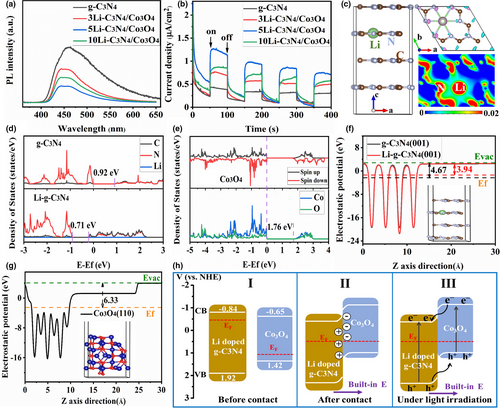
To investigate the charge separation and transfer property of Li-C3N4/Co3O4 composite photocatalyst, photocurrent response (I–t), and electrochemical impedance spectra (EIS) were performed, as shown in Figure 4b and Figure S4, Supporting Information. Compared with the pristine g-C3N4, the Li-C3N4/Co3O4 composites displayed increased photocurrent and decreased arc radius values. Theoretically, the larger photocurrent intensity and smaller arc radius mean the higher charge separation efficiency and lower charge transfer resistance.[41] Therefore, it can conclude that the Li doping and Co3O4 integration improved the charge separation and transfer efficiency in g-C3N4. Moreover, the 5Li-C3N4/Co3O4 exhibited the highest charge separation and transfer efficiency, which illustrated the above photocatalytic activity results.
To further reveal the mechanism of Li doping and Co3O4 integrating on the enhanced photocatalytic activity of g-C3N4, some DFT calculations were performed. We firstly optimized the bulk structures of g-C3N4, Li-doped g-C3N4, Co3O4 and then optimized the Li-doped g-C3N4 (001) and Co3O4 (110) surfaces for investigating the charge separation and transfer path in the interface. Besides the TEM results, we chose the Co3O4 (110) surface because this surface is a frequently exposed surface in Co3O4 nanomaterials.[42-44] Finally, based on the stable structures, the electronic structures were calculated. The detailed DFT calculations were provided in the Supporting Information. As shown in Figure 4c, after structure optimization, the Li was doped in the cave position. The top view image of the enlarged structure is shown in Figure S5, Supporting Information. The differential charge density (Figure 4c, upper right) indicated the charge redistribution after Li doping in g-C3N4, and the 2D sectional drawing (Figure 4c, bottom right) clearly suggested the formation of Li-N covalent bond between Li and g-C3N4. The result further clarified the above XPS-Li 1s result (Figure 1e) that the Li was interacted with g-C3N4 by Li-N bond.[26, 27] The differential charge density (Figure 4c) indicated the charge redistribution and formation of Li-N covalent bond after Li doping, which clarified the above Li 1s XPS results. The calculated DOS of bulk g-C3N4 and Li-doped g-C3N4 are shown in Figure 4d. The valence band (VB) and conduction band (CB) of g-C3N4 were all constituted by C and N. The bandgap (Eg) of g-C3N4 was calculated to 0.92 eV, which was an agreement with the previous calculation result.[45] The calculated result was lower than that of the experimental value due to the well-known shortcoming of the GGA functional. However, it can still well-accurately describe the relative results and will not affect the electronic structure analysis.[46] After Li doping, Li did not contribute to the VB and CB, while made both VB and CB down-shift and also decreased the Eg of g-C3N4. The narrowed Eg could decrease the electron transition energy from VB to CB and thus improve the visible light absorption, which further clarified the above UV–vis result (Figure 3a). In addition, the Co3O4 bulk (Figure 4e) characterized the semiconductor properties. The VB and CB composed of Co and O. The Eg of Co3O4 was calculated to 1.76 eV, which well agreed with the previous DFT result.[43]
To study the charge separation and transfer path, we also calculated the work function of the samples. As shown in Figure 4f,g, the work function of g-C3N4 (001) and Co3O4 (110) was calculated to 4.67 and 6.33 eV, respectively, which were well consistent with the previous studies.[47, 48] As for Li-doped g-C3N4 (001), the work function was decreased to 4.03 eV, suggesting that the Li doping could be beneficial to the electrons overflow to the surface. The difference in work functions of Li-doped g-C3N4 and Co3O4 indicated the presence of charge transfer at the interface. The band position of Li-doped g-C3N4 and Co3O4 before contact is presented in Figure 4h-I according to the UV–vis (Figure 3a) and Mott–Schottky plots (Figure S6, Supporting Information). The Fermi levels (EF) of the n-type g-C3N4 and p-type Co3O4 were located near their CB and VB positions, respectively.[39, 40] From the results of the Mott–Schottky plots, the Li-doped g-C3N4 still characterized the n-type. Based on the well-known energy band theory, the greater work function of Co3O4 will cause the electron transfer from Li-doped g-C3N4 to Co3O4. Then, at the interface, the surface of Li-doped g-C3N4 was positively charged, and the surface of Co3O4 was negatively charged. Consequently, a build-in electric field with the direction from Li-doped g-C3N4 to Co3O4 was produced. The further electrons migration was suppressed by the electric field until their EF reached alignment. As a result, accompanied by the shift of their EF, the band positions of the Li-doped g-C3N4 shifted down while the band positions of the Co3O4 shifted up. At the same time, the band edge of Li-doped g-C3N4 was curled upward, while the band edge of Co3O4 was curled downward (Figure 4h-II). Under simulated sunlight irradiation, as presented in Figure 4h-III, the Li-doped g-C3N4 and Co3O4 were both excited to produce electron–hole pairs. The build-in electric field accelerated the photogenerated electrons transfer from CB of Co3O4 to Li-doped g-C3N4, and holes from VB of Li-doped g-C3N4 to Co3O4. Consequently, the composite photocatalyst effectively separated the photogenerated charge carriers.
Based on the above experimental and theoretical results, the Li-doped g-C3N4/Co3O4 composite photocatalyst was successfully constructed through a one-pot in situ thermal reduction of spent LiCoO2 by melamine. During the reduction process, LiCoO2 was decomposed and the Li element in situ participated in the formation of g-C3N4, and simultaneously, the Co3O4 integrated with g-C3N4, forming the Li-doped g-C3N4/Co3O4 composite photocatalyst. The Li doping and Co3O4 integrating can synergistically enhance the photocatalytic performance of g-C3N4. The synergistic enhancement mechanism can be summarized as follows. Firstly, the Li doping narrowed the bandgap and decreased the required electron transition energy from the VB to CB, thus enhancing the visible light absorption and producing more photogenerated charge carriers for photocatalytic reaction. Furthermore, the Co3O4 integrated with Li-doped g-C3N4 can benefit the transfer and separation of photogenerated electron–hole pairs, further enhancing the photocatalytic performance. The photogenerated electrons on the CB of g-C3N4 could react with the dissolved O2 producing •O2− for RhB degradation or react with H2O for H2 evolution. Meanwhile, the holes on the VB of Co3O4 could directly oxidize RhB or were captured by the sacrificial reagent. Therefore, compared with the g-C3N4, the Li-doped g-C3N4/Co3O4 composite photocatalyst demonstrated an enhanced photocatalytic performance owing to the synergetic Li doping and Co3O4 integrating.
As is known, in addition to LiCoO2, spent LIBs contained other electrodes such as LiMn2O4 and LiNixCoyMnzO2 (NCM). When these electrodes were sintered with melamine, they will also decompose and form some Li-doped g-C3N4-based composite photocatalysts. The applicability for NCM and mixed electrodes by this approach will be verified in our following studies.
3 Conclusions
The study transformed spent LiCoO2 into a Li-doped g-C3N4/Co3O4 composite photocatalyst through one-pot in situ thermal reduction by melamine. This special composite photocatalyst showed a significantly enhanced photocatalytic activity. The photocatalytic H2 evolution and RhB degradation rates of the heterojunction were 8.7 and 6.8 times higher than those of g-C3N4. Furthermore, the composite photocatalyst exhibited well photocatalytic stability. The experiments combined with DFT calculation revealed that the enhanced photocatalytic activity was due to the synergetic mechanism of Li doping and Co3O4 integrating heterojunction, which enlarged the broader visible light absorption (450–900 nm) and facilitated the efficient transfer and separation of photo-generated charge carriers. In short, this study utilizes waste as a high-efficient photocatalyst through a facile and sustainable process, which realizes waste utilization, low-cost preparation of catalyst, and environmental protection.
4 Experimental Section
Materials and Synthesis
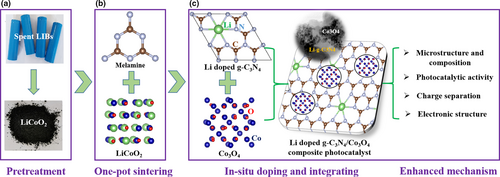
Spent LiCoO2 batteries were firstly discharged, and then the plastic shell, separator, cathode, and anode were manually dismantled to separate. Then, the LiCoO2 cathode powder was obtained,[11] as presented in Scheme 1a. The Li-doped g-C3N4/Co3O4 composite photocatalyst was prepared by simple one-pot sintering process, as shown in Scheme 1b. Melamine (10 g) and a certain amount of spent LiCoO2 powder (3, 5, and 10 wt%, relative to melamine) were uniformly mixed in a covered crucible and then sintered in a muffle furnace at 550 °C for 2 h with a heating rate of 3 °C min−1. During the sintering process, melamine served as both the reductant for LiCoO2 decomposition and the raw material for g-C3N4 production. The Li was doped in g-C3N4, and the generated Co3O4 particles were in situ integrated with the Li-doped g-C3N4, forming the Li-doped g-C3N4/Co3O4 composite photocatalyst, as shown in Scheme 1c. Since the toxic cyanide gas could be produced during the thermal decomposition of melamine, the muffle furnace was equipped with a gas outlet pipe to expel the cyanide gas, which was then collected and neutralized by alkaline liquor. After cooling naturally to room temperature, the products were washed thoroughly by deionized water follow by centrifugation and dried at 80 °C. The Li-doped g-C3N4/Co3O4 with the different LiCoO2 adding amount (3, 5, and 10 wt%) is denoted as 3Li-C3N4/Co3O4, 5Li-C3N4/Co3O4, and 10Li-C3N4/Co3O4, respectively. To determine the actual Co3O4 content in the composite photocatalysts, the products were sintered at 700 °C and then washed by water and dried to remove the Li-doped g-C3N4. The results indicated that the actual Co3O4 content in the composite photocatalysts was 8.6, 16.7, and 35.4 wt%, respectively, for the samples denoted with LiCoO2 adding amount (3, 5, and 10 wt%, relative to melamine). For comparison, the 5Li-C3N4 and 5-C3N4/Co3O4 (without Li doping) were prepared as the above process which LiCoO2 was, respectively, replaced by CH3COOLi and Co(NO3)3·6H2O according to the previous studies.[24, 26] The adding amount of CH3COOLi and Co(NO3)3·6H2O for the preparation of 5Li-C3N4 and 5-C3N4/Co3O4 was about 0.33 and 1.48 g, respectively, which accorded with the element weight of Li and Co in LiCoO2 (0.5 g). The pristine g-C3N4 and Co3O4 were, respectively, obtained by only using melamine and Co(NO3)3·6H2O as the above process. In this study, we mainly investigated the microstructure, composition, photocatalytic activity, charge separation, and electronic structure of this heterojunction (Scheme 1c). Ultimately, the enhanced synergy mechanism of photocatalytic activity was revealed.
Characterization
The detail material characterization, photocatalytic H2 evolution, and RhB degradation experiments as well as the DFT calculations were provided in the Supporting Information.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51534005), Postdoctoral Innovative Talent Support Program (BX20190200), and China Postdoctoral Science Foundation (2020M671129). Additionally, the authors also thank the Analytical and Testing Center of Shanghai Jiao Tong University of Science and Technology for providing experimental measurements.
Conflict of Interest
The authors declare no conflict of interest.




