Green roof irrigation management based on substrate water potential assures water saving without affecting plant physiological performance
Funding information: European Union, Grant/Award Number: POR FESR 2014-2020
Abstract
Irrigation management in extensive green roofs (EGRs) is crucial in Mediterranean and semi-arid climates, as it should guarantee efficient water use while ensuring plant survival and vegetation cover. However, benefits of maintaining moderately low substrate water potential (Ψs) have not been adequately investigated to date. An irrigation control unit based on Ψs thresholds for irrigation (MediWater Safe [MWS]) was compared to a common irrigation timer maintaining Ψs ⁓ 0 MPa (CTR) in shrub-vegetated Mediterranean EGR modules. The effect of the different irrigation regimes on substrate temperature, plant water relations (leaf conductance to water vapour, midday water potential and turgor loss point) and root vulnerability to heat stress via electrolyte leakage was tested in four shrub species. Decreasing Ψs thresholds to −0.4 MPa reduced irrigation volumes by 68% in 3 summer months. However, the MWS unit neither influenced plant water status and vegetation cover nor induced physiological acclimation responses. Brief irrigation cycles imposed by MWS in the warmest hours reduced substrate surface temperature by 3°C compared to CTR. Plant water status dynamics and root vulnerability to heat were species specific. Progressive stomatal closure and plant decline occurred only in Ceanothus thyrsiflorus and were associated to high root vulnerability to heat. Mortality occurred only in some Ceanothus plants in the CTR module, where higher Ψs favoured the expansion of Hyperucum x moserianum. The results suggest that selecting proper Ψs thresholds for irrigation could optimize EGR benefits, guaranteeing substantial water savings and proper plant establishment. Moreover, we claim root resistance to heat as a key parameter for plant selection in Mediterranean EGRs.
1 INTRODUCTION
Green roofs are engineered ecosystems included among urban greening techniques in the so-called nature-based solutions framework (European Commission & Directorate-General for Research and Innovation, 2015; Faivre et al., 2017). The benefits provided by green roofs comprise mitigation of the urban heat island effect (Bevilacqua et al., 2017), reduction of storm-water runoff (Brandão et al., 2017; Li & Babcock, 2014), air and noise pollution abatement (van Renterghem, 2018; Viecco et al., 2018), carbon sequestration (Agra et al., 2017), species conservation, increase in functional diversity (Braaker et al., 2017), habitat availability and connectivity (Partridge & Clark, 2018). Moreover, at the single-building scale, green roofs can provide better insulation and mechanical protection to the roof, reducing energy consumption for heating and cooling and prolonging its lifespan (Evangelisti et al., 2020; William et al., 2016). In particular, high albedo, shading and increased evapotranspiration reduce surface temperatures in vegetated roofs compared to conventional ones (William et al., 2016).
Extensive green roofs (EGRs) are characterized by substrate depth of <15–20 cm, where only small-sized vegetation such as herbs and small shrubs can thrive (Oberndorfer et al., 2007). EGRs are more affordable than intensive green roofs (characterized by deeper substrates) in terms of construction and maintenance costs and are thus suitable for retrofitting of many buildings. Green roof substrates consist of engineered growing media that are generally lighter and shallower and have lower organic matter content than most natural topsoils (Szota et al., 2017). This makes EGRs extremely challenging habitats for plants, especially in arid, semi-arid and Mediterranean climates, where warm and dry summer periods constitute a major constraint for EGR implementation. These conditions can compromise plant establishment and development, with negative impacts on plant cover and related green roof benefits (van Mechelen et al., 2015). These impacts are exacerbated by climate change, leading to more frequent and/or prolonged drought periods coupled with heat waves (Spinoni et al., 2018).
Some technological advancements have recently been applied to Mediterranean green roofs to improve their performance. These include modifications of substrate composition to increase water retention and available water content (WC) (Raimondo et al., 2015; Xue & Farrell, 2020) and the use of soil conditioners to improve water holding capacity (Papafotiou et al., 2013; Savi et al., 2014, 2015). Still, adequate selection of plant species to vegetate green roofs and proper irrigation management appear as the most promising strategies for further implementation of green roofs in harsh climates.
Selection of drought-tolerant plants including succulents (e.g., Sedum Spp.), grasses, herbs and shrubs (Savi et al., 2015) reduces water requirements of green roofs. In particular, shrubs are very good candidates for EGRs in Mediterranean climates (Raimondo et al., 2015; Savi et al., 2015) as they generally have a better stomatal control of transpiration (Farrell et al., 2013) and at the same time higher water use rates than herbaceous plants, ensuring higher storm water retention capacity (Brandão et al., 2017). However, studies on plant performance and trait-based selection on EGRs are still scarce (Du et al., 2019a). Plant selection for EGRs should be ideally based on physiological traits related to drought and heat resistance, including water use strategies (iso-anisohydry; Raimondo et al., 2015), safety/efficiency trade-offs, leaf water potential at turgor loss point (Ψtlp, Du et al., 2019b), vulnerability to embolism formation and vulnerability of shoots and roots to heat stress (Savi et al., 2016). However, selection based on water use strategies and climate of origin alone does not per se ensure higher drought survival in EGR systems (Du et al., 2019a), requiring specific physiological performance tests on EGRs.
A crucial issue in EGRs, especially in regions suffering recurrent drought periods, is irrigation management, which should ensure adequate water supply for plant survival and vigour, better substrate temperature control and energy performance (Gomes et al., 2019). In Mediterranean regions, additional irrigation is required in summer, especially during the growing season following transplant. However, effects of substrate WC on green roof thermal performance are still controversial, producing contradictory results (van Mechelen et al., 2015). On one hand, irrigation can partly buffer high substrate temperatures in dry, hot summers, as moist substrates enhance evaporative cooling effects (Chagolla-Aranda et al., 2017; Wang et al., 2017). On the other hand, higher WCs can increase substrate temperatures because water has higher thermal conductivity than air, enhancing heat transfer to the deeper substrate layers (Azeñas et al., 2018; Moody & Sailor, 2013; Theodosiou, 2003).
Freshwater is a major limiting natural resource worldwide (Vörösmarty et al., 2010), calling for efficient and sustainable water use solutions (UNESCO, 2020). Therefore, reducing irrigation volumes without compromising vegetation performance and EGR benefits, including thermal performance, should become a key target for the expansion of green roof technology (Azeñas et al., 2018; Schweitzer & Erell, 2014). ‘Deficit irrigation’ is a possible agronomical strategy based on the reduction of potential evapotranspiration to a threshold value assuring a satisfying crop yield, and it has been recently tested in EGRs (Azeñas et al., 2018; Ntoulas & Nektarios, 2015). Another irrigation strategy typically adopted in open field crops or greenhouse vegetable crops is based on thresholds of substrate WC (Thompson et al., 2007), which is related to water potential (Ψs) with substrate-specific patterns. Substrate WC can be easily and continuously monitored through dielectric sensors based on time domain, frequency domain reflectometry and capacitance (see Vaz et al., 2013) or even through plant microbial fuel cells (Tapia et al., 2017).
To the best of our knowledge, the effects of reduced irrigation and consequent decrease of Ψs on plant physiological performance and substrate temperatures have not been thoroughly addressed in green roofs. To this purpose, in this experiment, we applied two different irrigation control systems, i.e., a common irrigation timer and an irrigation control unit based on Ψs thresholds, on shrub-vegetated EGR modules during the first summer after planting. We hypothesized that a moderate decrease in water supply (and Ψs) would not negatively impact plant physiology and would be beneficial for substrate temperature control in Mediterranean EGRs. In addition, we evaluated the suitability of the four study species, based on relevant physiological traits, for EGR implementation.
2 MATERIALS AND METHODS
2.1 Experimental design and irrigation treatments
The experiment was carried out between spring and summer 2019 on a flat rooftop of the Biology building of the University of Trieste, Dept. of Life sciences (Trieste, Italy, 45°39′40.9″ N, 13°47′40.1″ E). Trieste is characterized by a sub-Mediterranean climate, with relatively mild winters and warm and dry summers. Average annual temperature is 15.9°C (average maximum of 28.0°C in July and August and average minimum of 5.0°C in January) and average annual rainfall sums up to 870 mm (reference period 1994–2020, http://www.osmer.fvg.it). During the experimental period, air temperature (Tair), relative humidity (RH; EE06-FT1A1-K300, E + E Elektronik GmbH, MA, USA) and precipitation (ARG 100 Raingauge, Environmental Measurements Limited, UK) were measured on a roof nearby the experimental modules and recorded every 5 min by a datalogger (CR1000, CAMPBELL SCIENTIFIC INC., USA).
In spring 2019, two rectangular (2.00 m × 1.25 m) experimental green roof modules were installed. The green roof profile comprised, from bottom to top: LDPE waterproofing root barrier (HarpoBarrier, mass = 240 g m−2); protection and water retention layer (MediPro MP300, retention capacity = 3 L m−2); 25 mm plastic profiled drainage layer (MediDrain MD25, PST; drainage capacity at 1% slope = 0.8 L m−1 s−1, water reservoir = 3 L m−2); geotextile polypropylene filtering layer (MediFilter MF1; pore size = 120 μm); 15 cm deep mineral-based substrate developed for EGR installations (TerraMediterranea TMT, Harpo Spa, Trieste, Italy). The substrate was a blend of lapillus, pumice and zeolite, enriched with organic matter (compost and peat, <60 g l−1). Substrate chemical and physical properties were the following: pH = 8.40; electric conductivity = 112.20 μS cm−1, cation exchange capacity = 28.25 meq/100 g, grain size = 0.05–20 mm, porosity = 60%–70% (v/v), dry bulk density = 1010.1 kg m−3, drainage rate = 15.8 mm min−1.
Six squared metal boxes (43 cm × 43 cm × 18 cm) were placed next to the shorter sides of each experimental module and equipped with drainage layer, filtering layer and 15 cm TMT substrate. The boxes had four small holes on the bottom to allow water drainage and were used to host plant species for destructive measurements of root vulnerability to heat stress (see below). A picture and the scheme of the modules and related boxes are shown in Figure S1.
Four shrub species commonly used in EGR installations were selected: Ceanothus thyrsiflorus Eschsch. (Rhamnaceae), Hypericum x moserianum (hybrid of Hypericum calycinum L. and H. patulum Thunb.; Clusiaceae), Lonicera ligustrina var. pileata (Oliv.) Franchet (Caprifoliaceae) and Myrtus communis L. (Myrtaceae). On 18 April, 24 potted plants (six Ceanothus, nine Hypericum, six Lonicera and three Myrtus) provided by a private nursery (‘Il Germoglio Cooperativa Sociale’, Salzano, Italy) were randomly arranged in each of the two modules. For each module, additional 12 plants (three per species) were planted in the six metal boxes, with two plants of different species per box, so that every box differed in species combination. Before planting, roots were gently rinsed in order to remove the original growing medium.
Modules and boxes were equipped with a drip irrigation system: Constant flow rate drippers (15 per module and 1 per steel box) were connected via HDPE tubes to the tap water system. From transplant to irrigation control installation, modules and boxes were manually irrigated every 2 days with 10 mm water, in order to favour plant establishment. Between April and July, three different fertilizers were applied to the substrate after being solubilized in water: Nutri One (Valagro Spa; 0.02 L per plant), Ferrilene 4.8% (Valagro Spa, 5 g per plant) and Asso di Fiori (Cifo Srl, 0.35 g per plant).
On 21 June, the irrigation tubing of one module and respective boxes (acronym CTR, Control) was connected to a common garden timer supplying every day at 8:00 h (solar time) a water volume corresponding to 7 mm precipitation, while the other one was connected to an irrigation control unit prototype (MediWater Safe, Harpo Spa, Trieste, Italy; acronym MWS). The MWS unit was connected to two WC sensors diagonally installed at 5–10 cm depth and to a temperature sensor installed at 2 cm depth. While the traditional irrigation system was addressed at maintaining the substrate at field capacity (i.e., Ψs ⁓ 0 MPa), the MWS was programmed to reduce irrigation, but avoiding Ψs dropping below a minimum target value of −0.4 MPa. Irrigation started when Ψs measured at 08.00 h approached the target value. Water volumes supplied were the same as the CTR module when Ψs reached the target, while were automatically corrected by reducing or increasing irrigation times when Ψs was above or below the selected threshold, respectively. The target Ψs was raised to −0.2 MPa on 14 August (after all plant physiological measurements ended) to slightly reduce soil drought stress. In addition, MWS was programmed to provide additional brief irrigations (1 min each) in the hottest hours of the day (at 12:00, 15:00 and 18:00 h), only when substrate temperature surpassed a threshold of 30°C. A pressure reducer was fitted at the faucet of both irrigation systems, to get comparable flow rates for drippers in the CTR (870 ml min−1) and in the MWS (930 ml min−1) module.
In order to compare substrate WC and related Ψs between the two irrigation treatments, on 28 June, five additional soil moisture sensors (EC-5, METER Group Inc. USA; three in the main module, two in randomly selected boxes) per treatment were diagonally placed in the substrate at 5–10 cm depth and connected to a datalogger (Em50, METER Group Inc. USA) recording WC every hour.
2.2 Substrate moisture release curve
The moisture release curve of the green roof substrate, relating WC to the respective water potential (Ψs) was obtained to calibrate the irrigation control unit (see above). Ψs > −0.88 MPa was measured with a two-tensiometer-based device (Hyprop 2, METER Group Inc., Pullman, USA) according to the measurement limit of tensiometers, while Ψs < −0.88 MPa was measured with a dewpoint hygrometer (WP4, Decagon Devices Inc., Pullman, USA).
For dewpoint hygrometer measurements, about 1 L of substrate was saturated to field capacity in a pot containing a filter paper to prevent particle loss, six to seven subsamples (about ca. 5 g) were placed in sample holders. Ψs was measured and coupled with its respective FW measured with a digital balance. All samples were dehydrated on the bench at progressive steps while measuring Ψs and FW, until reaching Ψs ~ −6 MPa. After measuring their DW as above, WC was calculated for each Ψs following Equation (1).
The moisture release curve for the substrate was obtained by pooling together the data obtained with the two methods, using the software Hyprop Fit (METER Group Inc. USA). The best fit function, chosen through the corrected ‘Akaike information criterion’ (AICc; Akaike, 1974), was the ‘Van Genuchten constrained unimodal’. The curve is shown in Figure S2.
2.3 Plant water status, leaf fluorescence and derived parameters
Water status of shrubs was assessed by measuring daily minimum leaf water potential (Ψmin, MPa) and leaf conductance to water vapour (gL, mmol m−2 s−1) in three campaigns: one before installation of MWS on 20–21 June when the modules were similarly irrigated, and other two on 16 July and 12 August. Measurements were carried out between 12.00 and 14.00 h (solar time), at the peak of irradiance, in three to four individuals per species and module. Given the small size of leaves, Ψmin was measured on ca. 4 cm twigs that were immediately sealed in plastic cling after harvest and placed in refrigerated bags with a piece of wet paper towel to avoid water loss until measurements, performed with a pressure chamber (1505D, PMS Instrument Company, Albany, USA). gL was assessed with a steady-state porometer (SC-1, METER Group Inc., Pullman, USA) on sun-exposed, intact, healthy leaves at the twig apex, and coupled to photosynthetic photon flux density (PPFD, μmol m−2 s−1) measured with a Quantum photo-radiometer (HD 9021, Delta OHM S.r.l., Padova, Italy) at the selected leaf surface. In the June and July campaigns, some additional photosynthetic parameters (relative chlorophyll content, PhiNPQ, Phi2, PhiNO, Fv/Fm; see Table S1 for a description) were additionally measured with an all-in-one fluorometer, chlorophyll meter and bench-top spectrometer (MultispeQ, PhotosynQ LLC, East Lansing, USA).
2.4 Substrate temperatures
To test for possible effects of the applied irrigation regimes on substrate temperatures and to assess the highest temperatures at which the plant root systems were exposed, substrate temperatures were monitored in three selected hot days (27 June, 24 July and 12 August) between 16.00 and 17.00 h, i.e., at the daily air temperature peaks. Superficial (T0cm, °C) and 5 cm depth (T5cm, °C) temperatures were measured with an infrared thermometer (805—Testo SE & Co. KGaA, Settimo M.se, Italy) and with a thermometer equipped with a thermistor probe (Temp 7 NTC and thermistor probe NT 7L, XS Instruments, Carpi, Italy), respectively, in 9 points positioned along the diagonals of the modules, and in the centre of each metal box.
2.5 Root vulnerability to heat stress
2.6 Vegetation cover and plant mortality
Possible effects of the irrigation regimes on plant vigour and growth were evaluated by measuring vegetation cover of the two modules at the end of the growing season (30 September). For each module, two pictures were taken with a digital camera (IXUS 185, Canon) fixed to a bubble levelled tripod. With the software ImageJ (US National Institutes of Health, http://imagej.nih.gov/ij/), the internal surface area in pixels of the module was measured, and a colour threshold adjustment was applied (HUE: 37–107; saturation 0–255; brightness: 95–255) to identify vegetation and measure the vegetated area (in pixels). Vegetation cover was calculated as the vegetated area divided by the total surface area of the module, expressed as a percentage. Plant mortality was monthly assessed in the modules during the whole experimental period.
2.7 Statistical analysis
Statistical analyses were performed with R software (R Core Team, 2019). For gL, Ψmin, ΨTLP, relative chlorophyll content, PhiNPQ, Phi2, PhiNO, a three-way analysis of variance (ANOVA) test was applied, using the ‘aov’ function of the ‘stats’ R package, with treatment (CTR and MWS), species, campaign and their interaction as predictive variables. For Fv/Fm, due to heteroscedasticity of the data, a generalized least square (GLS) model was calculated, using the ‘nlme’ package (Pinheiro et al., 2019), specifying a ‘varPower’ variance structure. For T5cm, a three-way ANOVA test was used, with treatment, campaign, container (module or metal box) and their interaction as predictive variables. Since normality of the residuals and homogeneity of variances assumptions for the T0cm model were not met, the variable was log-transformed, and a GLS model was performed as described above. For ΔREL, a three-way ANOVA test was used, with temperature, species and treatment and their interaction as predictive variables. For all significant tests (α = 0.05), differences between groups were tested with the Tukey honest significant difference (HSD) post hoc test, with P values adjusted using Bonferroni–Holm method, and the adjusted R2 of each model was calculated. For GLS models, the pseudo R2 was calculated using the Nagelkerke method (Nagelkerke, 1991).
3 RESULTS
During the experimental period, mean daily temperature was 15°C in May, 25°C in June, July and August, and 20°C in September (Figure S3). The highest temperatures were recorded between late May and July, with daily peaks frequently above 30°C. May was characterizad by relatively abundant precipitation (204 mm), whereas June was the driest month, with 35.2 mm of total rainfall, concentrated in one single day. In July and August, monthly precipitation was about 110 mm, but rain events were rare and often separated by long rainless, hot periods. Mean vapour pressure deficit (VPD) was 0.39 kPa in May, 1.3 kPa in June and July, 1.2 kPa in August and 0.8 kPa in September, with several peaks above 3.0 kPa over the whole summer.
3.1 Irrigation regimes, substrate WC and water potentials
Over 102 days, from installation of the MWS unit (June 21st) to the end of the experiment (30 September), the total irrigation volume supplied to the CTR and MWS modules was 1775 L (710 mm) and 569 L (228 mm), respectively (Figure 1a). Therefore, for about 3 months, the MWS irrigation control unit saved about 1200 L of water for a 2.5 m2 irrigated green roof surface.
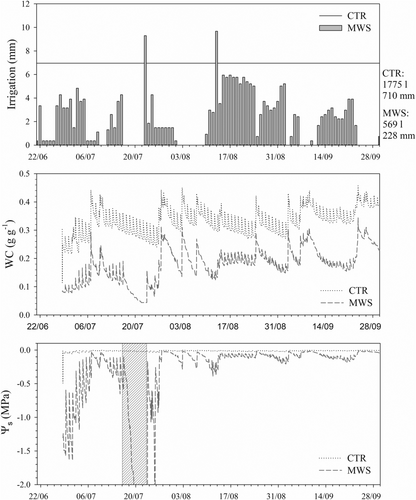
The MWS irrigation control unit reduced substrate WC, with a consequent decrease in substrate water potential (Ψs) with respect to the CTR treatment (Figure 1b,c). Except for the MWS trial stage (first two operation weeks) and during 1 week of malfunction in mid-July, in which WC fell below 0.10 g g−1 and the respective Ψs dropped below the permanent wilting point (−1.5 MPa), Ψs was maintained above the target threshold values (−0.40 and −0.20 MPa, before and after 12 August, respectively). At the end of the period of malfunction of the MWS unit, Ψs reached a minimum value of −8.6 MPa, and some plants growing near the edges of the module showed partial wilting. However, re-irrigation re-established the target Ψs regime within a few days and the affected plants fully recovered as well. Substrate WC in the CTR module ranged between 0.30 and 0.40 g g−1, approaching full saturation, and the respective Ψs was always above −0.05 MPa. In the MWS module, maximum (less negative) Ψs peaks were recorded after relatively abundant rainfall events, which often impeded to reach the target Ψs imposed to the irrigation unit. Progressive Ψs reductions occurred in warm rainless periods in between rainy days (compare Figures S3a and 1c).
3.2 Plant water status, leaf fluorescence and derived parameters
During the measurement campaigns, mean PPFD measured at the leaf surface was 1510 μmol m−2 s−1 and ranged between 1,200 and 1800 μmol m−2 s−1. Leaf conductance to water vapour (gL, Figure 2a–d) and minimum leaf water potential (Ψmin, Figure 2e–h) were not significantly affected by the irrigation treatment, whereas differences were detected among species, date and their interaction (Table S2). Hypericum maintained relatively high gL (around 600 mmol m−2 s−1), while Lonicera had relatively low gL (around 100 mmol m−2 s−1 on average) over the whole measuring period. In Myrtus, intermediate values (450 mmol m−2 s−1) were measured in June and August, with a drop (not significant) to 250 mmol m−2 s−1 in July. On the other hand, in Ceanothus, mean gL was 600 mmol m−2 s−1 in June, and progressively dropped during the season, falling below 100 mmol m−2 s−1 in August. Ψmin ranged between −1.0 and −2.0 MPa and did not change within the single species during the season except for Hypericum, in which it raised by about 1 MPa in July.

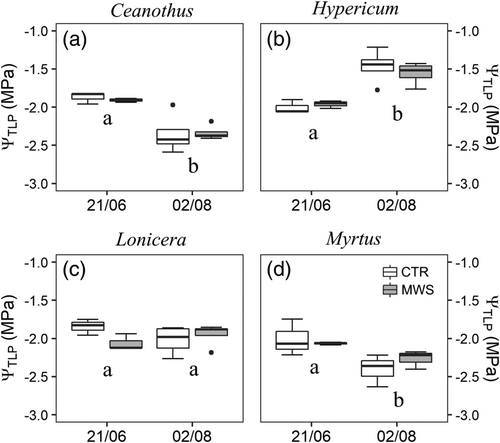
For all species, no significant difference was found between the two irrigation regimes in any photosynthetic parameter (Table 1). In all species, RCC, PhiNO and Fv/Fm slightly decreased from June to July (P < 0.05, Figures S4 and S5). Leaf water potential at turgor loss point (ΨTLP) was unaffected by the treatment in all species (Figure 3). In June, ΨTLP was around −2.0 MPa in all plant species. In August, 40 days after the activation of the MWS unit, ΨTLP of Lonicera did not change, whereas it dropped to ca. −2.5 MPa in Ceanothus and Myrtus, and it raised to −1.5 MPa in Hypericum.
| 20–21/06/2019 | 16/07/2019 | ||||
|---|---|---|---|---|---|
| CTR | MWS | CTR | MWS | ||
| Ceanothus | RCC | 35.691 ± 2.224a | 36.958 ± 2.097a | 30.025 ± 8.089b | 28.804 ± 4.589b |
| PhiNPQ | 0.406 ± 0.020a | 0.405 ± 0.030a | 0.605 ± 0.036a | 0.549 ± 0.008a | |
| Phi2 | 0.313 ± 0.003a | 0.393 ± 0.062a | 0.249 ± 0.011a | 0.281 ± 0.003a | |
| PhiNO | 0.281 ± 0.020a | 0.202 ± 0.036a | 0.146 ± 0.026b | 0.171 ± 0.005b | |
| Fv/Fm | 0.664 ± 0.016a | 0.609 ± 0.024a | 0.475 ± 0.045b | 0.536 ± 0.008b | |
| Hypericum | RCC | 47.587 ± 1.943a | 44.637 ± 0.931a | 37.834 ± 0.912b | 43.466 ± 5.626b |
| PhiNPQ | 0.585 ± 0.024a | 0.541 ± 0.036a | 0.543 ± 0.087a | 0.530 ± 0.094a | |
| Phi2 | 0.265 ± 0.012a | 0.300 ± 0.015a | 0.338 ± 0.060a | 0.346 ± 0.083a | |
| PhiNO | 0.150 ± 0.015a | 0.159 ± 0.021a | 0.118 ± 0.028b | 0.124 ± 0.016b | |
| Fv/Fm | 0.495 ± 0.027a | 0.518 ± 0.041a | 0.452 ± 0.077b | 0.480 ± 0.062b | |
| Lonicera | RCC | 24.331 ± 8.087a | 3.998 ± 1.339a | 10.515 ± 0.810b | 7.757 ± 2.188b |
| PhiNPQ | 0.565 ± 0.028a | 0.591 ± 0.141a | 0.651 ± 0.030a | 0.645 ± 0.035a | |
| Phi2 | 0.268 ± 0.022a | 0.290 ± 0.111a | 0.213 ± 0.005a | 0.197 ± 0.024a | |
| PhiNO | 0.166 ± 0.013a | 0.119 ± 0.031a | 0.136 ± 0.024b | 0.158 ± 0.048b | |
| Fv/Fm | 0.524 ± 0.023a | 0.439 ± 0.106a | 0.447 ± 0.043b | 0.460 ± 0.064b | |
| Myrtus | RCC | 18.810 ± 4.899a | 26.597 ± 8.375a | 15.298 ± 1.826b | 12.045 ± 1.691b |
| PhiNPQ | 0.679 ± 0.052a | 0.632 ± 0.036a | 0.717 ± 0.49a | 0.687 ± 0.011a | |
| Phi2 | 0.209 ± 0.022a | 0.234 ± 0.016a | 0.175 ± 0.028a | 0.202 ± 0.012a | |
| PhiNO | 0.112 ± 0.031a | 0.133 ± 0.021a | 0.107 ± 0.025b | 0.110 ± 0.014b | |
| Fv/Fm | 0.387 ± 0.078a | 0.452 ± 0.041a | 0.376 ± 0.057b | 0.399 ± 0.027b | |
- Note: A description of the parameters is given in Table S1. Different letters indicate significant differences among campaigns (P < 0.05).
3.3 Substrate temperatures
Figure 4 shows substrate temperatures measured at 5 cm depth (T5cm) and on substrate surface (T0cm) in the three measuring campaigns. Along the campaigns, T5cm and T0cm ranged between 30°C and 50°C, and between 31°C and 60°C, respectively. Overall, T5cm was only about 1°C higher in the metal boxes than in the modules (P = 0.03). However, these differences between container types were not significant within the single campaign and single irrigation treatment. Therefore, in Figure 4, box and module data are pooled together.
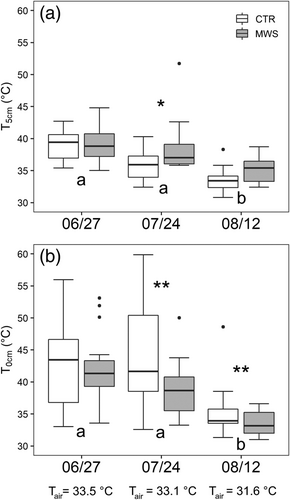
In June, i.e., before starting the MWS unit, substrate temperatures did not differ between modules. However, in July, T5cm was 2.7°C higher in MWS than in CTR substrates (P = 0.046), but again similar in August. Oppositely, T0cm were overall 3.2°C higher in CTR module (P = 0.02).
3.4 Root vulnerability to heat stress
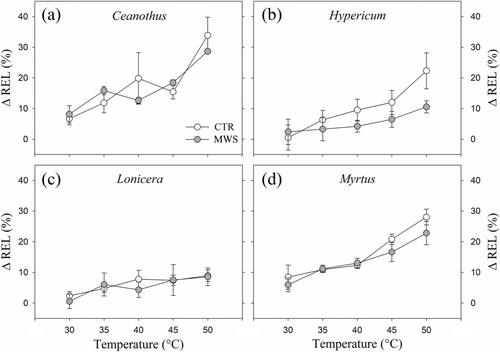
CTR and MWS plants did not differ in root vulnerability to heat stress, expressed as ΔREL, in any of the examined species (Figure 5). Significant differences were found between species (P < 0.0001): Ceanothus and Myrtus were the most, whereas Lonicera and Hypericum the least vulnerable. Ceanothus roots showed the highest vulnerability and overcame 30% ΔREL at 50°C, while Lonicera roots were the most resistant, never overcoming 10% ΔREL. Albeit not significantly, MWS roots of Hypericum tended to be less vulnerable than CTR roots at high temperatures, with an average ΔREL of 10% in the former and of 23% in the latter.
3.5 Vegetation cover
Vegetation cover at the end of the growing season was 71% and 61% in CTR and MWS modules, respectively. During the experiment, mortality was observed only in four individuals (one in July, three in September) of Ceanothus and only in the CTR module.
4 DISCUSSION
In this experiment, we tested the effects of reduced substrate water potentials (and irrigation volumes) on substrate temperature and on water status of the four selected shrub species in an EGR system. In the 3 months of operation of the MWS irrigation control unit, corresponding to the period with highest VPD (Figure S3c), irrigation volumes were reduced by 68% with respect to a classical irrigation timer used in the CTR module. If this irrigation were applied to a vegetated roof of modest surface area (100 m2), 48 m3 of water could had been saved for the same period of time. The reduction in available water for the plants did not significantly affect plant physiological performance and vegetation cover during the first year of plant establishment. However, independently on water availability, differences in water relations and root vulnerability to heat stress were generally observed among species.
4.1 Substrate temperatures
One of the aims of the experiment was to test the effectiveness of brief irrigation cycles in reducing substrate surface temperatures in hot summer days. In particular, the MWS unit was programmed to give small amounts of water in the hottest hours of the day when subsurface temperature overcame 30°C. This irrigation strategy was proven to be successful, reducing T0cm by about 3°C due to latent heat dissipation through evaporation. This result supports previous findings indicating a significant reduction in substrate subsurface temperature of green roof test modules after irrigation, with an effective decrease also in vegetation temperatures (Chagolla-Aranda et al., 2017).
On the other hand, the imposed decrease in WC in the MWS module was accompanied by a 3°C increase in substrate temperature at 5 cm depth (T5cm) in July at the daily air temperature peak. This result contrasts with our expectations and with a previous study showing a strong positive correlation between substrate WC and temperature in semi-arid climates (Reyes et al., 2016). Moreover, in a Mediterranean region, irrigation control based on deficit irrigation did not significantly affect temperatures at 7 cm depth (Azeñas et al., 2018).
In addition to substrate WC and leaf transpiration, light interception from the vegetation mixture is another factor influencing the ground heat-flux and, consequently, green roof substrate temperature especially in hot summer days. In this respect, plant canopy traits related to plant type (e.g., succulents vs. broadleaf perennials, Vaz Monteiro et al., 2017), species composition and cover (Bevilacqua et al., 2015) may play an important role. In our study, we did not address this aspect as a factor affecting substrate temperatures. Percentage of vegetation cover was not very high because plants were transplanted in spring of the same year of experiments. Therefore, it is possible that a higher cover would have positively influenced substrate temperature. However, vegetation cover was similar between the two treatments, likely not influencing the temperature results in our data set.
Irrigation tubes on the roof were not isolated and, therefore, high solar radiation overheated the water in the tubes that was then supplied to the modules during the refreshing cycles in the hottest hours of the day. We hypothesize that this warm water, due to the high permeability of the green roof substrate used, could quickly drain in depth and increase T5cm. Further experiments are required to test the effect of irrigation water temperature on substrate temperatures and whether colder water could solve the observed overheating.
4.2 Plant physiological responses
Plant physiological performance and vegetation were not influenced by the reduced irrigation volumes supplied by the MWS unit. In particular, the decrease in Ψs was likely not low enough to induce any ΨTLP adjustment. While in the first campaign performed in June all species and treatments showed very similar ΨTLP, later in the season different turgor loss point adjustments were observed among species. In August, Ceanothus and Myrthus had more negative values, whereas Hypericum did increase and Lonicera did maintain the same ΨTLP (Figure 3). These contrasting responses could indicate a species-specific plasticity in this trait and/or changes in substrate water availability between species along the season, perhaps as consequence of different root development (see below). We did not measure predawn water potentials, which could give additional information on substrate water availability for each single species. However, in the spring following the experiment, plants grown in metal boxes were gently removed, and visible differences in root development were observed between species, with Lonicera and especially Hypericum having the most, while Ceanothus having the least developed root system (see Figure S6). Leaf water potential at turgor loss point (ΨTLP) is recognized as one of the most important indicators of plant physiological drought resistance (Bartlett et al., 2012; Lenz et al., 2006), and its seasonal plasticity is common in plant species worldwide (Bartlett et al., 2014). However, the capability to adjust this trait along with changes in water availability is species specific and is primarily driven by osmoregulation, i.e., active accumulation of compatible solutes in the cells (Bartlett et al., 2012). Some species adopt other morphological/physiological strategies to cope with decreased water availability (Nicotra et al., 2010). This was possibly the case of Lonicera, which maintained the lowest gL over the whole summer, without showing decline symptoms. Conversely, Hypericum preserved the highest gas exchange rates along the whole summer despite maintaining Ψmin similar to those measured in the other species. This might indicate a relatively more water-spending behaviour, which could be also related to a higher substrate water availability due to a deeper and more developed rooting system.
In any case, for all species, ΨTLP was more negative than the lowest Ψmin measured along the season, theoretically ensuring a certain safety level to maintain adequate gas exchange and hydraulic function. Nevertheless, Ceanothus was the most vulnerable species in the experiment, as suggested by the progressive drops in gL along the season, by the marked drop in photosynthetic efficiency (see, e.g., Fv/Fm at Figure S4) and by the observed leaf shedding, plant decline and mortality despite high water availability. C. thyrsiflorus is native to California where it occurs in evergreen forests and chaparral sites (Conard et al., 1985). Species from this genus are deep rooted (Conard et al., 1985), likely guaranteeing access to stable water sources as superficial soil dries out during the dry summer season. In EGR systems, where substrate depth is a major limiting factor, Ceanothus seems (at least in the first establishment phase) to be vulnerable to hot summers, even when relatively abundant irrigation is supplied. Only some Ceanothus individuals died and, surprisingly, only in the CTR module. By the end of the experiment, Hypericum tended to dominate aboveground biomass in the CTR module. Thus, we hypothesize that in the CTR treatment this shrub could better profit from higher water availability, whereas in the MWS module, lower Ψs could have contributed to limit its expansion (see Figure S7 showing the modules at the end of the experiment). Neighbouring plant performance in green roofs can vary from facilitation to competition depending on availability of resources such as water (Butler & Orians, 2011) and light (Aguiar et al., 2019). Our results suggest that a moderate reduction in substrate water availability could provide the additional benefit of reducing negative competition-induced effects.
Heat is a major constraint for plant health and survival in Mediterranean EGRs (Savi et al., 2016). In our experiment, we have quantified the root electrolyte leakage in the range of values registered in the substrate during the experiment (Figure 4). Temperature effects on root plasma membrane integrity via electrolyte leakage tests have been previously examined only in perennial grasses (Zhang & Du, 2016), vegetable crops (Iglesias-Acosta et al., 2010) and some drought-adapted shrub species (Savi et al., 2016). Elevated temperatures can compromise plant growth and development and induce thermal stress in both plant shoots and roots, altering composition, fluidity and permeability of plasma membranes, as well as membrane protein activities with consequent leakage of ions and amino acids (e.g., Lindberg et al., 2005). Thermotolerance mechanisms can prolong the maintenance of protein stability and decrease degradation, ensuring growth during temperature stress (Huang et al., 2012). In our experiment, given that substrate temperatures at 5 cm depth were higher in the MWS than in the CTR module, we expected that MWS root systems could acclimate or, conversely, be damaged by these more unfavourable conditions. We did not observe any significant difference in root vulnerability between the two treatments (Figure 5). However, Hypericum roots tended to increase their heat tolerance in the MWS module, likely indicating a positive effect of the irrigation control on this species. Root vulnerability was rather species specific. Lonicera, never overcoming 10% ΔREL even at 50°C, was the most heat resistant species and might, therefore, represent a good candidate for Mediterranean EGR installations. The progressive decline of Ceanothus observed in the experimental modules might be explained by its high root vulnerability to heat stress, given that ΔREL ranged between 10% and 20% already at 30°C–35°C (Figure 5), when the average recorded substrate temperature peaks in summer were between ca. 35°C and 40°C (Figure 4). Accordingly, root resistance to high substrate temperatures was related to higher survival rates in shrub species grown in an EGR system (Savi et al., 2016). This belowground constraint in Ceanothus would likely reduce the efficiency of the root-to-leaf water pathway and contribute to the observed leaf gas exchange drop and plant decline. This underlines that water use and drought responses of a species in green roof installations are not necessarily comparable to those observed in its native distribution sites, where belowground root exploration does not encounter such limitations (Du et al., 2018). Therefore, shrub species selection based on their natural distribution does not necessarily guarantee greater survival and health in green roof installations (Du et al., 2019a).
5 CONCLUSIONS
In this experiment, we tested an irrigation control unit based on Ψs thresholds in a shrub-vegetated experimental EGR module. Given that the experiment was conducted in the first summer after transplant, which is crucial for plant establishment, the obtained results are promising.
Here, no acclimation to the new irrigation regime was detected, likely because of the short testing period (one summer) and/or the moderate Ψs thresholds used that, however, guaranteed remarkable water savings. In the future, plant physiological acclimation and development could be studied in the long term after the irrigation unit installation in the EGR system, considering also other crucial periods of the year for plant development such as spring. Moreover, the results suggest that lower Ψs thresholds could be set to further reduce irrigation volumes, identifying the Ψs limits that guarantee survival and satisfactory green roof vegetated cover. This study underlines the prospect of applying water potential-based irrigation systems on EGRs in Mediterranean and semi-arid regions to reduce water consumption while preserving or even enhancing green roof benefits.
ACKNOWLEDGEMENTS
The experiment was conducted in cooperation with the green roof company Harpo Spa (Trieste, Italy) within a POR FESR 2014-2020 FVG project (Programma Operativo Regionale cofinanziato dal Fondo Europeo di Sviluppo Regionale, CE 4814, 14th July 2015) funded by the European Union. Open Access Funding provided by Universita degli Studi di Trieste within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
The study was financed by Harpo SpA, which received the funding from the European Community and has developed the irrigation control unit tested during experiments. The co-author Sergio Andri is an employee of Harpo SpA.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




