Influence of landscape homogenization due to river damming on dragonfly (Odonata) community structuring in a subtropical forest in the southern Atlantic Forest
Funding information: Council for Scientific and Technological Development (CNPq), Grant/Award Numbers: 301867/2018-6, 402828/2016-0, 307984/2015-0, 482368/2013-6, 482986/2011-5, 132210/2018-5, 308648/2021-8; “Insetos e a metrópole - EDITAL N. 02/2020: PESQUISA/PRPPG/UFPR, Apoio a Atividades de Pesquisa”; International Dragonfly Fund (IDF)
Abstract
Human activities affect the structure, dynamics and energy flow of aquatic ecosystems. River damming, a common anthropic impact in Brazil, changes solar incidence, water flow and temperature of waterbodies, thereby affecting their fauna. Due to their high sensitivity to environmental changes, the Odonata may be indicators of these impacts. We sampled two ecologically distinct sites, (1) a quasi-pristine forested area and (2) a nearby human-impacted reservoir landscape, to evaluate the effects of damming on odonate community structure. The species composition of quasi-pristine communities was more heterogeneous and differed almost completely (indicating high turnover) from that of the reservoir-area communities. The capacity of the reservoir to maintain local fauna was almost nil. The communities in the changed landscape had the highest local diversity, which is related to the high occurrence of widespread generalist South American species. We also tested two recently proposed bioindication ratio tools based on the richness or abundance of high-level taxonomic categories; both effectively reflected the extent of the impacts of damming. The best performing ratios were Coenagrionidae/other Zygoptera richness ratio, Zygoptera/Anisoptera abundance ratio and Libellulidae/other Anisoptera richness ratio. The reservoir landscape promotes biotic homogenization. However, the water supply system entails the preservation of part of the native habitat in its surrounding areas, consequently maintaining forest-dependent biodiversity in quasi-pristine environments.
Practitioner points
- The capacity of the reservoir (the damming process) to maintain quasi-pristine forested communities was almost nil.
- The high diversity in the modified landscape (reservoir) is related to the high occurrence of widespread and generalist South American Odonata.
- Bioindication ratio tools were effective for demonstrating the extent of the impacts of damming. The best performing ratios were Coenagrionidae/other Zygoptera richness ratio, Zygoptera/Anisoptera abundance ratio and Libellulidae/other Anisoptera richness ratio.
1 INTRODUCTION
[…] globally, many more odonates occur in tropical ecosystems than in all others combined (Orr, 2006).
Forested areas in tropical regions play an essential role in providing the resources needed to maintain most of the dragonfly species at all stages of their life cycle; at least 70% of the earth's odonate diversity is forest-dependent (Corbet, 2006; Orr, 2006). Many dragonfly species are restricted to neotropical forests, for example, more than 80% of neotropical dragonfly genera have forest species (Paulson, 2006). Even the species that do not depend on forests can spend a part of their adult life there (Cordero-Rivera, 2006), benefiting from the physical, physiological and intra- and inter-biotic features of forests or just using them as their daily and seasonal refuge (Paulson, 2006).
Dragonflies are considered sun-loving insects (Cordero-Rivera, 2006). Sunlight is one of the most important factors influencing the success of the Odonata (Paulson, 2006) and is a major environmental filter of odonate species occurrence (De Marco et al., 2015). As predominantly ectothermic insects, adults of most species fly when the incidence of daylight is highest (Corbet, 1999). Cordero-Rivera (2006) compiled several factors supporting the importance of forests for maintaining Odonata biodiversity: Forests are places for feeding, larval development, protection and reproduction.
A common approach to estimating anthropic impacts on dragonflies is the evaluation of the conservation status of riparian forests (Oliveira-Junior et al., 2017; Petersen & Robert, 1992; Simaika & Samways, 2009; Smith et al., 2007), which has been recognized by some authors as the main determinant of the survival of Odonata assemblages (Oliveira-Junior et al., 2017). Therefore, human impacts on the landscape, such as river damming, deforestation, stream diversion, channelling and pollution, are causes of local extinction (see Calvão et al., 2016; Cordero-Rivera, 2006; Daga et al., 2019; Suhling et al., 2015).
The damming of rivers and streams to supply water or to produce hydroelectric energy is a frequent intervention (see Moretto et al., 2012). The impacts on the Odonata communities due to the river damming process involve changes in key environmental factors that affect dragonflies, such as increased incidence of sunlight, extended duration of water permanence, higher temperatures, excessive accumulation of material (e.g., silt) on the river bottom (Dijkstra & Lempert, 2003) and increased abundance of littoral aquatic macrophytes (Butler & deMaynadier, 2007), particularly invasive species (Mormul et al., 2010), conditions quite different from those found in lotic waters.
The impact on the incidence of sunlight, the light that reaches the forest floor, varies from less than 25% in forested areas to 100% in impacted areas (cf. Paulson, 2006), and these differences in light incidence act as an environmental filter for odonate community composition (De Marco et al., 2015). According to the ecophysiological hypothesis, this effect can be explained by body size. Slender-bodied odonates (most Zygoptera) are more susceptible to wide temperature variation, overheating and desiccation (Corbet, 1999; Paulson, 2006; see also Oliveira-Junior et al., 2017; Oliveira-Junior & Juen, 2019. Pereira et al., 2019), due to their thermoregulation made by simple heat-convection. On the other hand, robust-bodied odonates (most Anisoptera) are more tolerant to temperature variation given their higher efficiency considering convective and irradiative heat exchange - explained by their relative higher total surface - as well as most display a heliothermic behaviour for thermoregulation (De Marco et al., 2015).
1.1 Community responses to damming
Sporadic or continuous disturbances in watercourses generate individual and community responses, which may include (1) loss of diversity, (2) species substitution with the arrival of opportunistic species and (3) decrease in the average body size of dominant species (Gray, 1989). The loss of diversity and the arrival of opportunistic species (e.g., Ferreira-Peruquetti & Fonseca-Gessner, 2003) may reflect a regional decrease in the local endemic fauna, which helps to explain Vellend's ‘biodiversity conservation paradox’ (Vellend, 2017). Indeed, environmental degradation at different levels may or may not result in an increase in species richness due to colonization by common widespread species (Oliveira-Junior & Juen, 2019; Pereira et al., 2019). In this sense, the evaluation of how different facets of biodiversity change in pristine and impacted areas is key. With the recent increase in studies using metacommunity frameworks (Leibold et al., 2004), human-made ecosystem changes can be investigated by considering metacommunity structuring (Tonkin et al., 2016) and beta diversity patterns that are used to identify biotic homogenization phenomenon (Olden et al., 2018). This phenomenon is defined as the increase in similarity between communities over time because of the substitution of many rare and endemic species for a few widespread and common species—the mass extinction event driven by human activities in ecosystems (McKinney & Lockwood, 1999).
The effects of environmental degradation on odonate biodiversity of Brazilian tropical forests have been well studied for the Brazilian Amazonia (Brasil et al., 2014; Faria et al., 2017; Monteiro-Júnior et al., 2015; Nessimian et al., 2008; Oliveira-Junior & Juen, 2019; Pereira et al., 2019). For the Atlantic Forest, the most degraded Brazilian biome, there is still a paucity of studies (Dalzochio et al., 2018; Ferreira-Peruquetti & De Marco, 2002; Renner et al., 2016).
The purpose of this study is to understand how odonate biodiversity and community structure differ between quasi-pristine rivers (a lotic system) in a subtropical forest and a nearby man-made large reservoir (a lentic system). Given the known physicochemical differences between lotic and lentic systems (e.g., in temperature, dissolved oxygen and abundance of macrophytes) and between pristine and disturbed water systems (e.g., in riparian vegetation and solar incidence), we assume that river damming causes homogenization of the landscape and hence of community structure. We predict high species turnover (loss of forest-dependent endemic species and increase in open area widespread species), given the likelihood of high occurrence of species typical of South American lentic systems and reduction in species, typical of lotic environments, with a restricted distribution (De Marco et al., 2015; Gray, 1989). We also expect lower compositional variation in local communities in the reservoir area compared to variation in quasi-pristine forested sites. If this is the case, we would be able to demonstrate the biotic homogenization phenomenon, even though our analyses are based on the space-for-time approach (see Olden et al., 2018).
Finally, we applied two bioindication tools to determine their accuracy in subtropical forest water system conversion. The first one was proposed by Oliveira-Junior and Juen (2019), the Zygoptera/Anisoptera ratio as a bioindication tool for evaluating the habitat quality of Amazonian streams. This bioindication tool has as main premise the association of species of the suborder Zygoptera (with high surface area/volume ratio) with unaltered shaded streams, while Anisoptera species with open degraded habitats, premise supported by some studies (Oliveira-Junior et al., 2015, 2017). This may be due to differences in thermoregulation, dispersal capacity and environmental sensitivity of each suborder (Oliveira-Junior et al., 2017). The second one was proposed by Šigutová et al. (2019) that drew attention to odonate families that do not conform to the pattern represented by its suborder. The richness ratio between the Coenagrionidae and other Zygoptera and that between the Libellulidae and other Anisoptera can be used to identify habitat degradation: The higher these proportions, the greater the degradation.
2 METHODS
2.1 Study area
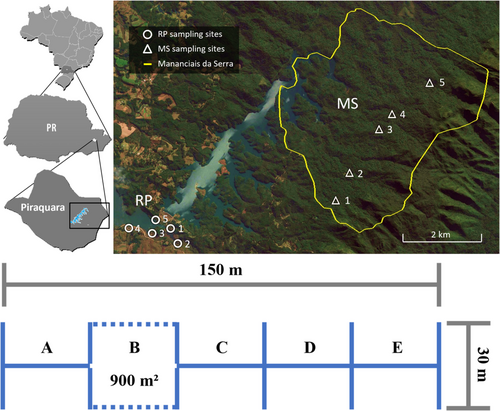
This study was carried out in a quasi-pristine remnant of the Atlantic Forest in Mananciais da Serra (headquarters 25°29′32″S, 48°59′34″W, 976 m a.s.l.) in Piraquara municipality, Paraná State, southern Brazil. A comprehensive checklist of the dragonflies of this region, with descriptions of the sampling sites and biotypes, is available in Araujo and Pinto (2021). The region encompasses a well-preserved area of the Atlantic Forest with many streams dammed to form two large reservoirs, maintained by the water and waste management company of the State of Paraná (SANEPAR), to supply water to Curitiba city and its metropolitan area (Figure 1). The vegetation of this area is characteristic of an ecotone between the Araucaria Forest and the Tropical Forest (Reginato & Goldenberg, 2007) with two distinct landscapes: an upstream preserved area at the watershed and a downstream modified area at the level of large artificial reservoirs.
2.2 Sampling
We selected five sampling sites to represent the forest-dependent communities of the metacommunity of odonates in the forested area and an additional five sampling sites to represent those in the modified landscape from forest and natural grasslands to open areas. The local community of a metacommunity was defined based on local knowledge of the area, given the goal to represent local and regional diversity measures, a common approach of the metacommunity framework (see Leibold et al., 2004). In addition, we sampled each sampling site twice for 6 h in total to improve the effort to represent the local community of each area. The sampling sites were selected based on their accessibility and to represent most of the mesohabitats found in the investigated area. Forested sites in Mananciais da Serra (MS) represent the native fauna of quasi-pristine habitats, in which the water system is predominantly lotic and is shaded by the forest. The open landscape on the banks of the Piraquara II reservoir (RP) represents the human-impacted area, in which the water system is predominantly lentic with a higher level of primary production by macrophytes (Figure 1, Table 1).
| Collection site | Coordinates | Mesohabitats | ||
|---|---|---|---|---|
| Latitude | Longitude | |||
| Lentic sites | ||||
| RP1 | RPIV. Piraquara II reservoir bank | −25.509284° | −49.038360° | Composite site, predominantly lentic, banks lacking macrophytes, riparian area with grassland; lotic system composed by small second order stream from the grassland flowing into the reservoir |
| RP2 | RPV. Piraquara II reservoir bank | −25.507368° | −49.031490° | Lentic with abundant macrophytes and forested riparian area |
| RP3 | RPIII. Piraquara II reservoir bank | −25.510484° | −49.032375° | Composite site, predominantly lentic, partially inhabited for macrophytes, banks lacking macrophytes; semi-lotic spots formed by small tributaries of Rio Piraquara river throughout the site |
| RP4 | RPI. Piraquara II reservoir bank | −25.509353° | −49.027647° | Lentic, with abundant macrophytes of many species; riparian area composed of open grass field and forest with semi-lotic channels near reservoir |
| RP5 | RPII. Piraquara II reservoir bank | −25.512844° | −49.025808° | Composite site, lentic spot composed by swamps with grasses and many macrophytes species; lotic spot is as fourth order river tributary of the Piraquara II reservoir |
| Lotic sites | ||||
| MS1 | Salto catchment. Stream in forested area | −25.502778° | −48.985278° | Composite site, lentic spot formed by damming of a stream rock bottom, a partially shaded artificial pool with dense leaf litter bottom |
| MS2 | Carvalho catchment. Stream in forested area | −25.496389° | −48.980000° | Composite site with open and forested areas; lentic spot composed by the largest artificial pool with concrete bottom; lotic system composed by first and second order streams with bottom with abundant leaf litter, sand, and rocks; |
| MS3 | Aqueduto. Forested area with small streams | −25.486289° | −48.974170° | Lotic, first order streams/streamlets with a well-preserved riparian forest; semi lentic shallow pools in flooded areas |
| MS4 | Cayguava catchment. Stream in forested area | −25.482792° | −48.970836° | Composite site; lotic formed by a second order stream with well-preserved riparian forest, rocky and sandy bottom; lentic corresponds to the artificial pool of the Cayguava catchment with sand and leaf bottom |
| MS5 | Ipiranguinha river. River in forested area | −25.475547° | −48.961192° | Composite site; lotic is a third order river with well-preserved riparian forest, rocky bottom; lentic correspond to the artificial pool of the Ipiranguinha catchment with sandy and leaf bottom |
- Note: ‘Catchments’ refers to small dams of the old water supply system.
Samplings occurred on consecutive days from December 2018 to March 2019 with an interval of 30–60 days between the first and second sampling effort in each site. A modified scheme of the sampling method of Peck et al. (2006) was adopted in each site delimited by a transect of 150 m, which was perpendicularly subdivided every 30 m, to obtain a total sampling area of 45,000 m2 per site (Figure 1). Similar sampling strategies have largely been adopted in studies of metacommunities in the Brazilian Amazonia (e.g., Nessimian et al., 2008; Oliveira-Junior et al., 2017). All sampling events occurred on sunny days with each event lasting 3 h between 09:30 AM and 13:30 PM (time zone: UTC−03:00). The choice of the location of sampling sites was largely based on a former study (Araujo & Pinto, 2021), as well as on pilot collecting expeditions, considering the accessibility of sampling sites and the representation of the biota. The sampling sites described in Figure 1 were those that we considered more suitable to represent dragonfly communities in both MS and RP areas.
2.3 Curation and taxon identification
We followed the procedures of Araujo and Pinto (2021). The specimens were dried in absolute acetone and deposited in the Entomological Collection Pe. Jesus Santiago Moure, Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Brazil (DZUP) and the entomological collection of the Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (MNRJ).
2.4 Analysis
The following analyses were conducted using the software R (packages: lmPerm, vegan and labdsv; Oksanen et al., 2019; Roberts, 2019; Wheeler & Torchiano, 2016) to compare the MS (quasi-pristine) and RP (human-impacted) metacommunities: (1) One-factor analyses of variance (ANOVAs) with permutations (given data did not met assumptions) were used to test for differences among sites considering the local communities' species richness, the Shannon–Wiener diversity index and Pielou's evenness. (2) Multivariate permutational ANOVA (PERMANOVAs) and ‘betadisper’ with PERMDIST (Anderson et al., 2006) analyses using Hellinger transformation (abundance data) and Raup–Crick distance were used to evaluate the variation in species composition within and between MS and RP assemblages. To demonstrate differences in composition, we used an nMDS ordination. (3) Dufrêne–Legendre indicator species analysis was used to identify species typical of each metacommunity (MS or RP) (sensu Dufrêne & Legendre, 1997). This analysis was performed only to characterize typical species from MS and RP, with the expectation according to the literature that typical species from MS would have restricted distributions, whereas those from RP would be common and widespread.
Given that we compared two clusters of sites (MS vs. RP), it was pointless to control for spatial autocorrelation, which is important to exclude spatial dependency of sampling sites in correlation-based analyses. Although with a clustered distribution, our design simulates the effect of damming given that without reservoir formation, all this region would have environmental features similar to those observed in streams sampled in the near-pristine sites. Also, in the damming area, the landscape is more homogeneous and open, which may facilitate dispersion and decrease spatial variation in impacted sites, in line with our expectations.
2.5 Application of bioindication tools
The bioindication ratio tools Zygoptera/Anisoptera (Oliveira-Junior & Juen, 2019) and Coenagrionidae/other Zygoptera and Libellulidae/other Anisoptera (Šigutová et al., 2019) were applied using species richness and abundance of RP and MS communities to compare their accuracy in subtropical forests or in lotic–lentic conversion.
3 RESULTS
3.1 Community structure and application of bioindication tools
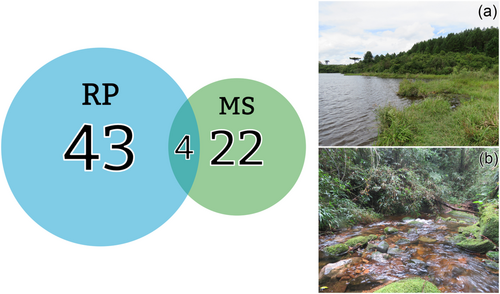
We sampled 950 specimens from seven families, 36 genera and 61 species (see Table S3). Twenty-two species were exclusive to MS, while 43 were from RP, and the following four occurred in both sampled areas: the libellulids Dasythemis mincki mincki (Karsch, 1889), Erythrodiplax melanorubra Borror, 1942, Miathyria marcella (Selys in Sagra, 1857) and the gomphid Progomphus complicatus Selys, 1854 (Figure 2). The most abundant taxa in MS were the Coenagrionidae (212 specimens) at family level and Argia (178) at generic level, while the Libellulidae (322) and Erythrodiplax (186) were the most abundant in RP.
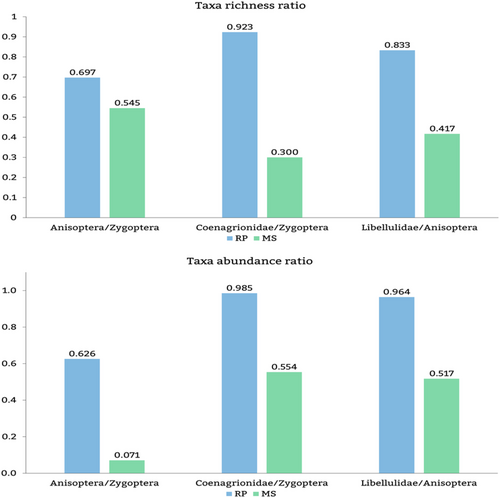
The bioindication ratio tool Anisoptera/Zygoptera richness ratio (Oliveira-Junior & Juen, 2019) status based on Ribeiro et al. (2021) were 0.69 for RP (>0.67 = altered) and 0.54 for MS (0.46 > 0.67 = moderately altered), and for abundance ratio, Anisoptera had 0.62 for RP (>0.52 = altered) and 0.07 for MS (<0.33 = preserved). The species richness ratios of Coenagrionidae/other Zygoptera and Libellulidae/other Anisoptera (Šigutová et al., 2019) were 0.300 and 0.417, respectively, in MS and 0.923 and 0.833, respectively, in RP (Figure 5). For abundance, the ratios for Coenagrionidae/Zygoptera were 0.554 (MS) and 0.985 (RP), and those for Libellulidae/Anisoptera were 0.517 (MS) and 0.964 (RP) (Figure 5).
3.2 Local diversity and differences in composition
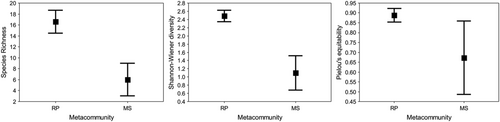
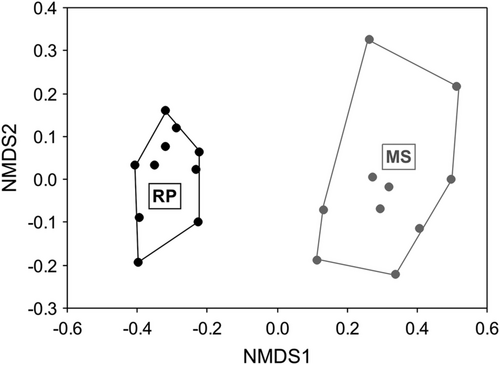
The odonate community in RP significantly (one-factor ANOVAs with permutations always produced p < 0.05) had the highest species richness (Figure 3a). Shannon–Wiener diversity (Figure 3b) and Pielou's evenness differed between sites (Figure 3c). The PERMANOVA indicated that species composition differed between areas (p = 0.001, pseudo-F = 95.04, R2 = 0.84). Only two typical species were detected in MS, whereas 11 were identified in RP (Table 2). Compositional variation also differed and was greater in MS (betadisper approach using the Raup–Crick index: p = 0.023, F = 7.78; Figure 4).
| Typical species | Assemblage | Indicator value | Probability | Mesohabitat preference |
|---|---|---|---|---|
| Argia sordida Hagen in Selys, 1865 | MS | 0.70 | 0.002 | Lotic water in shaded forested areas |
| Forcepsioneura sancta (Hagen in Selys, 1860) | MS | 0.70 | 0.001 | Shallow semi-lotic water in forested areas |
| Erythrodiplax melanorubra Borror, 1942 | RP | 0.97 | 0.001 | Lentic |
| Acanthagrion lancea Selys, 1876 | RP | 0.90 | 0.001 | Lentic |
| Erythrodiplax media Borror, 1942 | RP | 0.90 | 0.001 | Lentic |
| Homeoura chelifera (Selys, 1876) | RP | 0.90 | 0.001 | Lentic |
| Oxyagrion terminale Selys, 1876 | RP | 0.90 | 0.001 | Lentic |
| Acanthagrion gracile (Rambur, 1842) | RP | 0.70 | 0.001 | Lentic |
| Erythrodiplax fusca (Rambur, 1842) | RP | 0.70 | 0.003 | Lentic |
| Erythrodiplax sp. A | RP | 0.70 | 0.002 | Lentic |
| Perithemis mooma Kirby, 1889 | RP | 0.70 | 0.004 | Lentic |
| Planiplax erythropyga (Karsch, 1891) | RP | 0.70 | 0.005 | Lentic |
| Telebasis willinki Fraser, 1948 | RP | 0.70 | 0.004 | Lentic |
4 DISCUSSION
4.1 Community structure and the Odonata as bioindicators of habitat integrity
The flooding of the local forest transforms mesohabitats of shaded running water into less complex slow water systems (the homogenization process). Our results show which facets of Odonata biodiversity differ from a quasi-pristine environment to a human-impacted open area reservoir. The habitats altered by river damming have no forest-dependent species but maintain a community dominated by common species with a wide distributional range that are usually found in lentic systems (Corbet, 1999; Ferreira-Peruquetti & De Marco, 2002). This difference highlights the importance of maintaining quasi-pristine forest remnants to preserve Odonata biodiversity in damming projects.
The altered human-impacted RP community was characterized by the lack of forest-dependent species, instead composed by species typical of open areas. Some of these species are considered opportunistic, with a wide distribution and great potential for dispersal and colonization (see de Almeida et al., 2013; Oliveira-Junior & Juen, 2019; Paulson, 2006). Indeed, it is astonishing that the altered landscape (RP) with more lentic environments enabled the maintenance of 39 species not specialized in breeding in forested habitats; this number is almost double the species richness of the forest-dependent (MS) community (see Araujo & Pinto, 2021) and explains the higher biodiversity indices of the metacommunity in the altered environment.
On the one hand, one could argue that the higher local biodiversity indices of the more homogeneous landscape indicate that the intensity and duration of the perturbations have not reached the threshold to decrease biodiversity, in line with the intermediate disturbance hypothesis (see Petraitis et al., 1989). On the other hand, higher biodiversity may hide an impact that is not obvious: the promotion of colonizing species with a wide geographical distribution to the detriment of local and endemic species, as explained by the ‘biodiversity conservation paradox’ (see Vellend, 2017). In this study, we demonstrated that this paradox is not only valid for species richness as the biodiversity indicator but also for other commonly used indices such as Shannon–Wiener and Pielou's evenness.
Despite the lower values of local diversity indices, the MS (quasi-pristine) community represented a refuge for local and unique biodiversity. The species typical of the quasi-pristine forested communities were those dependent on the forest; their adults tended to breed in the same or very similar sites where larvae developed, had low dispersion ability and were mesohabitat specialists (see Paulson, 2006). We argue that such a community is extremely sensitive to changes in its habitat and is largely dependent on intact forest environments with running water (see Clausnitzer et al., 2009). We suggest that future studies should explore the phylogenetic and functional structures of specimens associated with human-impacted areas to better describe which groups of taxa might replace local endemic species in the Anthropocene.
The use of the dichotomy between Zygoptera and Anisoptera abundance/richness for evaluating the impact of the damming process can be biassed. Habitat preferences and, consequently, ecological responses are heterogenous within each suborder; species of both damselflies (Zygoptera) and dragonflies (Anisoptera) breeds in phytotelmata and both suborders have species that can be considered riverine, lentic, crepuscular, forest-dependent, generalist, and so on.
Both tools were effective for showing the impact of damming. Although Šigutová et al.'s tool uses only richness, if applied to abundance data, it also provides effective results. The taxon ratios that best represented the alterations caused by damming were Coenagrionidae/other Zygoptera (using richness data), Zygoptera/Anisoptera (using abundance data) and Libellulidae/other Anisoptera (using richness data) (Figure 5). Šigutová et al.'s approach increases the accuracy of the taxon response to environmental changes and refines the understanding of the factors driving odonate composition and the efficacy of bioindication tools. Therefore, this field is open to innovation, and refined strategies should capture the actual ecological signal from other combinations of comparison juxtapositions (such as low taxonomic levels, forest-dependent vs. non-forest-dependent species, lotic vs. lentic species, fliers vs. perchers, heliothermic vs. endothermic).
For example, Oliveira-Junior and Juen (2019) showed that environment factors explained less than 0.25 of the variation in composition of stream communities. Therefore, they suggested that the major determinants of the Odonata community in small streams may not be environmental factors; instead, biotic interactions or variables still not explored or measured, such as the capacity of an area to maintain different mesohabitats, especially a high number of specific habitats, may be more important. In addition, biological parameters not evaluated, such as competition and trophic cascades, could be key factors differentiating pristine forest species from those occupying degraded areas (Dijkstra & Clausnitzer, 2006; Knight et al., 2005; Šigutová et al., 2019)—another perspective to understand the drivers of Odonata biodiversity.
Studies analysing habitat quality indicate the replacement of endemic species or species with higher habitat specificity by species that are more widely distributed and are generalist with low demands on environmental parameters (Ferreira-Peruquetti & De Marco, 2002; Roque F et al., 2017). However, it is important to note that the prevalence of Anisoptera species in samples could be explained in part by sampling bias; most studies show a tendency to sample abundant generalist species instead of the specialist Anisoptera (see de Almeida et al., 2013).
Comparisons of lower-level taxonomic categories increase the accuracy of predictions of taxon responses to environmental changes and refine the understanding of the factors driving Odonata composition and the use of bioindication tools. However, the use of less inclusive taxa, for example, genus, presumably will improve the prediction of disturbance, but it also results in more complex correlations, making it difficult to apply such taxa in monitoring programmes. For example, even within the Coenagrionidae, there are non-generalist species that occur in forested areas, such as Forcepsioneura sancta (Hagen in Selys, 1860), and the same is true of the Gomphidae, which is composed mostly of strongly forest-dependent lotic species, while also having species, such as Aphylla spp., with a preference for lentic habitats in open areas. The use of the dichotomy between Zygoptera and Anisoptera is too simple and is certainly strongly biassed. This dichotomy overlooks the fact that despite members of each suborder having a common evolutionary history, that is, both suborders are monophyletic, each lineage independently diversified spatiotemporally and underwent distinct selective pressures leading to unique histories at the genus level or even at closely related species level.
It has been suggested that the occurrence of larger dragonflies in modified areas can hinder the occurrence of smaller ones due to competition even in favourable environments (Pereira et al., 2019); thus, smaller species are limited to pristine sites. However, it may be dependent on local conditions. In our study, larger Zygoptera and Anisoptera were observed in more the pristine habitats (e.g., the calopterygid Hetaerina brightwelli Kirby, 1823 and the aeshnid Rhionaeschna decessus Calvert, 1953) while minute individuals, such the coenagrionids of Telebasis willinki Fraser, 1948 and Ischnura capreolus, were only sampled in the altered human-impacted area of the reservoir.
The conversion from a lotic to a lentic system caused high turnover, with only four species co-occurring in MS and RP (see Figure 2; Dasythemis mincki mincki; Erythrodiplax melanorubra; Miathyria marcella; Progomphus complicatus). They are typical of southern South America or are widespread in the Western Hemisphere as a whole. Only Dasythemis m. mincki can be considered persistent, a local species that lives in the forested areas and is adapted to the reservoir, while the others are ‘invaders’ in the lotic systems (see Ferreira-Peruquetti & De Marco, 2002).
It is interesting to note that lentic systems, such as lakes and bogs, are not abundant in tropical forests; in addition, lentic species are usually found in artificial reservoirs (Sahlén, 2006). Therefore, these common and widespread species from rare natural lentic systems in forests may be considered local, or they may have originated from another degraded area (see Renner et al., 2016). If these species are considered local, the contribution of the reservoir to the maintenance of local biodiversity rises from almost nil to about 20% (this conclusion is based on the overall data on the regional pool from Araujo & Pinto, 2021).
4.2 Local diversity, compositional differences and biotic homogenization
In this study, we demonstrated the complexity of interpreting habitat heterogeneity and community structuring. It is worth noting that it is not always true that human-impacted sites have lower environmental heterogeneity. A recent study found relatively high heterogeneity in altered streams in the eastern Amazon (Oliveira-Junior & Juen, 2019), casting doubt on the assumption that dams always cause environmental and biotic homogenization, since the number of mesohabitats provided by reservoirs is large. Indeed, in our study area, there were banks with several species of macrophytes (Silva et al., 2014), mesohabitats of semi-running water that flowed into the reservoir and a few remnants of well-preserved riparian forests (Araujo & Pinto, 2021).
However, the elimination of lotic waters and original forests is an undeniable modification of the landscape, causing an enormous impact on the area. For the Odonata, such an impact is not easily detected using local diversity indices (as discussed above). However, the high turnover and differences in community composition between MS and RP reflect the impacts of river damming on the odonate community. Almost all species of the local community have been replaced, most probably due to the physical and biotic changes caused by the conversion of the water system and homogenization. The communities found in the human-impacted (RP) and quasi-pristine (MS) water systems are almost totally different. It appears that the system at large determines the occurrence of most species and the mesohabitats and biological interactions refine the composition. The complexity of mesohabitats should be proportional to species richness. The forest mesohabitats are the most fragile and the most difficult to recover. Therefore, the mesohabitats that are easily reproducible harbour the most common species.
However, more important than the changes in species in the metacommunity, we also showed that the metacommunity of impacted sites, even with higher species numbers, is less variable across the impacted landscape compared to the compositional variation among quasi-pristine forested areas. Although our analyses did not show a temporal or directional change towards increasing similarity between local communities (see Olden et al., 2018), we posit that the landscape transformation had a negative effect by promoting biotic homogenization. Moreover, the impacted reservoir areas were ecologically similar to the quasi-pristine sites before human impacts (i.e., they are located nearby; see Figure 1); such a space-for-time substitution demonstrates how pervasive biotic homogenization is (Padial et al., 2020).
We can also speculate that the mesohabitats of the quasi-pristine MS areas may be more heterogenous than those in the RP areas (Figure 4). Also, homogenization of RP sampling sites may be reinforced by the fact that species dispersal is likely increased in forested-open areas nearby large water bodies. The analysis of typical species supports this conclusion due to the low number of such species in MS compared to RP and since these species have different mesohabitat preferences: Forcepsioneura sancta (Hagen in Selys, 1860) and Argia sordida Hagen in Selys, 1865 are species of shaded forested areas, although the first occurs in a very specific type of semi-flowing shallow water, and the second characteristically occurs in lotic systems along the Atlantic Forest in south-eastern and southern Brazil. The 11 species typical of RP show that the sampling sites in this area are quite similar; in addition, all these species occur in lentic systems and are associated with macrophytes. These results indicate that the environmental conditions of RP are more homogeneous that those of MS.
5 CONCLUSIONS
Our results demonstrate the reservoir formed by damming process in Atlantic Forest does not maintain a huge portion of the Odonata biodiversity dweller to the forested mesohabitats; therefore, the conservation of these species depends on preserving pristine areas. In other words, the maintenance of lotic forest species, the more threatened (see Clausnitzer et al., 2009), and those with a low range of distribution needs greater attention to the preservation of the original characteristics of their habitats. The use of reservoirs for water supply entails the preservation of the surrounding areas with forest landscape and, consequently, the local biodiversity, and it is a desirable side effect. We recommend that, in addition to preserving large areas of forest, vulnerable areas inhabited by sensitive local fauna are identified and protected for effective maintenance of biodiversity. To complement the understanding of the effects of damming in the biodiversity, studies with non-aquatic groups, such as terrestrial and soil-dependent species, should be developed. Finally, the bioindication tools tested here can be an effective device using both richness and abundance of Odonata to evaluate aquatic environment integrity in the Atlantic Forest.
ACKNOWLEDGEMENTS
This study was partially supported by grants from the International Dragonfly Fund (IDF) and “Insetos e a metrópole - EDITAL N. 02/2020: PESQUISA/PRPPG/UFPR, Apoio a Atividades de Pesquisa” to APP and a master's scholarship from the Council for Scientific and Technological Development (CNPq proc. 132210/2018-5) via PPGEnto/UFPR to BRA. AAP also acknowledges CNPq for continued financial support (proc. 482986/2011-5; 482368/2013-6; 307984/2015-0; 402828/2016-0; 301867/2018-6; 308648/2021-8). Thanks are due to the Water and Waste Management Company of the State of Paraná (SANEPAR), especially to Ana Cristina Rego Barros for supporting this study. We also thank 'Instituto Água e Terra do Paraná (IAT) and ICMBIO/SISBIO for collecting licences. This study was significantly improved after the review of Master in Biological Sciences (Entomology) degree committee for Gabriel A.R. Melo, Leandro Juen and Maurício O. Moura, and after anonymous reviews in previous submitted drafts.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study will be available as supplementary files or in online open access repository such as Figshare after publication.




