Genomic Phylogenetic Analysis of Physaliastrum and Archiphysalis (Solanaceae): Insights From Chloroplast Genomes Indicate Distinct Evolutionary Relationships
Funding: This study was supported by the National Natural Science Foundation of China (grant no. 32260051), the Guizhou Provincial Foundation for Excellent Scholars Program (grant no. GCC[2023]077), and the National Wild Plant Germplasm Resource Center.
ABSTRACT
This research involved the sequencing and analysis of chloroplast genomes from nine species across four genera of the Solanaceae family, specifically two species of Archiphysalis, five species of Physaliastrum, and the taxa Tuberowithania pengiana and Tubocapsicum anomalum. We conducted a comprehensive series of analyses, including assessments of genome structure, gene content, codon usage, selection pressure, and other genomic features. The chloroplast genomes of Archiphysalis and Physaliastrum exhibit typical quadripartite structures, with lengths ranging from 156,345 to 156,924 bp. Notably, Archiphysalis contains 130 genes, while the species of Physaliastrum have a slightly higher gene count of 132. Phylogenetic analysis reveals that A. sinensis and A. chamaesarachoides cluster together within the subtribe Withaninae, which is consistent with their fruit morphology. Divergence time analysis indicates that Physaliastrum separated from Archiphysalis approximately 41.2 million years ago (95% HPD: 38.6–43.5 Mya), resulting in intergeneric differences in chloroplast gene features that are significantly greater than intrageneric differences, particularly in gene count, structure, and lengths of gene duplications. These findings provide genomic-level support for the classification of species within these genera and underscore the close relationship between A. chamaesarachoides and A. sinensis.
1 Introduction
The genus Physaliastrum Makino was first established by Makino in 1914, initially comprising two Japanese species: P. echinatum (Franch. et Sav.) Honda and P. savatieri Makino. These species were originally classified under the American genus Chamaesaracha (A.Gray) Benth. & Hook.f., but were later transferred to Physaliastrum due to distinct morphological characteristics, including a campanulate (not rotate) corolla, induplicato-vatvate (not plicate) corolla lobes, and a much enlarged fleshy persistent calyx (Makino 1914). Over time, the genus Physaliastrum expanded to include a total of nine species. Among these, five are exclusively endemic to China: P. heterophyllum (Hemsl.) Migo, P. kweichouense Kuang & A.M.Lu, P. sinense (Hemsl.) D'Arcy & Z.Y.Zhang (syn. Archiphysalis sinensis (Hemsl.) Kuang), P. sinicum Kuang & A.M.Lu, and P. yunnanense Kuang & A.M.Lu. Additionally, P. chamaesarachoides (syn. A. chamaesarachoides (Makino) Kuang) and P. echinatum are widely distributed across China and Japan, with the latter also found in Korea and Russia. The remaining two species, P. kimurai Makino and P. savatieri, are found only in Japan (Kuang and Lu 1965; Zhang et al. 1994).
In 1966, Kuang established the genus Archiphysalis Kuang, initially including three species: A. chamaesarachoides, A. kwangsiensis Kuang, and A. sinensis, and distinguished it from Physaliastrum based on the degree to which the berry fills the fruiting calyx (Kuang 1966; Kuang and Lu 1978). Species of Archiphysalis were characterized by a bladdery calyx that significantly exceeds the berry in size, whereas Physaliastrum typically features a spiny calyx that closely adheres to the fruit (Figure 1). However, this distinction was later challenged. D'Arcy and Zhang (1992) argued that the variation in calyx morphology was insufficient to justify the separation of Archiphysalis from Physaliastrum. Consequently, Zhang et al. (1994) reclassified all species of Archiphysalis back into Physaliastrum, merging A. kwangsiensis and A. chamaesarachoides into a single species, P. chamaesarachoides (Makino) Makino.

However, the classification of Archiphysalis sinensis or A. chamaesarachoides within Physaliastrum has not been supported by subsequent phylogenetic analyses. Li et al. (2013) used three DNA sequences (ITS, ndhF, and trnL-F) to analyze the taxonomic positions of P. heterophyllum and A. chamaesarachoides. Their results revealed that these two species belong to different subtribes within Solanaceae: A. chamaesarachoides is placed in the subtribe Withaninae Bohs & Olmstead, while P. heterophyllum is classified within the subtribe Physalinae Reveal. This supports the independent status of Archiphysalis from Physaliastrum, which has also been corroborated by Huang's recent research based on orthologous genomic data (Huang et al. 2023).
Research by Deanna et al. (2019) utilized four DNA markers (ITS, LEAFY, trnL-F, and waxy) to conduct a phylogenetic study of the tribe Physalideae. Their results supported the independent status of A. chamaesarachoides as the sole member of the genus Archiphysalis, whereas the type species A. sinensis remained classified under Physaliastrum. In contrast, a later study by Wang et al. (2024), based on the same datasets but including the newly described genus Tuberowithania Ze H.Wang & Yi Yang, did not support this finding. This latter study revealed that, although the two species formerly placed in Archiphysalis did not cluster together, they each formed distinct branches with other genera of the subtribe Withaninae.
In summary, current molecular systematic studies rely on a limited number of genetic markers (Li et al. 2013) and primarily focus on broader taxonomic levels, such as the tribe Physalideae (Deanna et al. 2019; Wang et al. 2024) or the family Solanaceae (Huang et al. 2023), resulting in limited representation of samples from the genera Physaliastrum and Archiphysalis. Additionally, the morphological similarities between A. chamaesarachoides and A. sinensis (Kuang 1966) raise questions about their classification within different subtribes or lineages of the subtribe Withaninae. Furthermore, the studies by Deanna et al. (2019) and Wang et al. (2024) indicate inconsistencies in the phylogenetic relationships revealed by different genetic markers, highlighting the ongoing complexity and unresolved nature of their taxonomic relationships. This highlights the need for further research to determine their definitive systematic positions.
In this study, we utilize next-generation sequencing technology to obtain complete chloroplast genome sequences for Archiphysalis sinensis, A. chamaesarachoides, and five species of Physaliastrum. Our goal is to elucidate the systematic relationships among these seven species, with a particular focus on the two species of Archiphysalis.
2 Materials and Methods
2.1 Plant Material Information, Sequencing, Assembly, and Annotation
To investigate the relationships between Archiphysalis and Physaliastrum, we conducted field surveys for these genera and their related taxa Tuberowithania and Tubocapsicum (Wettst.) Makino. Mature leaves were collected from individuals at these localities and dried using silica gel, with voucher specimens deposited in the herbarium of Guizhou University of Traditional Chinese Medicine, China (GZTM). All samples were sent to the Molecular Biology Experiment Center, Germplasm Bank of Wild Species, Kunming, China, for DNA extraction and library preparation. The libraries were sequenced on the DNBSEQ-T7 platform, yielding 150 bp paired-end reads and approximately 20 G of raw data.
The sequencing results underwent quality control using fastp v0.23.2 (Chen et al. 2018) with default settings, which involved removing adapters and filtering out low-quality sequences to obtain high-quality next-generation sequencing (NGS) data. The NGS data were then assembled into chloroplast genomes with GetOrganelle v1.7.5 (Jin et al. 2020), using the genomes with the highest BLAST values as references: Withania sp. (OR426647) for Physaliastrum, and Nothocestrum latifolium (OR400642) for Archiphysalis, Tuberowithania pengiana Ze H.Wang & Yi Yang, and Tubocapsicum anomalum (Franch. & Sav.) Makino.
Annotation of the chloroplast genomes was performed using GeSeq (Tillich et al. 2017) and further manually corrected with Geneious Prime 9.0.2 (Kearse et al. 2012), incorporating insights from Qu et al. (2023). The physical map of the chloroplast genome was constructed using OrganellarGenomeDRAW (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html, Lohse et al. 2007), and the annotated protein-coding sequences, rRNA genes, and tRNA genes were summarized using CPJSdraw (Li et al. 2023). Finally, the chloroplast genome sequences were deposited in NCBI with accession numbers PV472654–PV472662.
2.2 Phylogenetic Analysis
To enhance the reliability of our results, we included Tuberowithania pengiana and Tubocapsicum anomalum in our phylogenetic analysis, along with two species of Archiphysalis and five species of Physaliastrum, as both Tuberowithania and Tubocapsicum are closely related to Archiphysalis (Deanna et al. 2019; Wang et al. 2024). We retrieved 47 chloroplast genomes from the NCBI database (https://www.ncbi.nlm.nih.gov/) based on Deanna et al. (2019) and excluded four sequences: T. anomalum (MW829600) and Withania somnifera (MN746302, MK142783, OR166175). MK142783 and OR166175 were excluded for aligning with our selected OR166174, simplifying the dataset. MW829600 and MN746302 were removed due to concerns about their accuracy, as the phylogenetic trees (Figures T1 and T2: Appendix S1) showed inconsistent placements within the Physalideae compared to the results of several published studies (Olmstead et al. 2008; Li et al. 2013; Deanna et al. 2019; Huang et al. 2023; Wang et al. 2024). We resampled T. anomalum to improve data reliability. This resulted in a total of 56 genomes, including 47 from NCBI and 9 newly sequenced species, which were aligned using MAFFT in PhyloSuite v1.2.3 (Zhang et al. 2020). Capsicum lycianthoides (NC_026551) and Lycianthes radiata (NC_062492) served as outgroups, following the methodology and findings of Deanna et al. (2019).
Phylogenetic trees were constructed using both Bayesian Inference (BI) and Maximum Likelihood (ML) methods. The best substitution model for both analyses was determined using ModelFinder in PhyloSuite v1.2.3 (Zhang et al. 2020). For the Bayesian method, the best-fit model selected according to the Bayesian Information Criterion (BIC) was GTR + F + I + G4. For the maximum likelihood method, the best-fit model chosen based on the Akaike Information Criterion (AIC) was TVM + F + I + I + R6. Subsequently, phylogenetic trees were built using MrBayes and IQ-TREE within the same software, applying the selected model, with MrBayes running for 2,000,000 generations and IQ-TREE for 1000 generations, while all other parameters were set to their defaults. Finally, we visualized and edited the phylogenetic trees using FigTree v.1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/, Rambaut 2018).
2.3 Divergence Time Estimation
The MCMCtree module in PAML v4.9 (Yang 2007) was employed to estimate divergence times among species based on the phylogenetic tree constructed from 56 chloroplast genomes. To calibrate this tree, we incorporated three estimated divergence times: the tribe Capsiceae (31.0–45.4 Mya), the tribe Physalideae (27.8–42.7 Mya), and the combined divergence time for both tribes (52.2 Mya), as reported by Deanna et al. (2019). Additionally, we referenced a fossil of a lantern fruit, which exhibits features characteristic of Physalideae within Solanaceae, dating back to the early Eocene (ca. 52 Ma) from the Laguna del Hunco site in Chubut, Argentina (Deanna et al. 2020; Wilf et al. 2017). Following these estimations, we used Tracer v. 1.6 (Rambaut et al. 2018) to assess parameter convergence, ensuring that Effective Sample Size (ESS) values exceeded 200.
2.4 Genomic Structure Comparison and Sequence Divergence Analysis
To explore the structural variations in the chloroplast genomes of Archiphysalis and Physaliastrum, we analyzed the genome size, lengths of the LSC, SSC, and IR regions, GC content, and gene numbers of the seven species using Geneious v9.0.2 (Kearse et al. 2012), along with the scripts calculate_gc.py and count_genes.py from CPStools 2.0.9 (Huang et al. 2024). We utilized the online tool IRscope (https://irscope.shinyapps.io/irapp/, Amiryousefi et al. 2018) to investigate the expansion and contraction of the Inverted Repeat (IR) regions. To assess sequence divergence between the two species of Archiphysalis and five species of Physaliastrum, we employed mVISTA (https://genome.lbl.gov/vista/index.shtml, Frazer et al. 2004) in Shuffle–LAGAN mode, using the chloroplast genome of P. kweichouense, obtained in this study as the reference.
Following this analysis, we aligned the chloroplast genome sequences of the seven species using MAFFT within Geneious Prime 9.0.2 (Kearse et al. 2012). To further investigate sequence divergence and identify mutation hotspots, we conducted a nucleotide diversity analysis with DnaSP v6 (Rozas et al. 2017), setting the sliding window to a length of 600 bp and a step size of 200 bp.
2.5 Repeat Analysis
Simple sequence repeats (SSRs) were identified using the SSRS_annotation.py script in CPStools 2.0.9 (Huang et al. 2024), which effectively categorizes the types of c and c* repeat sequences. To further analyze repeat sequences in the chloroplast genome, we employed the online tool REPuter (https://bibiserv.cebitec.uni-bielefeld.de/reputer, Kurtz et al. 2001). This tool facilitated the identification of forward, reverse, complementary, and palindromic repeat sequences, with parameters set to a maximum repeat length of 5000 bp, a Hamming distance of 3, and a minimum repeat length of 30 bp.
2.6 Condon Usage Analysis
To investigate codon usage preferences and influencing factors in the chloroplast genomes of Archiphysalis and Physaliastrum, we utilized the rscu.py script from CPStools 2.0.9 (Huang et al. 2024) to analyze codon counts and relative synonymous codon usage (RSCU) values. The script excludes sequences shorter than 300 bp and ensures that all analyzed sequences use ATG as the start codon and either TAA, TAG, or TGA as stop codons. An RSCU value of 1 indicates no codon preference, while values greater than 1 indicate a strong preference, and values less than 1 indicate lesser usage.
To analyze codon usage bias and its driving factors, we generated ENC-GC3s, Neutrality, and PR2-bias plots. ENC-GC3s plots reveal whether codon usage bias is driven by mutation (genes along the standard curve) or selection (genes below the curve). The expected ENC baseline is established through a theoretical curve, and points clustering near this curve suggest mutation is the primary driver, whereas deviations indicate that natural selection may play a significant role (Novembre 2002). Additional analyses using discrepancies between empirical and predicted ENC values further quantify these influences (Kawabe and Miyashita 2003; Zhang et al. 2007).
Neutrality plots assess the correlation between GC content at different codon positions to distinguish between mutation and selection effects. PR2-bias plots examine preferences for A/T and C/G, with deviations from the center point indicating the degree and direction of codon usage bias. Together, these analyses elucidate the factors affecting codon usage in chloroplasts.
2.7 Selection Pressure Analysis
To reveal the types of selection pressures and their differences experienced by the genes of Archiphysalis, Tuberowithania, Tubocapsicum, and Physaliastrum during evolution, we conducted an intergeneric selection pressure analysis. The nonsynonymous to synonymous substitution rate (Ka/Ks) values were calculated using the Genepioneer platform (http://112.86.217.82:9929/#/tool/alltool/detail/305) provided by Nanjing Jisi Huiyuan Biotechnology Co. Ltd. We used the annotated gb file of P. kweichouense as the reference and imported the annotated gb files of Archiphysalis, Tuberowithania, and Tubocapsicum as input files for comparative analysis. By utilizing the platform's built-in tools, including BLASTN v2.10.1, MAFFT v7.427 (Katoh and Standley 2013), and Perl scripts, we automated the calculation of Ka/Ks values between the input and reference files, selecting genetic code table 11 (bacterial and plant plastid code) and employing the Maximum Likelihood with Weighted Likelihood (MLWL) method.
3 Results
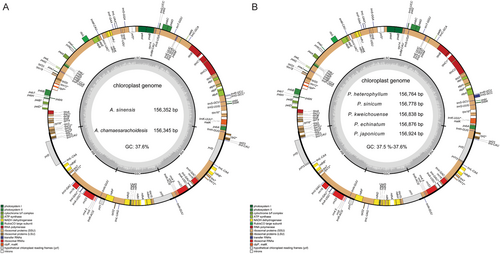
3.1 Features of the Chloroplast Genome
The complete chloroplast genomes of two Archiphysalis species and five Physaliastrum species have been successfully assembled and annotated. All seven genomes exhibit a circular DNA molecule characterized by a typical quadripartite structure, as illustrated in Figure 2. This structure comprises a large single-copy (LSC) region from 86,944 to 87,450 bp, a small single-copy (SSC) region from 18,435 to 18,565 bp, and a pair of inverted repeat (IR) regions spanning from 50,446 to 51,308 bp. The total length of these genomes varies from 156,345 to 156,924 bp, with an overall GC content of 37.5%–37.6%. GC content is unevenly distributed among the four genomic regions: the LSC region exhibits a GC content of 35.58% to 35.67%, while the SSC region has the lowest content, ranging from 31.40% to 31.89%. In contrast, the IR regions have the highest content, between 43.05% and 43.12%. Additionally, the GC content in the LSC and SSC regions of the two Archiphysalis species is higher than that in Physaliastrum, while the IR region in these two species exhibits a lower GC content compared to Physaliastrum (Table 1).
| Species | Length (bp) | GC% | PCG | tRNA | rRNA | Total genes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cp. genome | LSC | SSC | IR | cp. genome | LSC | SSC | IR | |||||
| A. sinensis | 156,352 | 87,442 | 18,444 | 50,446 | 37.60 | 35.66 | 31.90 | 43.05 | 85 | 37 | 8 | 130 |
| A. chamaesarachoides | 156,345 | 87,450 | 18,435 | 50,460 | 37.60 | 35.67 | 31.89 | 43.05 | 85 | 37 | 8 | 130 |
| P. heterophyllum | 156,764 | 86,944 | 18,514 | 51,306 | 37.60 | 35.58 | 31.45 | 43.10 | 87 | 37 | 8 | 132 |
| P. kweichouense | 156,838 | 86,993 | 18,539 | 51,306 | 37.60 | 35.58 | 31.44 | 43.10 | 87 | 37 | 8 | 132 |
| P. sinicum | 156,778 | 86,948 | 18,522 | 51,308 | 37.60 | 35.59 | 31.41 | 43.10 | 87 | 37 | 8 | 132 |
| P. echinatum | 156,876 | 87,038 | 18,548 | 51,290 | 37.60 | 35.59 | 31.43 | 43.12 | 87 | 37 | 8 | 132 |
| P. japonicum | 156,924 | 87,069 | 18,565 | 51,290 | 37.50 | 35.58 | 31.40 | 43.11 | 87 | 37 | 8 | 132 |
Annotation of the chloroplast genomes revealed that the two Archiphysalis species contain 130 genes, including 85 protein-coding genes (PCGs), 37 tRNA genes, and 8 rRNA genes. In contrast, the five Physaliastrum species have 132 genes, which possess two copies of the ycf15 gene compared to those of the Archiphysalis species (Table 2). Based on their functions, these genes are categorized into four major groups: genes related to photosynthesis (44 genes), self-replication (59 genes), other functional genes (including matK, clpP, cemA, accD, ccsA), and genes of unknown function (ycf1, ycf2, ycf3, ycf4, ycf15). Among all the annotated genes, 15 contain one intron, 3 genes (rps12, clpP, ycf3) contain two introns, and 18 genes are duplicated due to their presence in the IR regions. Specifically, the genes ndhB, rpl2, trnA-UGC, and trnI-GAU contain one intron and are duplicated, while the gene rps12, which contains two introns, is also duplicated, with its 5′ and 3′ exons located in the LSC and IR regions, respectively.
| Category of genes | Group of genes | Name of genes |
|---|---|---|
| Photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of NADH dehydrogenase | ndhA,a ndhBa (×2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB,a petD,a petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF,a atpH, atpI | |
| Large subunit of rubisco | rbcL | |
| Self-replication | Large ribosomal subunit | rpl14, rpl16,a rpl2a (×2), rpl20, rpl22, rpl23(×2), rpl32, rpl33, rpl36 |
| Small ribosomal subunit | rps11, rps12b (×2), rps14, rps15, rps16,a rps18, rps19, rps2, rps3, rps4, rps7(×2), rps8 | |
| RNA polymerase | rpoA, rpoB, rpoC1,a rpoC2 | |
| Ribosomal RNAs | rrn16(×2), rrn23(×2), rrn4.5(×2), rrn5(×2) | |
| Transfer RNAs | trnA-UGCa (×2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC,a trnG-UCC, trnH-GUG, trnI-CAU(×2), trnI-GAUa (×2), trnK-UUU,a trnL-CAA(×2), trnL-UAA,a trnL-UAG, trnM-CAU, trnN-GUU(×2), trnP-UGG, trnQ-UUG, trnR-ACG(×2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(×2), trnV-UAC,a trnW-CCA, trnY-GUA, trnfM-CAU | |
| Other function | Maturase | matK |
| Protease | clpP b | |
| Envelope membrane protein | cemA | |
| Acetyl-CoA carboxylase | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Unknown function | Conserved open reading frames | ycf1, ψycf1, ycf2(×2), ycf3,b ycf4, 1ycf15(×2) |
- Note: (×2): duplicated gene; 1: gene only present in Physaliastrum; ψ: pseudogene.
- a Gene contains one intron.
- b Gene contains two introns.
3.2 Phylogenetic Analysis
In this study, we successfully assembled the complete chloroplast genomes of both species of Archiphysalis and five out of seven species of Physaliastrum, facilitating an exploration of the phylogenetic relationships among these genera. Furthermore, we also sequenced and obtained the complete chloroplast genomes of Tuberowithania pengiana and Tubocapsicum anomalum, as previous studies have indicated their close relationship with Archiphysalis (Deanna et al. 2019; Wang et al. 2024). Sampling these two genera is essential for accurately determining the phylogenetic placement of the Archiphysalis species. Detailed collection information for all newly sequenced samples is provided in Table 3.
| Species | Collector and collection no. | Location | GenBank accession no. |
|---|---|---|---|
| Archiphysalis sinensis | Yi Sirong YSR202311-01 | Nanchuan District, Chongqing, China | PV472654 |
| Archiphysalis chamaesarachoides | Li Hongqing et al. Lihq0393 | Kuaihua County, Quzhou City, Zhejiang Province, China | PV472655 |
| Physaliastrum heterophyllum | Wang Zehuan 202307-01 | Qianshan City, Anqing City, Anhui Province, China | PV472656 |
| Physaliastrum kweichouense | Sangzhi Forestry Science Institute 0995 | Sangzhi County, Zhangjiajie City, Hunan Province, China | PV472657 |
| Physaliastrum sinicum | Liu Bing, Feng Yalei, Zhou Xinxin 12357 | Fangshan District, Beijing City, China | PV472658 |
| Physaliastrum echinatum | Wang Zehuan 202408-01 | Huairou District, Beijing City, China | PV472659 |
| Physaliastrum japonicum | Miyoshi Furuse 44638 | Yama-kita-choo, Ashigara-kami-gun, Kanagawa Prefecture, Japan | PV472660 |
| Tubocapsicum anomalum | Wang Zehuan & Xu Jiaju 202407-01 | Wulingyuan District, Zhangjiajie City, Hunan Province, China | PV472661 |
| Tuberowithania pengiana | Wang Zehuan & Chen Li 202208-01 | Shuangjiang County, Lincang City, Yunnan Province, China | PV472662 |
To construct a robust dataset for phylogenetic analysis, we retrieved 47 chloroplast genomes from the NCBI database (https://www.ncbi.nlm.nih.gov/) based on the phylogenetic research conducted by Deanna et al. (2019) on the Physalideae tribe. This resulted in a final dataset comprising 56 genomes, including two outgroup species: Capsicum lycianthoides (NC_026551) and Lycianthes radiata (NC_062492). The final aligned dataset of chloroplast genomes for phylogenetic analysis spans 165,714 bp, including 3692 parsimony-informative sites.
The phylogenetic trees constructed using the Bayesian Inference (BI) and Maximum Likelihood (ML) methods exhibit identical topologies, though there are minor differences in branch support values. Through the analysis of chloroplast genomes, we have clearly resolved the phylogenetic relationships within the genera Archiphysalis and Physaliastrum, with particular emphasis on the phylogenetic position of A. sinensis. As illustrated in Figure 3, we identified three subtribes within the tribe Physalideae: Iochrominae Reveal, Withaninae, and Physalinae. Notably, the five species of Physaliastrum are nested within the Physalinae subtribe, while A. chamaesarachoides is placed in the Withaninae subtribe, consistent with previous studies (Deanna et al. 2019; Huang et al. 2023; Li et al. 2013; Wang et al. 2024).
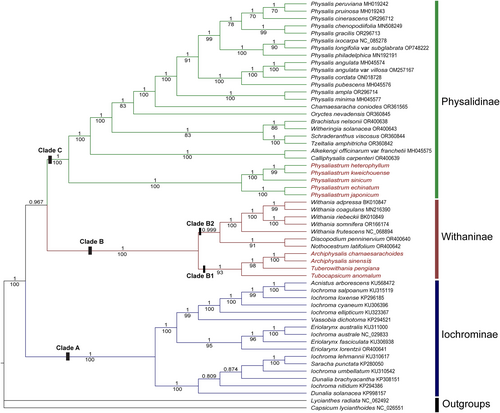
However, our findings also indicate a significant discrepancy from previous studies (Deanna et al. 2019; Huang et al. 2023; Li et al. 2013; Wang et al. 2024), as Archiphysalis sinensis, the type species of the genus Archiphysalis, clusters with A. chamaesarachoides within the subtribe Withaninae (BS = 100, PP = 1), rather than grouping with Physaliastrum. This clustering, which aligns with our expectations, forms a single clade alongside Tuberowithania pengiana and Tubocapsicum anomalum (BS = 93, PP = 1).
3.3 Divergence Time Estimation
The differentiation times of the major clades within the Physalideae tribe are illustrated in Figure 4. Divergence time estimates indicate that the crown age of the clade formed by the Physalinae and Withaninae subtribes separated during the middle Eocene, specifically around 41.2 million years ago (Mya) (95% HPD: 38.6–43.5 Mya). Within the Physalinae subtribe, five species of Physaliastrum constitute a basal clade, with a stem age dating back to the late Eocene at approximately 38.1 Mya (95% HPD: 34.7–41.4 Mya), and the crown age estimated to be around 19.4 Mya (95% HPD: 10.6–29.0 Mya) in the early Miocene.
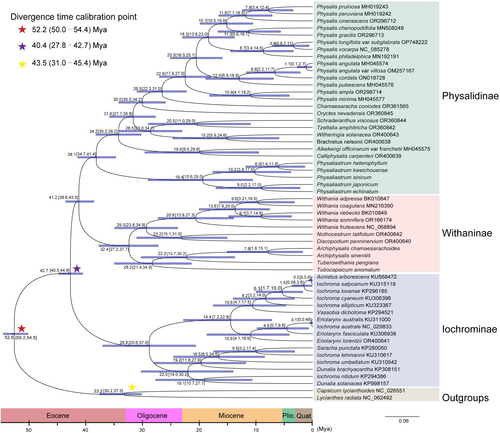
In the Withaninae subtribe, the two Archiphysalis species, along with Tuberowithania pengiana and Tubocapsicum anomalum, form a sister clade to all other members of the Withaninae subtribe. This clade has a stem age from the late Eocene, estimated at 32.4 Mya (95% HPD: 27.2–37.7 Mya), and a crown age of approximately 28.2 Mya (95% HPD: 21.4–34.9 Mya) in the middle Oligocene. For the two Archiphysalis species, the stem age is estimated to be 22.2 Mya (95% HPD: 13.7–30.2 Mya), while their crown age is around 7.8 Mya (95% HPD: 1.8–15.1 Mya).
3.4 Comparative Genomic Analysis
3.4.1 IR Contraction and Expansion
A comparative analysis of the inverted repeat (IR) boundaries was conducted on the complete chloroplast genomes of seven species from the genera Physaliastrum and Archiphysalis. The results, illustrated in Figure 5, indicate that the boundary genes at JLB (LSC/IRb), JSB (IRb/SSC), JSA (SSC/IRa), and JLA (IRa/LSC) exhibit complete identity across the species, demonstrating a high degree of conservation. Overall, the IR region of Physaliastrum is longer than that of Archiphysalis, measuring between 415 and 421 bp. Additionally, the IR lengths among different species within each genus are relatively consistent, differing by 8–9 and 3 bp, respectively.
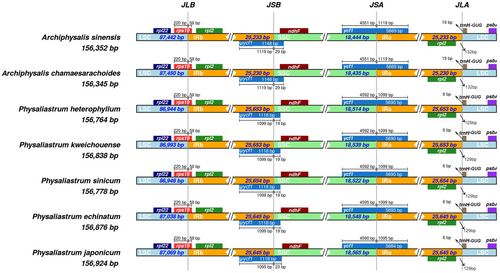
Further observations have shown that the rps19 gene, which spans the LSC/IRb boundary, consistently measures 220 bp in the LSC region and 59 bp in the IRb region across all species. Similarly, the IRa/LSC boundary is consistently located between the rpl2 and the trnH-GUG genes in the analyzed chloroplasts. In Archiphysalis, the distances between the IRa/LSC boundary and the rpl2 and trnH-GUG genes are 132 and 19 bp, respectively, whereas in Physaliastrum, these distances are 129 and 6 bp. In contrast, the ycf1 gene, which spans the IRb/SSC and SSC/IRa boundaries, demonstrates variability in length among species. Specifically, in the five Physaliastrum species, the ycf1 gene ranges from 5684 to 5693 bp in the IRb/SSC region, while the two Archiphysalis species consistently measure 5669 bp. Additionally, at the JSA boundary, the pseudogene ψycf1 has lengths of 1118 bp in Physaliastrum and 1148 bp in Archiphysalis, both extending into the SSC region by 19–23 and 29 bp, respectively (Figure 5).
3.4.2 Comparison of Chloroplast Genomes and Hotspot Identification
The annotated complete chloroplast genome of Physaliastrum kweichouense served as the reference sequence for global mVISTA visualization and comparative analysis. The results indicated that there was no gene rearrangement observed in both Archiphysalis and Physaliastrum. The coding regions (exons) across the seven species showed higher conservation compared to the noncoding regions (CNS), with the IR regions being more conserved than the LSC and SSC regions (Figure 6).
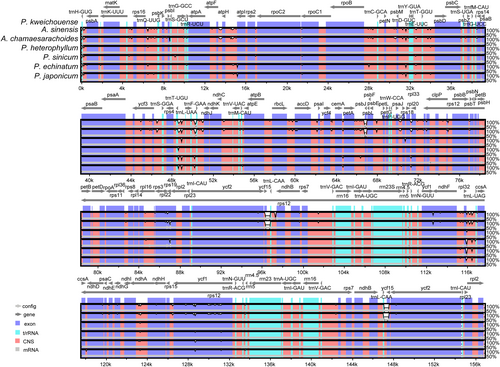
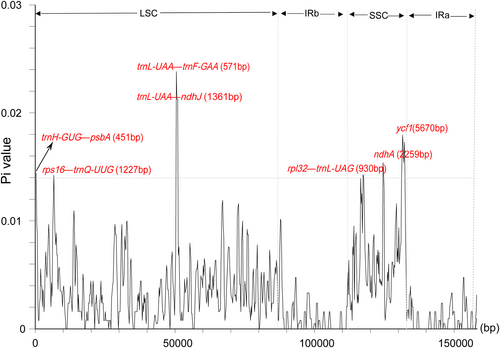
DnaSP identified a total of 774 polymorphic sites in the chloroplast genomes, with an average nucleotide diversity (Pi) value of 0.00330. Notably, the regions trnH-GUG–psbA, rps16–trnQ-UUG, trnL-UAA–trnF-GAA, trnF-GAA–ndhJ, and rpl32–trnL-UAG, along with the genes ndhA and ycf1, displayed significant variability, with Pi values exceeding 0.01400 (Figure 7, Table S1).
3.4.3 Repeat Analysis
A total of 52–61 SSR sites were detected in the complete chloroplast genomes of the studied species, with Physaliastrum heterophyllum showing the highest number (61) and Archiphysalis chamaesarachoides the lowest (52). Mononucleotide repeats were the most abundant (61.40%–68.97%), primarily composed of A/T repeats, while C repeats were only found in A. chamaesarachoides and P. echinatum. AT/TA repeats were the second most abundant (6.90%–12.96%). Pentanucleotide repeats were absent in A. chamaesarachoides and A. sinensis but present in all Physaliastrum species (1 each). Unique repeats, such as ATTTT and TTCTAT, were specific to certain species (Figure 8A–C). SSRs were predominantly located in the LSC region (73.58%–80.76%), with intergenic spacers (IGS) harboring the majority (56.60%–69.23%) (Table S2).
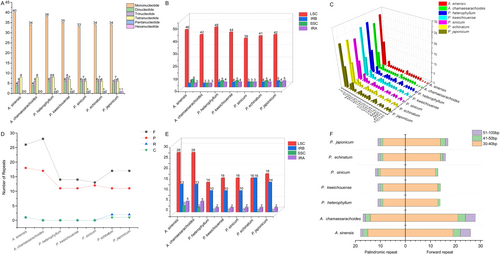
Long repeat sequences, including palindromic (P), forward (F), reverse (R), and complementary (C) repeats, were also identified. Overall, the two Archiphysalis species exhibit a significantly higher number of long repeats compared to the five Physaliastrum species, with counts ranging from 48 to 49 for Archiphysalis and 26–34 for Physaliastrum. Specifically, A. sinensis has the highest count at 49 long repeats, while P. heterophyllum has the lowest at just 26 (Table S3). Long repeat sequences were predominantly located in the LSC region (47.05%–58.33%) and IRb region (26.53%–47.06%). Palindromic and forward repeats with lengths of 30–40 bp were the most abundant. Notably, A. chamaesarachoides, P. heterophyllum, P. kweichouense, and P. sinicum lacked reverse and complementary repeats, whereas A. sinensis had one reverse repeat and one complementary repeat. Additionally, P. echinatum and P. japonicum each had two reverse repeats and one complementary repeat (Figure 8D–F).
3.4.4 Codon Usage Bias Analysis
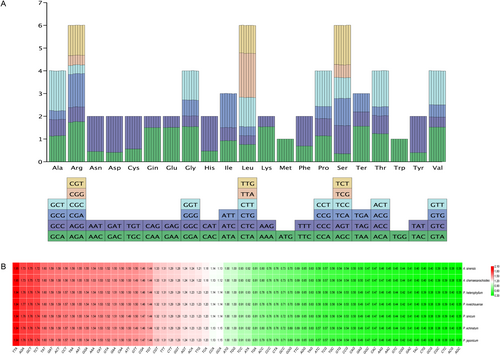
The total number of codons in the protein-coding genes of the chloroplast genomes ranged from 20,949 to 20,960 across the seven species. Analysis of the 64 codons encoding 20 amino acids revealed that leucine (Leu), arginine (Arg), and serine (Ser) were the most abundant, each represented by six codons, whereas methionine (Met) and tryptophan (Trp) were the least abundant, with only one codon each. Leu was the most frequently used amino acid, accounting for 10.50%–10.54% of total codons across species. Ser ranked second (7.49%–7.52%), followed by Arg (5.98%–6.11%). The number of codons was relatively conserved, with no significant differences among species.
Codon usage bias was assessed using the RSCU values. Except for Met and Trp, which had RSCU values of 1 (indicating no bias), the remaining amino acids showed varied RSCU values: 30 codons had RSCU values greater than 1, and 32 had values less than 1 (Figure 9; Table S4).
The complete chloroplast genomes of seven species were analyzed using the rscu.py script, identifying 52 protein-coding genes in each genome. The length of the protein-coding regions ranged from 62,847 to 62,880 bp, with GC content varying between 38.03% and 38.14%. The GC content was highest in the first codon position (46.22%–46.34%), followed by the second (38.17%–38.27%) and third positions (29.69%–29.81%).
The ENC-GC3s plots indicated that most genes did not fall along the expected curve, suggesting a significant discrepancy between the actual ENC values and the expected ENC values (Figure 10A–G). Further analysis using the ENC ratio frequency revealed that among the 52 analyzed genes, all seven species exhibited 15 genes with ENC ratios within the range of −0.05 to 0.05, indicating that these genes are relatively close to the expected ENC values and are significantly influenced by mutation. In contrast, the majority of the remaining 37 genes fall outside this range, indicating a stronger influence of natural selection on their codon usage (Figure 10H; Table S5). Overall, it can be concluded that, for the genera Physaliastrum and Archiphysalis, the codon usage preference of the vast majority of genes is primarily shaped by selection, while a smaller subset of genes is more impacted by mutation.
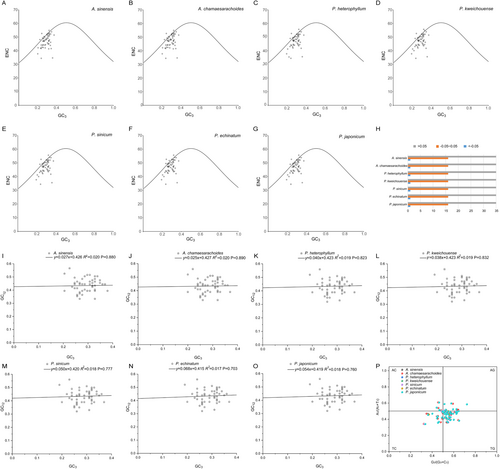
Neutrality plots demonstrated that the correlation between GC12 and GC3 content was not significant (p = 0.703–0.890), indicating distinct base compositions in the first and second codon positions compared to the third position. This further supports the notion that codon usage is predominantly influenced by selection (Figure 10I–O; Table S6). Additionally, the PR2-bias plot revealed an uneven distribution of the 52 genes across four regions, with most genes concentrated in the lower right quadrant (Figure 10P; Table S7). This pattern highlights a higher frequency of T over A and G over C usage in the third codon position.
3.5 Selection Pressure Analysis of Protein Coding Genes
Seventy-nine unique protein-coding genes (PCGs) without duplication were extracted, and their Ka/Ks values were analyzed to identify significant selection pressures. During this process, we excluded genes with Ka/Ks values of “NA” (indicating unavailable data) or 0, as these values typically represent cases with no substitutions or complete matches and thus do not provide meaningful information about selection pressures. This filtering step ensured that only genes with measurable evolutionary dynamics were included in the final analysis.
Using Physaliastrum kweichouense as the reference, we identified 36 protein-coding genes with valid Ka/Ks values. Among these, Tubocapsicum anomalum had three genes with Ka/Ks > 1: accD (1.51), rbcL (1.03), and rpl20 (3.69). Archiphysalis sinensis and A. chamaesarachoides had four and three such genes, respectively, with the former including accD (1.33), ccsA (1.02), clpP (1.22), and rpl20 (3.69), and the latter having clpP (1.22), rbcL (1.03), and rpl20 (3.69). Tuberowithania pengiana had seven genes with Ka/Ks > 1: accD (1.07), ccsA (1.06), clpP (1.44), petA (1.25), rbcL (1.74), rpl20 (3.69), and ycf2 (1.27). These results suggest that these genes may be under positive selection, where nonsynonymous mutation rates exceed synonymous mutation rates. For the remaining protein-coding genes in all species, Ka/Ks values were less than 1, indicating purifying selection, where nonsynonymous mutations are eliminated to maintain functional conservation (Figure 11A; Table S8).
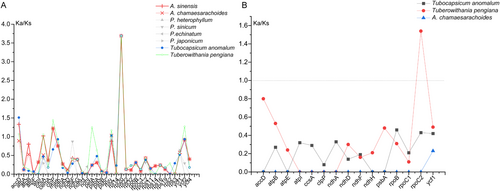
When using Archiphysalis sinensis as the reference protein sequence, we identified 15 protein-coding genes with valid Ka/Ks values. Among these, only the rpoC2 gene in Tuberowithania pengiana exhibited a Ka/Ks value greater than 1, while the Ka/Ks values of the other genes were all less than 1, suggesting purifying selection (Figure 11B; Table S8). This further supports the prevalence of purifying selection in maintaining gene function across species.
Combining the Ka/Ks analysis with the phylogenetic tree in Figure 3, we observed that species within the same branch exhibited higher similarity in their protein-coding sequences. This indicates that the closer the evolutionary relationship between species, the higher the conservation of their gene sequences. The consistency between the Ka/Ks analysis results and the phylogenetic relationships further supports the reliability of Ka/Ks values as an effective tool for assessing selection pressures in gene evolution.
4 Discussion
This study presents the complete chloroplast genomes of nine Chinese taxa, which include two species of Archiphysalis, five species of Physaliastrum, as well as Tuberowithania pengiana and Tubocapsicum anomalum. To elucidate the phylogenetic position of A. sinensis, we downloaded 47 chloroplast genomes from the NCBI database. Our genomic phylogenetic analysis effectively resolves the relationships between the genera Archiphysalis and Physaliastrum, supporting their distinct generic status. Notably, our findings indicate that A. sinensis clusters with A. chamaesarachoides within the subtribe Withaninae, which is consistent with their morphological traits. This close relationship is further corroborated by analyses of chloroplast gene characteristics. A detailed comparative analysis of the chloroplast genomes revealed significant differences in gene features between Archiphysalis and Physaliastrum, particularly regarding gene count, gene structure, and the lengths of gene duplications. The intergeneric differences in chloroplast gene features were markedly greater than the intrageneric differences. Our study provides genomic-level support for the classification of species within these two genera and further highlights the close relationship between A. chamaesarachoides and A. sinensis.
4.1 Phylogenomic Comparison of Chloroplasts in Physaliastrum and Archiphysalis
This study successfully assembled the complete chloroplast genomes of two species of Archiphysalis and five species of Physaliastrum, along with two related genera, Tuberowithania (T. pengiana) and Tubocapsicum (T. anomalum), using second-generation sequencing technology. With the exception of P. yunnanense, which exhibited poor quality and could not be well assembled, all species of Archiphysalis, Physaliastrum, Tubocapsicum, and Tuberowithania distributed in China were included in our research.
Through a detailed analysis of the chloroplast genomes of the two Archiphysalis species and five Physaliastrum species, we found that intergeneric differences in chloroplast gene features were significantly greater than the intrageneric differences. Divergence time analysis indicates that Physaliastrum split from Archiphysalis during the middle Eocene, approximately 41.2 million years ago (95% HPD: 38.6–43.5 Mya; Figure 4). As a result, there are notable differences in chloroplast gene features between Archiphysalis and Physaliastrum. For instance, our research revealed that the chloroplast genomes of Archiphysalis contain 85 protein-coding genes, while those of Physaliastrum have 87. This difference arises from the absence of two copies of the ycf15 gene in the Archiphysalis species (Table 2).
In terms of genomic structure, we also identified distinctions between the two genera. Overall, the Large Single Copy (LSC) region of Archiphysalis species is about 450 bp longer than that of Physaliastrum, while the Inverted Repeat (IR) region is approximately 850 bp shorter (Table 1). Analysis of repetitive sequences indicated that the primary differences between the two genera lie in the Forward repeats and Palindromic repeats, with the most significant variations occurring in lengths of 30–40 bp. These two types of repeats are predominantly located within the LSC region of Archiphysalis, which may explain why this region is considerably longer than that of Physaliastrum (Figure 8D–F). The shorter IR region in Archiphysalis can also be attributed to the absence of the two ycf15 genes, while in Physaliastrum, the presence of these genes and their adjacent intergenic regions contributes approximately 900 bp to their IR length.
Results from the IR contraction and expansion analysis show that the boundary genes are identical in both genera: rps19 spans the LSC/IRb boundary, ψycf1 spans the IRb/SSC boundary, and ycf1 spans the SSC/IRa boundary. The IRa/LSC boundary is located between the rpl2 and the trnH-GUG genes, indicating a high degree of conservation. In species of Physaliastrum, the ycf1 gene and ψycf1 pseudogene in the IR region extend into the SSC region by 19–23 bp, while in the genus Archiphysalis, both extend into the SSC region by 29 bp (Figure 5).
The mVISTA analysis reveals genetic variations between the species of Physaliastrum and Archiphysalis, with the most significant difference being the absence of two ycf15 genes in the latter. In terms of gene type, the variation points are primarily located in the exons, followed by noncoding regions (CNS). Regarding gene distribution, the IR region exhibits very few variations aside from the absence of ycf15, making it the most conserved region (Figure 6). The DnaSP analysis identified four high mutation regions in the LSC and three in the SSC, each with Pi values greater than 0.014. Based on the lengths of these segments, these regions can serve as potential barcoding segments for the two genera (Figure 7).
The analysis of codon usage preference shows that both intra- and inter-generic codon usage among the species of these two genera is largely consistent, with no significant differences observed (Figure 9). ENC-GC3s plots and Neutrality plots indicate that most genes in these species are influenced more strongly by natural selection regarding their codon usage, while a smaller subset is more affected by mutation. The PR2-bias plot highlights a higher frequency of thymine (T) over adenine (A) and guanine (G) over cytosine (C) usage in the third codon position (Figure 10).
Moreover, in the analysis of selection pressure, we found that protein-coding sequences of species on the same branch were more similar, indicating that these species have close phylogenetic relationships and may have experienced similar selection pressures during evolution. Overall, among the 37 protein-coding genes with valid Ka/Ks values, only eight genes exhibit Ka/Ks values greater than 1, indicating they are subject to positive selection, whereas 29 genes exhibit Ka/Ks values less than 1, reflecting purifying selection that eliminates nonsynonymous mutations to maintain functional conservation. These findings further support the prevalence of purifying selection in preserving gene function across species, consistent with many previous studies (Bai et al. 2022; Meng et al. 2023; Palmé et al. 2008; Zhang et al. 2023).
Based on the above analysis, the chloroplast genomes of species within the genera Physaliastrum and Archiphysalis display relatively minor differences and are largely consistent. However, notable distinctions exist between the two genera concerning gene count, gene structure, and the lengths of gene duplications. These findings provide genomic-level support for the classification of species within these genera and further highlight the close relationship between A. chamaesarachoides and A. sinensis.
4.2 Phylogenetic Analysis and Taxonomic Implications
Our research indicates that chloroplast genome data effectively resolve the relationships between the genera Archiphysalis and Physaliastrum (Figure 3). The phylogenetic tree based on this data shows that all species within Physaliastrum cluster into a single evolutionary branch, receiving high support (BS = 100, PP = 1). Similarly, the two species of Archiphysalis also cluster together (BS = 100, PP = 1) and form an independent evolutionary branch within the subtribe Withaninae, alongside the genera Tuberowithania and Tubocapsicum (BS = 93, PP = 1).
The initial molecular phylogenetic study by Li et al. (2013) indicated a distant relationship between Physaliastrum heterophyllum and Archiphysalis chamaesarachoides, with the former in the subtribe Physalinae and the latter in the subtribe Withaninae. This relationship has been supported by subsequent studies (Deanna et al. 2019; Huang et al. 2023; Wang et al. 2024). Deanna et al. (2019) utilized four DNA markers (ITS, LEAFY, trnL-F, and waxy) to analyze the tribe Physalideae, including both species of Archiphysalis (A. chamaesarachoides and A. sinensis) and three species of Physaliastrum (P. heterophyllum, P. japonicum, and P. echinatum). The phylogenetic tree revealed that the two Archiphysalis species did not cluster together; instead, A. chamaesarachoides grouped with Tubocapsicum anomalum, falling within one of its two major branches of the subtribe Withaninae, while the type species A. sinensis clustered with the three species of Physaliastrum, forming a basal branch of the subtribe Physalinae. Although this study highlighted distinct phylogenetic positions for the two Archiphysalis species, it still supports the generic status of both Archiphysalis and Physaliastrum. Notably, the unexpected result of A. sinensis not clustering with A. chamaesarachoides is significant, given their similar fruit morphology. Furthermore, it is important to note that in Deanna's study, the sequence for A. sinensis was only available for the LEAFY fragment, and phylogenetic positions varied when constructed independently from different molecular fragments. For example, samples of Withania clustered together in trees constructed from the ITS and trnL-F fragments but did not cluster in trees based on the waxy and LEAFY fragments.
In 2023, Huang utilized nearly 1700 orthologous nuclear genes from transcriptomic/genomic datasets to reconstruct a highly resolved phylogenetic tree for the Solanaceae family. Their study included only Archiphysalis chamaesarachoides and Physaliastrum heterophyllum, confirming their phylogenetic positions as consistent with Li et al. (2013), thereby supporting their independent status within the subtribes Physalinae and Withaninae, respectively.
In 2024, Wang et al. (2024) published a new genus, Tuberowithania. While using fragments from Deanna's work, they revealed a different systematic position for Archiphysalis. Their results indicated that the inclusion of Tuberowithania shifted the systematic placement of A. chamaesarachoides within the subtribe Withaninae. Additionally, A. sinensis, previously grouped with the Physaliastrum species in the subtribe Physalinae, has now been reclassified to cluster with the genera Tubocapsicum, Discopodium, Nothocestrum, and three species of Withania, forming an evolutionary branch within Withaninae. Notably, A. sinensis is still represented only by the LEAFY gene, and in trees based on this gene, it clusters with species of Physalis L. It is important to highlight that constructing matrices based on LEAFY fragments is challenging due to difficulties in obtaining a reliable aligned matrix. The authors also noted that A. chamaesarachoides exhibits significantly more shared base variations with Tuberowithania than with Tubocapsicum. Morphologically, A. chamaesarachoides appears more closely related to Tuberowithania than to Tubocapsicum, as both possess 10 longitudinally thickened ribs on their fruiting calyx, further supporting its distinct taxonomic placement. Importantly, the authors cautioned that the relationships revealed in the deeper phylogenetic analysis may change with the inclusion of different molecular markers, consistent with Deanna et al. (2019). Therefore, we should remain cautious, as the phylogenetic positions of A. chamaesarachoides may vary with the addition of new samples or the use of different molecular markers.
This study, based on chloroplast genome phylogenetics, classifies the tribe Physalideae into three major clades: Iochrominae (Clade A), Withaninae (Clade B), and Physalinae (Clade C). Notably, our findings reveal that A. sinensis, the type species of Archiphysalis, clusters with A. chamaesarachoides within the subtribe Withaninae (BS = 100, PP = 1), rather than grouping with Physaliastrum, which aligns with our expectations based on morphological characteristics. This grouping forms a cohesive Clade B1 alongside Tuberowithania pengiana and Tubocapsicum anomalum (BS = 93, PP = 1). Furthermore, the five species of Withania also cluster together (BS = 100, PP = 1), associating with representative species of Discopodium and Nothocestrum to form Clade B2.
To minimize the impact of uniparental inheritance of chloroplast genes on the phylogenetic relationships of the two Archiphysalis species, we utilized GeneMiner (Xie et al. 2024) to extract four fragments (ITS, LEAFY, trnL-F, and waxy) from our next-generation sequencing data. These fragments were incorporated into a previous research matrix (Wang et al. 2024), and we constructed trees for each fragment as well as a combined matrix to observe any changes in the phylogenetic relationships of the two Archiphysalis species. Due to the absence of data for A. sinensis in three out of four fragments, we only utilized sequences from three Archiphysalis samples obtained in this study, which had nearly complete data for tree construction. The results showed that, regardless of whether we analyzed the combined data or each fragment separately, A. chamaesarachoides and A. sinensis consistently clustered together (BS = 96–100, PP = 1) within the Withaninae subtribe (Figures S1–S10).
Based on these findings, the authors suggest that while sample availability limits this study, the absence of samples from Aureliana Sendtn. and Deprea Raf. in this chloroplast phylogenetic analysis does not preclude the possibility that the phylogenetic relationships within Withaninae may change with the addition of chloroplast genome data from these genera. However, it is likely that the systematic relationships between the two Archiphysalis species will remain stable, even with more samples. To validate this, the authors plan to expand the representation of species within the Withaninae subtribe and increase sequencing depth, enabling the use of abundant orthologous genes for a more detailed examination of the phylogenetic relationships between Archiphysalis species and their relatives. This expanded dataset will enhance our understanding of the evolutionary dynamics and systematic placements within the Withaninae subtribe.
Author Contributions
Jiaju Xu: data curation (equal), formal analysis (equal), methodology (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Zehuan Wang: conceptualization (equal), funding acquisition (equal), investigation (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Qianqian Zhong: data curation (equal), formal analysis (equal), methodology (equal), validation (equal). Li Yan: data curation (equal), validation (equal), visualization (lead). Sirong Yi: investigation (equal), resources (equal). Qingwen Sun: funding acquisition (equal), supervision (equal).
Acknowledgments
We sincerely thank the Molecular Biology Experiment Center, Germplasm Bank of Wild Species, Kunming, China, for their invaluable support and resources, which greatly contributed to the success of this research. We would also like to express our gratitude to the editors and two anonymous reviewers for their valuable comments, which significantly helped improve this paper. This study was supported by the National Natural Science Foundation of China (grant no. 32260051), the Guizhou Provincial Foundation for Excellent Scholars Program (grant no. GCC[2023]077), and the National Wild Plant Germplasm Resource Center.
Ethics Statement
Experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data in this study are deposited in the NCBI (https://www.ncbi.nlm.nih.gov/), with accession numbers PV472654–PV472662 for complete chloroplast.




