Examining the associations between a generalist feeder and a highly toxic host
Abstract
Understanding the often antagonistic plant–herbivore interactions and how host defenses can influence herbivore dietary breadth is an area of ongoing study in ecology and evolutionary biology. Typically, host plants/fungi that produce highly noxious chemical defenses are only fed on by specialists. We know very little about generalist species that can feed and develop on a noxious host. One such example of generalists feeding on toxic host occurs in the mushroom-feeding Drosophila found in the immigrans-tripunctata radiation. Although these species are classified as generalists, their acceptable hosts include deadly Amanita species. In this study, we used behavioral assays to assess associations between one mushroom-feeding species, Drosophila guttifera, and the deadly Amanita phalloides. We conducted feeding assays to confirm the presence of cyclopeptide toxin tolerance. We then completed host preference assays in female flies and larvae and did not find a preference for toxic mushrooms in either. Finally, we assessed the effect of competition on oviposition preference. We found that the presence of a competitor's eggs on the preferred host was associated with the flies increasing the number of eggs laid on the toxic mushrooms. Our results highlight how access to a low competition host resource may help to maintain associations between a generalist species and a highly toxic host.
1 INTRODUCTION
Understanding the oftentimes antagonistic interactions between plants and their herbivores remains an active area of research (Berenbaum & Feeny, 1981; Ehrlich & Raven, 1964; López-Goldar et al., 2022). These interactions lead to the evolution of a variety of defense mechanisms in plants and fungi (Agrawal et al., 2009; Fraenkel, 1959; Hanley et al., 2007), which influence the breadth of host usage in herbivores. Specifically, hosts that produce highly noxious compounds are consumed by specialists with the necessary adaptations to tolerate/detoxify the compounds, but lost the ability to feed on a wide variety of hosts as a result (Cornell & Hawkins, 2003; Ehrlich & Raven, 1964; Krieger et al., 1971; Whittaker & Feeny, 1971). Conversely, generalist feeders evolve mechanisms of detoxification that are effective against the less toxic host secondary metabolites found across many of their acceptable hosts (Ali & Agrawal, 2012). Despite these predictions, there are cases where generalist herbivores are able to feed on heavily defended hosts even though they represent only a small portion of their acceptable host range (Dussourd & Denno, 1994; Hartmann et al., 2005). In this study, we assessed the associations between Drosophila guttifera, a generalist mushroom-feeder, and a highly toxic host, Amanita phalloides.
Within the genus Drosophila, species breed and feed on a wide variety of host resources (reviewed in Markow & O'Grady, 2008). Although many of the ~2000 species are classified as saprophagous feeders that consume decaying plant material, they vary in their preferred stage of ovipositional host decay. For example, Drosophila suzukii lays eggs in ripe fruits (Lee et al., 2011), while its close relatives Drosophila biarmipes and Drosophila mimetica use soft, rotting fruits (Atallah et al., 2014). Among species that use decaying hosts, variation is even observed in the preferred level of decay (Grimaldi, 1985; Kimura, 1980; Werner et al., 2018). Along with exhibiting preferences in stage of decay, Drosophila species also vary in the range of acceptable hosts and include both specialist and generalist feeders. Drosophila melanogaster and Drosophila simulans are generalist feeders of decaying fruits that can contain up to 7% ethanol and evolved resistance to this compound (McKenzie & McKechnie, 1979; McKenzie & Parsons, 1972). However, both species are susceptible to more noxious compounds found in the morinda fruit hosts of specialist feeders like Drosophila sechellia and Drosophila yakuba (R'Kha et al., 1991; Yassin et al., 2016). While these host associations typically follow the predictions arising from more than 50 years of work on plant–insect interactions, a group of generalist mushroom-feeders in the immigrans-tripunctata radiation of the Drosophila subgenus are tolerant to highly toxic compounds found in a small portion of their hosts (reviewed in Scott Chialvo & Werner, 2018).
The mushroom-feeding Drosophila in the immigrans-tripunctata radiation are classified as generalist feeders of fleshy-white Basidiomycota, and their acceptable developmental hosts include Amanita mushrooms that produce deadly cyclopeptide toxins (Scott Chialvo & Werner, 2018). These flies can develop at the mean concentration of cyclopeptides found in toxic Amanita; however, at higher concentrations, some species are unable to reach adulthood while adult fitness is impacted in others (e.g., reduced thorax length, malformed eyes; Jaenike, 1985). This suggests that the mechanisms of cyclopeptide tolerance is not absolute, which is not surprising given that these compounds are only present in a small portion of the flies' hosts. Furthermore, this adaptation is rapidly lost (~1 million years) when species transition to feeding on hosts other than mushrooms (Spicer & Jaenike, 1996), which provides further evidence that there are fitness costs associated with maintaining tolerance in these flies. Given that cyclopeptides are not present in all or even most of the mushroom-feeding flies' acceptable hosts, we know very little about the conditions that favor using these toxic hosts.
Among the mushroom-feeding Drosophila, several species occur in the quinaria species group, a young adaptive radiation (~19.5 million years; Izumitani et al., 2016). In this group, mushroom-feeding and cyclopeptide toxin tolerance are ancestral traits (Erlenbach et al., 2023; Scott Chialvo et al., 2019; Spicer & Jaenike, 1996). Prior studies (Jaenike, 1985; Perlman et al., 2003) demonstrated that using toxic mushrooms as developmental hosts offers fly larvae an escape from parasitic nematodes that can sterilize adults. To benefit from access to enemy-free space, the adult flies must exhibit a preference for the toxic hosts. In this study, we use a variety of behavioral assays to assess the associations between D. guttifera, a toxin-tolerant member of the quinaria group (Stump et al., 2011), and deadly A. phalloides. This species is found in eastern North America and uses gilled and pored fungi as developmental hosts (Sturtevant, 1921). Furthermore, some stocks show a higher probability of survival on diets with cyclopeptides than without (unpublished data). We specifically focus on identifying whether flies exhibit preferences for the toxic host and examining the functional role of competition (intra- and interspecific) on host preference. In sum, our results suggest that female flies and larvae do not exhibit a preference for toxic host mushrooms, but the ability to use a host that reduces competition may act as a selective pressure that contributes to the maintenance of cyclopeptide tolerance.
2 MATERIALS AND METHODS
2.1 Fly stocks
For this study, we worked with two, unique stocks of D. guttifera (KD – 15130-1971.00; TW – 15130-1971.10). These two stocks were originally maintained in the Drosophila species stock center; however, they are no longer available and were provided by Kelly Dyer (University of Georgia; 15130-1971.00) and Thomas Werner (Michigan Technological University; 15120-1971.10). The flies were maintained on a standard diet consisting of Carolina 4–24 Drosophila instant medium supplemented with a piece of fresh, white mushroom (Agaricus bisporus). A dental cotton roll was placed in each vial as a substrate for pupation. The stocks and experiments were maintained at 23°C with a 12:12 light–dark cycle for the toxin tolerance and oviposition assays. Due to an incubator failure, the fly colonies and experiments for the larval preference and competition assays were maintained at room temperature with a 12:12 light–dark cycle.
2.2 Toxin tolerance assay
To quantify tolerance to an individual toxin and a complex mix in both D. guttifera stocks, we conducted larval feeding assays in 7.5-mL glass scintillation vials containing 250 mg of an instant Drosophila medium (73.5%) and freeze-dried portabella mushrooms (A. bisporus; 26.5%) mixture. The dried mix was resuspended using 1 mL of water or a solution containing the toxin(s): α-amanitin (250 μL α-amanitin and 750 μL deionized water) and natural toxin mix (185 μL toxin mix and 815 μL deionized water). The final concentration of the α-amanitin diet was 250 μg/g, which is equivalent to the mean concentration of this compound in Amanita bisporigera (Tyler et al., 1966; Yocum & Simons, 1977). The concentration of the natural toxin mix was 100 μg/g. We found that toxin susceptible species do not survive on diets containing 50 μg/g (unpublished data).
We extracted the natural toxin mix from dried A. phalloides that were collected along Point Reyes, CA in December 2017. As cyclopeptides are thermostable (Li & Oberlies, 2005), it is not expected that drying the mushrooms would affect the potency of toxins. The extraction was performed using an accelerated solvent extractor and two solvents, methanol: water (5:4 v/v) and methanol following the protocol described in Scott Chialvo et al. (2020). We used these two solvents because A. phalloides contains a mixture of 14 known cyclopeptide toxins with differing polarities (Faulstich et al., 1975; Munekata et al., 1978; Wieland, 1968, 1983). The extracted solution was dried down in a rotary evaporator. As the phallotoxin subclass of cyclopeptides are less polar than amatoxins, the toxin concentrate was resuspended in a mixture of methanol and deionized water (325 and 550 mL, respectively). We confirmed the concentration of amatoxins, the only cyclopeptide class to readily pass through the gut lining (Diaz, 2005; Li & Oberlies, 2005), using HPLC analysis and commercially available chemical standards (i.e., α- and β-amanitin). The solution contained 0.541 μg/μL amatoxins (0.215 μg/mL α-amanitin and 0.326 μg/mL β-amanitin). To account for the potential impact of methanol on survival, we added 56 μL of methanol to both the control and α-amanitin treatments. After adding the solutions to the instant food-mushroom mix, the scintillation vials were placed uncovered in a fume hood for 96 h to allow the methanol to evaporate. Because some water could have evaporated, we then added 200 μL of DI water to each of the food vials.
Prior to adding fly larvae, a small piece of watercolor paper was placed into each vial for pupation. For each scintillation vial, we picked and placed 15 early, first instar larvae into each environment. For 30 days, the vials were monitored daily for survival to adulthood, which was based on successful emergence of adult flies from their pupal case. For both stocks, we completed five replicates for each of the three dietary treatments.
2.3 Oviposition preference
To characterize oviposition preference for both D. guttifera genotypes, we quantified the number of eggs laid on each of the three media: edible mushroom, toxic mushroom, and tomato. We used the tomato-based medium as a negative control because D. guttifera does not naturally use fruits, such as tomatoes, as hosts (Sturtevant, 1921). The mushroom media consisted of 200 mL deionized water, 8.3 g dehydrated and ground up mushroom, 4 g fly agar, and 200 mg tegosept (an antifungal agent). The edible medium used dried portabella mushrooms (A. bisporus), whereas the toxic medium used dried deathcaps (A. phalloides). The tomato medium was made with a 1:1 ratio of tomato juice to deionized water (100 mL each), 4 g fly agar, and 200 mg tegosept. These recipes are based on media used in oviposition assays of other fly species in the immigrans-tripunctata radiation (Jaenike, 1986). The media were allowed to set in 15-mL falcon tubes.
Oviposition assays were conducted in sterile Petri dishes (94 mm × 16 mm). The media were sliced into 5-mm-thick disks that were placed in equal distance from each other. A mated, female D. guttifera (7–10 days after emerging from pupation) was placed in the center of the plate (Figure S1). The assays were kept in a 22.5°C incubator with a 12:12 light dark cycle for a total of 72 h. The female fly was then removed and the number of eggs present on each medium was counted. We completed at least 50 complete replicates for both stocks. A replicate was only considered successful if three or more eggs were laid.
2.4 Larval preference assay
To assess whether larval flies exhibit host preference for either toxic or edible mushrooms, we quantified the change in mass of three different media (plain agar, edible mushroom, and toxic mushroom). Due to the availability of dried A. phalloides and the results of the oviposition assay, we limited the larval feeding preference assay to the KD D. guttifera stock. Plain agar medium was made with 200 mL water, 4 g fly agar, and 200 mg tegosept. The edible and toxic mushroom media used the same components as described above in the oviposition assays. Each of the three media were set using silicone square ice cube trays that produce 1″ × 1″ cubes. To these trays, we added 8 mL of liquid media in each cube.
A cube of plain medium was placed in a small Petri dish (35 mm × 10 mm). The dish was slotted into a 6 oz square bottom Drosophila bottle (Genesee Scientific) containing ≥50 adult D. guttifera KD flies (a mix of males and females) that were 7–10 days post emerging from their pupal case and are expected to be sexually mature. This setup was maintained at room temperature for 14 h to allow for oviposition to occur. The number of eggs laid on each cube were counted, and we cut the cubes down the center so that each half had approximately equal numbers of eggs. A cube was not used if <10 eggs were present on each half. After the cube was cut in half, the mass was taken prior to placing onto a large Petri dish (94 mm × 16 mm) for the larval preference assay.
For the preference assay, a half cube of plain medium was placed between a half cube of each mushroom medium. The cubes were arranged so as not to be in direct contact and separated by approximately 3 mm. As the majority of the eggs were found on the top of the cube and to minimize potential bias related to the placement of the cubes, one half of the plain agar medium was oriented toward the edible mushroom medium, and the other half was oriented toward the toxic mushroom medium (Figure S2). Prior to placing the agar cubes, we took the mass (mg) of both mushroom media and placed them on the plates. The plates were maintained at room temperature (22°C) for 96 h to allow the eggs to hatch and the resulting larvae to feed. Under these environmental conditions and this length of time, D. guttifera larvae reach late third instar just prior to pupation (personal observation). After 96 h, we recorded the mass of each media again.
2.5 Competition assay
To characterize how competition effects oviposition preference in the D. guttifera KD stock, we measured how inter- and intraspecific competition impacts the number of eggs laid and the location in comparison to no competition. The interspecific competitor used was D. tripunctata (2007 iso 1 Athens, GA line; provided by Kelly Dyer). Both D. guttifera and D. tripunctata occur in eastern North America from Texas to Florida and north into Canada (Werner et al., 2020). Thus, we expect these species would compete for resources in their natural distributions. For the intraspecific competition, we used eggs laid by females from the TW D. guttifera stock.
The competition assays included the same media types made using the same protocols that we used in the oviposition assay (i.e., edible mushrooms, toxic mushroom, and tomato (negative control)). For this assay, we allowed the three media to set in small Petri dishes (35 mm × 10 mm). These Petri dishes were filled to a depth of 5 mm.
For the assays, we compared the number of eggs laid in the presence of no competition and inter- and intraspecific competition. A replicate included each of these treatments run concurrently using female D. guttifera KD from the same brood. The competitors laid their eggs on the edible mushroom medium. We slotted the Petri dish containing edible medium into a 6 oz square bottom Drosophila bottle containing ≥50 adults (mix of males and females; 7–10 days post emergence) of either D. guttifera TW or D. tripunctata. After 14 h, we removed the dishes and checked for eggs.
We conducted the assays in a large Petri dish (94 mm × 16 mm) filled with plain agar to a depth of 7 mm. The plain agar was made using the same protocol as in the larval preference assay. We used a 9-mm-diameter leather awl to punch holes that were equidistant for the oviposition media (Figure S1). A 7-mm-diameter hole was placed in the center of the dish for the female fly. To limit the access of D. guttifera KD flies to the top surface of the oviposition media, we placed the disks of the three media (9 mm wide) into equivalently sized holes in the plain agar. For the inter- and intraspecific competition treatment, the edible medium contained ≥4 eggs. The no competition treatment used a disk of edible medium without eggs or exposure to other flies. We placed a D. guttifera KD female into the central hole and maintained the dish at room temperature for 72 h. After 72 h, we removed the flies and documented the number of eggs laid as well as the locations (edible, toxic, tomato, plain, and near each oviposition medium). Eggs laid in the 7-mm hole that the fly was placed in were coded as being laid in plain medium. Eggs laid within 5 mm of an oviposition medium were coded as being near to that medium. We completed 50 replicates; replicates were only used if ≥3 eggs were laid in two of the treatments.
2.6 Statistical analyses
All collected data (i.e. survival to adulthood, number of eggs laid, and change in mass) were analyzed using JMP Pro v 16.0.0 (SAS Institute Inc.). For the toxin tolerance assay, we coded the survival of each individual larvae in a vial using a binary strategy (0 = deceased, 1 = adult successfully emerged). To assess the contribution of genotype (stock), treatment (diet), and the interaction between the two factors on the probability of survival, we performed a logistic regression analysis using a generalized linear model with binomial distribution, logit link, and Firth bias-adjusted estimates (Firth, 1993). Additionally, we also calculated a chi-square for the effect of treatment in each genotype to determine whether survival was significantly affected by the toxins.
For the other assays (oviposition preference, larval preference, and competition), we assessed the contribution of the factors of interest with a regression analysis implemented using a standard least square model and an effect leverage emphasis. For the oviposition preference assay, we assessed how genotype (stock), oviposition media, and the interaction between these factors influenced the variation in the number of eggs laid. We also conducted a one-way ANOVA of the number of eggs laid on each media type (edible mushroom, toxic mushroom, and tomato) for each genotype to examine their preference for each of the medias. We compared the mean number of eggs laid on each media using a Tukey HSD analysis. In the larval preference assay, we assessed how the number of eggs on the plain media, media type, and the interaction between these two factors affected the variation in the change in mass. A one-way ANOVA of change in mass by media and Tukey HSD were used to compare the mean change in mass of each media type. For the competition assay, we first conducted a one-way ANOVA with Tukey HSD to assess how this factor influenced the total number of eggs laid. We used the regression analysis to assess the impact of competition, media, and their interaction on the variation where eggs were laid. To examine how oviposition preference varied under each competition treatment, we completed a one-way ANOVA of eggs laid by media with a Tukey HSD.
3 RESULTS
To better understand the impact of a novel adaptation, cyclopeptide tolerance, on the life history of D. guttifera, we combined feeding assays with characterizations of both adult and larval host preference tests. Our goal was to examine preferences for the toxic host mushroom at both the adult and larval stages and assess whether access to a resource with less competition contributes to the maintenance of this trait.
3.1 Toxin tolerance
To confirm that both available D. guttifera stocks possess the trait of interest, cyclopeptide tolerance, we reared larvae on diets with and without a natural concentration of α-amanitin (250 μg/g) or a mix of cyclopeptide toxins (100 μg/g amatoxins) and measured survival to adulthood. While the probability of survival in both stocks on each of the three diets was >0.1, the responses of the two stocks differed on the diet containing the single toxin (Figure 1). For the KD stock, the probability of survival was highest on this diet (α-amanitin), but the TW stock showed a decrease in comparison to the no toxin diet. In both stocks, developing on a diet containing the toxin mix reduced the probability of survival in comparison to the no toxin diet. Although both stocks showed differences in the probability of survival across the three diets, they were only significant in the TW stock (p < .0001; Table S1). In our examination of the variation in probability of survival across both D. guttifera stocks, the stock accounted for the greatest contribution (p = .00002; Table 1). Stock by diet interactions (p = .0277) and dietary treatments (p = .0009) also contributed significantly to the variation in this phenotype.
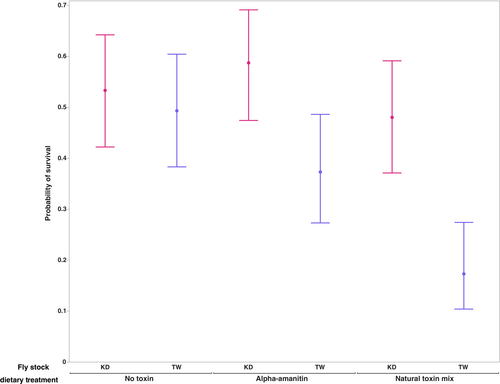
| Phenotype | Source | df | LogWorth | L–R χ2 | Prob > χ2 |
|---|---|---|---|---|---|
| Probability of survival | Stock | 1 | 4.619 | 17.839 | .00002*** |
| Diet | 2 | 3.065 | 14.115 | .0009*** | |
| Stock × Diet | 2 | 1.558 | 7.175 | .0277* |
- *p < .05, **p < .01, ***p < .001.
3.2 Oviposition preference
To assess whether the female flies in both D. guttifera stocks are partial to laying eggs on toxic mushroom hosts, we documented the number of eggs laid and the location when the flies were provided three host options (edible mushroom, toxic mushroom, and a negative control – tomato). Both genotypes laid the most eggs on the edible mushroom medium (mean number of eggs laid >10; Figure 2). This was significantly higher than the mean number of eggs laid on either the toxic mushroom or tomato (negative control) media (p < .001; Table 2). Somewhat surprisingly, the flies laid slightly more eggs on the tomato-based medium (negative control) than the toxic mushroom medium. However, this difference was not significant (p > .05). When examining the sources of variation in this data, only the oviposition media type made a significant contribution (p < .0001; Table 3). Genetic stock and the interaction between it and media did not contribute significantly (p = .273 and p = .418 respectively).
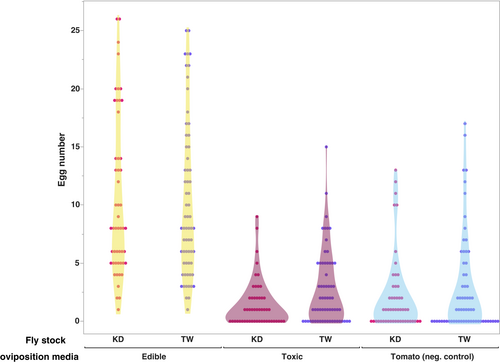
| Media | Stock | Mean eggs laid | 95% CI |
|---|---|---|---|
| Edible mushroom | KDb | 10.510*** | 9.251, 11.769 |
| Toxic mushroom | 1.431 | 0.173, 2.690 | |
| Tomatoa | 2.177 | 0.918, 3.435 | |
| Edible mushroom | TWc | 10.172*** | 8.965, 11.379 |
| Toxic mushroom | 2.734 | 1.528, 3.941 | |
| Tomatoa | 2.906 | 1.700, 4.113 |
- a Negative control.
- b 51 replicates.
- c 64 replicates.
- *p < .05, **p < .01, ***p < .001.
| Phenotype | Source | df | Sum of squares | F-ratio | Prob > F |
|---|---|---|---|---|---|
| Oviposition preference | Stock | 1 | 27.177 | 1.207 | .273 |
| Media | 2 | 4890.743 | 108.623 | <.0001*** | |
| Stock × Media | 2 | 39.369 | 0.874 | .418 |
- *p < .05, **p < .01, ***p < .001.
3.3 Larval preference
During the completion of the oviposition preference assay, we observed that larvae would migrate from the medium where their eggs were laid (edible mushroom) to another (e.g., toxic medium). To assess whether host preference occurs in larval D. guttifera of the KD stock, eggs were laid on a plain agar medium with no nutritional value, and we measured the change in mass of the two mushroom based media (Figure 3). After 96 h, the change in mass of the two media containing mushrooms (mean mass change >1 g for both media) was significantly higher (p < .001; Table 4) than for the plain medium (mean mass change = 646.53 mg) where the eggs were laid. When comparing the two, mushroom-based media, the change in mass did not differ significantly (p > .05). In characterizing the sources of variation in these data, we found that media type made the greatest contribution (p < .0001; Table 5). Additionally, the number of eggs laid on the plain medium was a significant source of variation (p = .0012). The interaction between media and the number of eggs was not significant (p = .563).
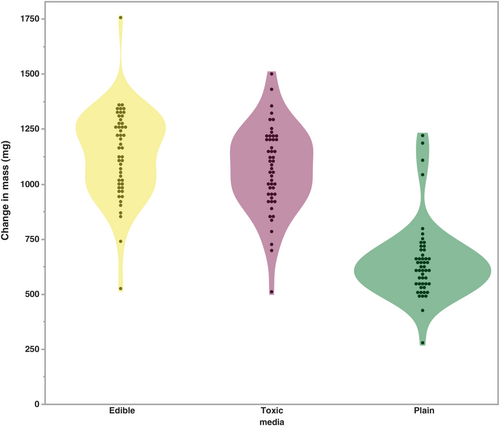
| Media typeb | Mean change (mg) | 95% CI |
|---|---|---|
| Edible mushroom | 1135.06 | 1082.0, 1181.1 |
| Toxic mushroom | 1067.61 | 1014.6, 1120.6 |
| Plaina | 646.53*** | 593.5, 699.6 |
- a Media eggs were laid on.
- b 50 replicates.
- *p < .05, **p < .01, ***p < .001.
| Phenotype | Source | df | Sum of squares | F-ratio | Prob > F |
|---|---|---|---|---|---|
| Change in mass | Media | 2 | 7008512.3 | 103.405 | <.0001*** |
| Egg # | 1 | 372547.8 | 10.9933 | .0012** | |
| Media × Egg # | 2 | 39120.5 | 0.5772 | .563 |
- *p < .05, **p < .01, ***p < .001.
3.4 Competition
To characterize whether competition could alter oviposition preference in the D. guttifera KD stock, we completed assays where the preferred oviposition site (edible mushroom medium) was either free from competition or already had eggs laid on it by either an inter- or intraspecific competitor. We counted the number of eggs laid on each medium and in close proximity (Figure 4). The mean number of eggs laid by the D. guttifera KD differed significantly between the inter- and intraspecific competition treatments (p < .05; Table 6). The flies laid fewer eggs (mean = 1.0714) when an interspecific competitor (D. tripunctata) had previously laid eggs on the edible mushroom medium. When considering the contribution of different factors in the data variation, competition was a significant variable (p = .0018; Table 7). Similar to the oviposition assay, the greatest source of variation in the number of eggs laid was media (p < .0001). However, there was also a significant interaction between media and competition (p = .0001).
| Competition state1 | Mean eggs laid | 95% CI |
|---|---|---|
| Intraspecific | 1.706a | 1.446, 1.966 |
| Interspecific | 1.0714b | 0.812, 1.331 |
| None | 1.489ab | 1.229, 1.749 |
- Note: Means not sharing any letters are significantly different in a Tukey test at p < .05.
- 1 52 replicates.
| Phenotype | Source | df | Sum of squares | F-ratio | Prob > F |
|---|---|---|---|---|---|
| Oviposition preference | Media | 6 | 333.621 | 9.339 | <.0001*** |
| Competition | 2 | 75.738 | 6.361 | .0018** | |
| Media × competition | 12 | 230.698 | 3.229 | .0001*** |
- *p < .05, **p < .01, ***p < .001.
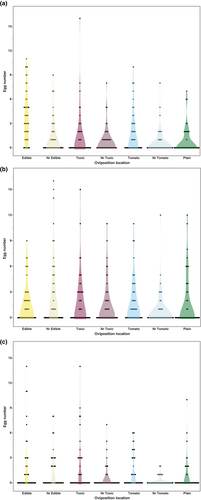
In our examination of the oviposition locations across the three treatments (Figure 4), we observed different patterns of host usage under each. However, the fewest eggs were laid in the plain media closest to the tomato across all assays (Table 8). The no competition treatment (Figure 4a) produced similar results to the original oviposition assay (Figure 2). The mean number of eggs laid was significantly higher on the edible mushroom medium (3.827, p < .05) in comparison to all other locations. In addition, the flies continued to lay more eggs on the tomato medium (mean = 1.731) than the toxic mushroom medium (mean = 1.346). When an intraspecific competitor laid eggs on the edible medium, the KD stock laid the most eggs near, but not on, the edible medium (Figure 4b). The mean number laid at this location was significantly higher (2.673, p < .05) than the near tomato location (0.827). The mean number of eggs laid at the other locations did not differ significantly from either of these two. Under the interspecific competition treatment (Figure 4c), the mean number of eggs laid in both the edible and toxic mushroom mediums (1.712 and 1.596, respectively) were significantly higher (p < .05) than the mean number laid near the tomato medium (0.231). Under both competition treatments, the number of eggs laid on the toxic mushroom medium was higher than the tomato medium.
| Media | No competition | Intraspecific (Drosophila guttifera) | Interspecific (Drosophila tripunctata) | |||
|---|---|---|---|---|---|---|
| Mean eggs laid | 95% CI | Mean eggs laid | 95% CI | Mean eggs laid | 95% CI | |
| Edible | 3.827a | 3.216, 4.438 | 1.596ab | 0.813, 2.379 | 1.712a | 1.126, 2.297 |
| Nr. Edible | 1.231b | 0.620, 1.841 | 2.673a | 1.890, 3.456 | 1.288ab | 0.703, 1.874 |
| Toxic | 1.346b | 0.736, 1.957 | 1.865ab | 1.0823, 2.649 | 1.596a | 1.011, 2.181 |
| Nr. Toxic | 0.865b | 0.255, 1.476 | 1.500ab | 0.717, 2.283 | 0.577ab | −0.0082, 1.162 |
| Tomato (NC) | 1.731b | 1.120, 2.341 | 1.827ab | 1.0438, 2.610 | 1.308ab | 0.723, 1.893 |
| Nr. Tomato | 0.615b | 0.0049, 1.226 | 0.827b | 0.0438, 1.610 | 0.231b | −0.354, 0.816 |
| Plain | 0.808b | 0.197, 1.418 | 1.654ab | 0.871, 2.437 | 0.788ab | 0.203, 1.374 |
- Note: Means not sharing any letters are significantly different in a Tukey-test at p < .05.
4 DISCUSSION
Within the study of plant–herbivore interactions, understanding how the defenses of host plants and/or fungi influence the dietary breadth of herbivore is an active area of research (Berenbaum & Zangerl, 1998; Bernays & Graham, 1988; Hardy et al., 2020; Katsanis et al., 2016). Host defenses include structures that prevent/limit feeding damage, production of secondary metabolites to deter feeding, and phenological shifts (Agrawal et al., 2009; Hanley et al., 2007). The potency of the defensive chemicals produced by plants and fungi to deter feeding, ranges from highly toxic to digestibility-reducing. The expectation of what organisms can feed on chemically defended hosts varies based on the toxicity of the compound. Specialists evolve mechanisms that allow them to develop on hosts containing highly toxic compounds, but lose the ability to feed on a wide range of hosts (Cornell & Hawkins, 2003; Whittaker & Feeny, 1971). In turn, the detoxification mechanisms that are expected to evolve in generalists are those that will be useful against less toxic defenses that are common across multiple hosts (Ali & Agrawal, 2012). We know far less about generalist species that are able to feed on hosts containing highly noxious compounds even though those hosts represent only a small portion of their diet. In this study, we examine this question in the mushroom-feeding D. guttifera, which is broadly polyphagous on fleshy Basidiomycota (Sturtevant, 1921), including toxic Amanita species. We used a combination of behavioral assays to assess whether preference for the toxic hosts exists within D. guttifera and the conditions under which this species will choose to use a toxic mushroom host.
We first examined the occurrence of the adaptation of interest, cyclopeptide toxin tolerance, in the two available D. guttifera stocks. Phylogenetic examinations of the evolution of this adaptation in the quinaria species group (Spicer & Jaenike, 1996) and the immigrans-tripunctata radiation (Erlenbach et al., 2023) suggest that tolerance arose once and has been lost multiple times. In species that transition away from mushroom feeding, toxin tolerance is lost in ~1 million years (Spicer & Jaenike, 1996). As both D. guttifera stocks have been maintained in the lab without exposure to cyclopeptide toxins for over a decade, we completed feeding assays where we reared larvae on diets without toxins and with either a single cyclopeptide (α-amanitin) or a natural toxin mix. The larvae of both stocks survived on all diets; however, the patterns of survival varied between them. While treatment (the presence or absence of cyclopeptide toxins) did not significantly affect survival of the KD stock, it did significantly lower survival in the TW stock. However, survival in both stocks decreased on the diet containing the natural mixture of toxins. The lowered survival on the toxin mix could be due to synergistic and/or antagonistic interactions that occur within the mixture. Prior studies (Dyer et al., 2003; Richards et al., 2016) on tolerance of host secondary metabolites found that the potent bioactivity of some compounds is due to these types of interactions. When examining potential sources of the variation in the survival data across both stocks, we found that there is a significant interaction between stock and treatment (environment). This suggests the potential for significant genetic variation in toxin tolerance within D. guttifera. With only two available stocks, we are limited in the conclusions we can draw regarding genetic variation within the species. However, this finding is consistent with studies of cyclopeptide tolerance in other mushroom-feeding species within the immigrans-tripunctata radiation (Jaenike, 1989; Kokate et al., 2022). Both studies identified a significant genotype by environment interaction for survival to adult when larvae are reared on diets containing cyclopeptide toxins. Kokate et al. (2022) identified this pattern in two other members of the quinaria group, D. recens and D. falleni. Thus, our results demonstrate that cyclopeptide tolerance is present and potentially a complex genetic trait.
Tolerance to highly toxic plant/fungal compounds can allow organisms to exploit new niches (Ehrlich & Raven, 1964; Fraenkel, 1959). For this to happen though, the herbivore must be able to identify the plant/fungus as an acceptable host and exhibit some preference for it. In generalists that can feed on highly toxic hosts, the toxic hosts make up only a small portion of the acceptable host range (Dussourd & Denno, 1994; Hartmann et al., 2005; Scott Chialvo & Werner, 2018). A question that arises is to what degree will these species make use of the toxic host. Mushroom-feeding flies in the immigrans-tripunctata radiation and more specifically the quinaria species group offer the potential to examine preference for a highly toxic host at both the adult and immature stages. To characterize a potential preference for the toxic host in adults D. guttifera, we conducted oviposition assays using both stocks where female flies selected among edible and toxic mushrooms along with a negative control. As D. guttifera can develop on diets containing a mix of toxins equivalent to what is found in toxic Amanita mushrooms, we would expect that females exhibit a preference for cyclopeptide containing mushrooms over a host that they do not use in the wild. Such a pattern can be found in other Drosophila species, including D. sechellia, an ecological specialist on the chemically defended noni fruit of Morinda citrifolia (Álvarez-Ocaña et al., 2023). With our oviposition assays, we found that both stocks exhibited similar preferences and laid the most eggs on the edible mushroom medium (p < .001). Somewhat surprisingly, both stocks laid more eggs on the negative control (tomato) than the toxic mushroom medium. When examining the effect of different factors on oviposition variation, only media was significant. While these results suggest that female flies show no preference for toxic mushrooms and are slightly more likely to lay eggs on a host that they do not use in the wild (tomatoes), work with other Drosophila species found that oviposition preference can be influenced by the proximity of the preferred substrate (Miller et al., 2011; Schwartz et al., 2012; Sumethasorn & Turner, 2016). In these situations, female flies chose to lay eggs on a suboptimal substrate for larval development if the larvae could move to the optimal source. During the completion of the oviposition assays, we observed that larvae would migrate between the media in some replicates. Furthermore, this observed negative correlation between the female's preference and a known host could be due to missing cues that would be present in a natural setting (Nylin et al., 1996; Thompson, 1988). While it does not appear that a preference for toxic mushrooms is present in the female flies, there is the potential that our laboratory setting is missing ecological cues that drive usage of these hosts and thereby maintain cyclopeptide tolerance.
For polyphagous insect species, the addition of a new host source requires females to lay their eggs on the host as well as the larvae developing successfully. However, oviposition preference and larval performance are not always positively correlated (Gripenberg et al., 2010; Mayhew, 1997; Murphy, 2004). For species whose larvae are unable to move between distant hosts, such as Drosophila, it is counter-intuitive that females would exhibit a preference for laying their eggs on sub-optimal locations/hosts, but this strategy has been observed in Drosophila species when media/host options are close enough to allow movement (Schwartz et al., 2012; Yang et al., 2008). Given the absence of an oviposition preference for toxic mushrooms—and in fact a potential distaste for this host—as well as our observations that D. guttifera larvae moved among the oviposition media types, we conducted a feeding assay to assess whether larvae from the KD genotype showed a preference for edible or toxic mushrooms. To quantify preference, we assessed the change in mass of the two mushroom media and a plain agar medium after 96 h. Studies of other Drosophila species found that larvae prefer specific yeast strains (da Cunha et al., 1951; Lindsay, 1958) and that the stage of fungal decay impacts larval preference in other fungal-feeding species in the immigrans-tripunctata radiation (Kimura, 1980). We found that both the type of media and the number of eggs present on the plain agar medium accounted for a significant portion of the variation. However, the interaction between these variables was not significant. When we compared the mean changes in mass of the media, we found that the larvae consumed significantly more (p < .001) of the media containing mushrooms than the plain medium. These results suggest that the larvae preferred food sources that contain necessary nutrients and that when more larvae are present more media is consumed, but the consumption of a specific type of mushroom medium is not influenced by the number of eggs. It does not appear that a preference for toxic mushrooms is present within the larvae. These results in combination with our findings from the oviposition assay raise the question of how cyclopeptide tolerance is being maintained in D. guttifera.
In a natural setting, the ability to feed on a highly toxic host offers a potential escape from competition (Harrison & Karban, 1986; Viswanathan et al., 2005; Zytynska & Preziosi, 2013) and access to enemy free space (Atsatt, 1981; Denno et al., 1990; Mulatu et al., 2004). Thus, the benefits of escaping from enemies and/or gaining access to a resource with fewer competitors can outweigh the negative costs associated with larvae developing on chemically defended hosts (Alzate et al., 2017; Craig et al., 2000; Murphy, 2004; Singer et al., 2004). For mushroom-feeding Drosophila, Perlman et al. (2003) found that developing on a toxic mushroom reduced the load of Howardula nematodes, which can sterilize adults. While this suggests that the larvae of these species benefit from access to enemy-free space, adults that are infected with nematodes do not alter their behavior to seek out mushrooms containing cyclopeptide toxins (Debban & Dyer, 2013). As mushrooms are an ephemeral resource, it is likely that one benefit of cyclopeptide tolerance is that the flies will experience lower levels of competition on the toxic host (Buxton, 1960; Grimaldi & Jaenike, 1984; Lacy, 1984). As such, we conducted additional oviposition assays to determine how intra- and interspecific competition affected host preference using the KD stock of D. guttifera. In these assays, we provided the flies with the same oviposition media types, but the edible mushroom medium contained eggs laid by a different D. guttifera stock or D. tripunctata, a mushroom-feeding species with overlapping distribution. In our no competition treatment, the results were consistent with what we had observed originally (i.e., edible mushrooms strongly preferred over all other options). With the competition treatments, we found that the responses of the flies varied depending on the competitor. When eggs from an intraspecific competitor where present on the edible mushroom medium, the flies laid significantly more eggs (p < .05) than in the interspecific treatment. The preferred host medium varied between the two competition treatments (near edible—intraspecific and edible/toxic—interspecific). With both competitive treatments, the mean number of eggs laid on the toxic medium was greater than the tomato medium, unlike the oviposition assays without competition. Additionally, the interaction between media and type of competition significantly influenced variation in oviposition preference. These findings are congruent with the work of Grimaldi and Jaenike (1984) who found that on edible mushrooms larvae experienced food shortages due to competition. In D. recens, another member of the quinaria group, higher levels of toxin tolerance were associated with lowered competitive ability (Kokate & Werner, 2023). Our findings suggest competition and more specifically interspecific competition could play a role in selecting for the use of toxic host mushrooms. Thus, the ability to use resources with lower levels of competition could act to maintain cyclopeptide tolerance in D. guttifera.
Overall, the results of our study suggest that in lab conditions and the absence of competition D. guttifera is unlikely to use toxic mushrooms as a developmental host. Although cyclopeptide tolerance is present in both stocks, neither the adults or larvae exhibit a preference for toxic mushrooms. Furthermore, female flies lay eggs on toxic Amanita at rates equivalent to non-natural hosts (tomatoes). Given how rapidly this adaptation can be lost (~1 million years; Spicer & Jaenike, 1996), we assessed the impact of intra- and interspecific competition on toxin tolerance maintenance. Our results suggest that competition can cause flies to make use toxic host mushrooms. Further studies could investigate the role of parasitoid wasps and the ephemeral nature of mushrooms in host choice in this species. Overall, our assays provide further evidence for the role of competition in driving the selection of hosts with associated costs in larval fitness.
AUTHOR CONTRIBUTIONS
Grace Kropelin: Data curation (lead); formal analysis (supporting); investigation (lead); methodology (supporting); validation (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (equal). Clare H. Scott Chialvo: Conceptualization (lead); data curation (supporting); formal analysis (lead); funding acquisition (lead); investigation (supporting); methodology (lead); resources (lead); validation (equal); visualization (lead); writing – original draft (lead); writing – review and editing (equal).
ACKNOWLEDGMENTS
We would like to thank Kelly Dyer and Thomas Werner for providing the fly stocks used in this project. This work was funded by grants from the National Science foundation (DEB-1737869 to Clare Scott Chialvo and Laura Reed - University of Alabama and DBI-2217912 to Clare Scott Chialvo) and startup funds provided by Appalachian State University to Clare Scott Chialvo.
CONFLICT OF INTEREST
The authors declare that we have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Raw data have been deposited in the Dryad Digital Repository (https://doi.org/10.5061/dryad.98sf7m0pm), and the JMP code is deposited in Zenodo (https://doi.org/10.5281/zenodo.8280572). Link for Peer Review: https://datadryad.org/stash/share/o4jW0TfoChE2j3zF69OQwFnM2fY0mXLU2ojg6s6TOkA.




