Premorbid body weight predicts weight loss in both anorexia nervosa and atypical anorexia nervosa: Further support for a single underlying disorder
Abstract
Objective
For adolescents, DSM-5 differentiates anorexia nervosa (AN) and atypical AN with the 5th BMI-centile-for-age. We hypothesized that the diagnostic weight cut-off yields (i) lower weight loss in atypical AN and (ii) discrepant premorbid BMI distributions between the two disorders. Prior studies demonstrate that premorbid BMI predicts admission BMI and weight loss in patients with AN. We explore these relationships in atypical AN.
Method
Based on admission BMI-centile < or ≥5th, participants included 411 female adolescent inpatients with AN and 49 with atypical AN from our registry study. Regression analysis and t-tests statistically addressed our hypotheses and exploratory correlation analyses compared interrelationships between weight loss, admission BMI, and premorbid BMI in both disorders.
Results
Weight loss in atypical AN was 5.6 kg lower than in AN upon adjustment for admission age, admission height, premorbid weight and duration of illness. Premorbid BMI-standard deviation scores differed by almost one between both disorders. Premorbid BMI and weight loss were strongly correlated in both AN and atypical AN.
Discussion
Whereas the weight cut-off induces discrepancies in premorbid weight and adjusted weight loss, AN and atypical AN overall share strong weight-specific interrelationships that merit etiological consideration. Epidemiological and genetic associations between AN and low body weight may reflect a skewed premorbid BMI distribution. In combination with prior findings for similar psychological and medical characteristics in AN and atypical AN, our findings support a homogenous illness conceptualization. We propose that diagnostic subcategorization based on premorbid BMI, rather than admission BMI, may improve clinical validity.
Public significance
Because body weights of patients with AN must drop below the 5th BMI-centile per DSM-5, they will inherently require greater weight loss than their counterparts with atypical AN of the same sex, age, height and premorbid weight. Indeed, patients with atypical AN had a 5.6 kg lower weight loss after controlling for these variables. In comparison to the reference population, we found a lower and higher mean premorbid weight in patients with AN and atypical AN, respectively. Considering previous psychological and medical comparisons showing little differences between AN and atypical AN, we view a single disorder as the most parsimonious explanation. Etiological models need to particularly account for the strong relationship between weight loss and premorbid body weight.
1 INTRODUCTION
A core feature of anorexia nervosa (AN) is a significantly low body weight, commonly defined by a body mass index (BMI) < 18.5 kg/m2 in adults or a BMI-centile-for-age <5 in children and adolescents (American Psychiatric Association, 2013). If a non-underweight patient (i.e., BMI ≥ 18.5 kg/m2 or BMI-centile ≥ 5), who has experienced ‘significant weight loss,’ fulfills the DSM-5 (B) (fear of gaining weight or persistent behavior interfering with weight gain) and (C) (disturbance in the way body weight and shape are experienced, undue influence of body weight or shape on self-evaluation or lack of recognition of the seriousness of the low body weight) criteria for AN, the appropriate diagnosis is atypical AN within the diagnostic category Other Specified Feeding or Eating Disorder (OSFED).
The heterogeneous model of AN and atypical AN has notable shortcomings, including an extant research base illustrating generally similar psychological and medical characteristics in both illnesses (Brennan et al., 2023; Johnson-Munguia et al., 2024; Walsh et al., 2023). Thus, differentiating diagnoses with a ‘significantly low body weight’ has not been externally validated. ‘Significant weight loss,’ a unique criterion of atypical AN, also lacks empirical quantification. Rather, differences in eating pathology and distress have not been demonstrated when patients with atypical AN were differentiated by a “significant weight loss” of 5%, 10%, or 15% of their highest lifetime weight (Forney et al., 2017). Further, in a systematic review and meta-analysis by Walsh et al. (2023), only three of 24 studies required ‘significant weight loss’ to diagnose atypical AN.
Whereas AN and atypical AN are distinguished by a patient's admission BMI, differences in premorbid BMI are also evident despite repeated findings for commensurate mean weight loss in both disorders (Garber et al., 2019; Matthews et al., 2024; Peebles et al., 2010; Whitelaw et al., 2014). Compared to patients with atypical AN, there is a lower prevalence of premorbid overweight/obesity and lower maximal BMI in patients with AN (Walsh et al., 2023). Further, epidemiological studies have demonstrated associations between low childhood BMI and increased AN risk (Leth-Moller et al., 2023; Stice et al., 2017, 2022; Yilmaz et al., 2019) and genome wide association studies (Duncan et al., 2017; Watson et al., 2019) have identified genetic correlations between AN, low BMI, and associated traits. For example, in the Avon Longitudinal Study of Parents and Children (Abdulkadir et al., 2022), female participants with a high polygenic score for AN and a low polygenic score for BMI constituted a high-risk group for AN. Epidemiological and genetic data for atypical AN are lacking.
Notably, significant interrelationships between premorbid BMI and weight-specific variables have been demonstrated in AN. In hospitalized adolescents, a high premorbid BMI-centile (or BMI-standard deviation score (BMI-SDS)) predicts a high admission BMI (or BMI-SDS) (Coners et al., 1999; Föcker et al., 2015; Peters, Kolar, et al., 2021). A positive correlation between weight loss in kg/m2 and premorbid BMI-centile was demonstrated in a single study (Coners et al., 1999). In our group's recent registry-based study (Peters, Kolar, et al., 2021), a high premorbid BMI-SDS again predicted a high admission BMI-SDS in 360 hospitalized adolescents with AN. In a prior study, we assessed the effect of age on BMI/BMI-SDS at admission: BMI was positively and BMI-SDS was negatively correlated with admission age (Engelhardt et al., 2020). We proposed that this inverse correlation reflects the increment in percent body fat during female adolescence (Engelhardt et al., 2020). Compared to young adolescents with AN who have not yet experienced an increase in percent body fat of similar magnitude, older adolescents with AN can achieve a lower weight in relationship to the BMI distribution of the reference population adjusted for age (BMI-SDS). Interestingly, illness duration, defined as the difference between admission age and age at initial weight loss, showed no influence on admission BMI-SDS (Coners et al., 1999; Peters, Kolar, et al., 2021). In adult AN samples, similar relationships between premorbid and admission BMI have been reported, with maximum adult BMI (Kaufmann et al., 2021) and weight suppression (i.e., the difference between a patient's admission weight and highest historical adult weight positively correlated with admission BMI (Lowe et al., 2018)). In summary, the prediction of admission weight in AN has been a major research focus; in contrast, the explanation of weight loss particularly in atypical AN has received only limited attention.
Accordingly, our current study extends our previous registry based studies (Engelhardt et al., 2020; Peters, Kolar, et al., 2021) by aiming to assess whether differentiating AN and atypical AN with the 5th BMI-centile (at admission) induces discrepancies in (i) weight loss adjusted for age and premorbid weight, (ii) premorbid weight, and (iii) interrelationships between weight-specific variables. In our registry-based sample of 460 hospitalized female adolescents with AN (n = 411) and atypical AN (n = 49), with diagnoses differentiated by an admission BMI-centile <5 or BMI-centile ≥5, respectively, we specifically delineated two hypotheses: (i) Consider two female adolescents of equal age, height, and premorbid weight, who both fulfill the (B) and (C) criteria for AN following weight loss. One patient, whose admission BMI-centile <5, is diagnosed with AN, whereas the other patient, whose admission weight remains above the BMI cutoff, is diagnosed with atypical AN. We hypothesize that as a direct consequence of the diagnostic BMI-centile cutoff, patients with atypical AN lose less weight than their AN counterparts upon controlling for age, height, illness duration, and premorbid weight. (ii) Because prior studies demonstrate a similar mean weight loss in kg (unadjusted for the aforementioned variables) in samples of patients with atypical AN and AN (Garber et al., 2019; Matthews et al., 2024; Peebles et al., 2010; Whitelaw et al., 2014), the diagnostic weight cutoff should result in patients with a high premorbid BMI being less likely diagnosed with AN. In contrast, weight loss in patients with a low premorbid BMI would more likely result in their being below the weight cutoff resulting in a diagnosis of AN. Thus, we hypothesize an overrepresentation of high premorbid BMIs in atypical AN and an overrepresentation of low premorbid BMIs in AN. We additionally aimed to replicate prior findings demonstrating significant relationships between premorbid BMI and admission BMI (Coners et al., 1999; Föcker et al., 2015; Peters, Kolar, et al., 2021), and weight loss (Coners et al., 1999) in adolescents with AN and to analyze these relationships in adolescents with atypical AN.
2 METHOD
The current study was based on the German Registry of Children and Adolescents with AN aged <19 years (Bühren et al., 2017; Herpertz-Dahlmann & Hebebrand, 2017; Kolar et al., 2018) with 16 participating child and adolescent psychiatric hospitals. The treating clinicians were responsible for diagnostic assessment and enrollment. DSM-5 criteria for AN were provided verbatim in the data entry sheets to increase diagnostic reliability; all patients fulfilled the B and C criteria for AN. Upon analysis, the registry included data of 769 patients enrolled between August 2014 and May 2020. As data of only 22 males were available, we included females only. Complete data sets were available for 460 females, with 71.7% (n = 330) representing data from the patient's first inpatient hospitalization for AN/atypical AN. Both legal guardians and patients provided written consent. Ethics committees of participating universities (n = 14) and local research centers (n = 2) approved the registry study.
For the purpose of the current study and in contrast to the use of the 10th BMI-centile in our previous registry-based studies (Engelhardt et al., 2020), patients were strictly subdivided according to the DSM-5 weight criterion of the 5th BMI-centile. A diagnosis of AN (BMI-centile <5) was fulfilled by 411 patients; 49 patients were diagnosed with atypical AN (BMI-centile ≥5); the DSM-5 requirement of ‘significant weight loss’ was waived. The majority of patients with atypical AN presented with admission BMI-centile ≥5 but <10 (n = 34) and the remaining 15 patients had an admission BMI-centile ≥10.
Admission BMI was calculated by dividing measured body weight by the square of measured height (kg/m2). Premorbid BMI was estimated based on body weight prior to initial weight loss as recalled by patients and/or parents and measured admission height (Coners et al., 1999; Föcker et al., 2015). On the basis of German reference data for girls (Rosario et al., 2010), individual BMI-values and heights were transformed into BMI-SDS, BMI-centiles, or height-SDS using the method of Cole and Green (1992). Recalled age at initial weight loss and premorbid BMI allowed the estimation of premorbid BMI-centiles/BMI-SDS.
We estimated weight loss in kg and in percent of premorbid weight based on recalled premorbid weight and measured admission weight and height (see Peters, Kolar, et al., 2021). Illness duration (log-transformed (ln) because of right-skewed distribution) was defined as the elapsed time between recalled age at initial weight loss and admission age (Coners et al., 1999; Peters, Kolar, et al., 2021). We refer to premorbid weight (BMI), illness duration, and weight loss despite their being based on recalled data. Systematic effects of study center or year of ascertainment on admission BMI and age had not been detected (Engelhardt et al., 2020).
2.1 Statistical analyses
For our first hypothesis, we used a regression model with weight loss (kg) as dependent variable and age, body height at admission, premorbid weight in kg, illness duration (ln), and diagnosis (AN vs. atypical AN) as independent variables (principal model; model 1). Given the identified positive correlation between admission age and BMI and the negative correlation between admission age and BMI-SDS in adolescents with AN (Engelhardt et al., 2020), we calculated two additional models upon use of premorbid BMI (model 2) and BMI-SDS (model 3) as independent variables. Thus, models addressed weight adjusted for height (model 1), weight standardized for height (kg/m2; model 2) and BMI standardized to a sex- and age-related reference population (model 3). For age as independent variable, we sequentially tested admission age and age at initial weight loss in all three models and compared the respective results to assess if the two age variables can be used interchangeably. We also tested all three models upon additional inclusion of the independent variable ‘first versus re-hospitalization’.
For the second hypothesis, we calculated a t-statistic to test premorbid BMI-SDS against a test value = 0 for each patient group, thereby assessing the deviance in premorbid BMI from the population based BMI distribution for each diagnosis. On a descriptive basis, we categorized premorbid BMI of patients into BMI-centiles and cross-tabulated in relation to diagnosis.
Non-parametric Spearman's correlation coefficients were used to compare admission and premorbid weight and BMI (or BMI-SDS), age at initial weight loss or admission age, weight loss in kg or %, height (or height-SDS), and illness duration in both disorders. We labeled the strength of an association in accordance to recommendations of British Medical Journal (https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression; absolute values of r .0–.19 very weak; .2–.39 weak; .40–.59 moderate; .6–.79 strong, .8–.1 very strong). We additionally calculated descriptive statistics for the subgroup of patients hospitalized for the first time and compared these with patients who were re-hospitalized. We calculated a t-statistic to compare descriptive data; a χ2-test was performed to compare categorical measurements between AN and atypical AN. Exact two-sided significances were calculated with alpha = .05. We did not correct for multiple testing. Analyses were performed using IBM® SPSS® Statistics 29.0.0 for Windows.
3 RESULTS
Descriptive data for the total sample, AN only, and atypical AN only are shown in Table 1. The lower means for weight related admission parameters (body weight, BMI, BMI-SDS, BMI-centile) in AN are explained by use of the 5th BMI-centile as weight-cutoff. Means of all premorbid weight parameters were also lower in patients with AN. Means of weight loss parameters in kg, kg/m2,and % were similar between both patient groups with all patients losing an average of approximately 12.5 kg and 20% of their premorbid weight. Mean admission age and mean age at onset of initial weight loss differed by almost 12 months, with patients with atypical AN being younger. Mean illness duration was approximately 10 weeks longer in patients with AN. Slightly over 70% of all patients were hospitalized for the first time, the frequency being 13.4% higher in patients with atypical AN. Illness duration and admission age were significantly longer and higher, respectively, in re-hospitalized patients, whereas admission BMI-SDS was lower in this subgroup (Table S1).
| All patients (n = 460) | Anorexia nervosa (n = 411) | Atypical anorexia nervosa (n = 49) | p-valuea | Test-statisticsb | |
|---|---|---|---|---|---|
| Age at initial weight loss | 14.2 (1.5) [9.8;18.6] | 14.3 (1.50) [9.8;18.6] | 13.5 (1.23) [10.9;16.7] | 1 × 10−4 | t(df adj. = 66.4) = 4.15 |
| Admission age | 15.2 (1.61) [10.8;19.0] | 15.3 (1.61) [10.8;18.9] | 14.4 (1.31) [14.7;20.7] | 9 × 10−6 | t(df adj. = 66.6) = 4.82 |
| Admission weight (kg) | 41.2 (5.69) [21.7;57] | 40.7 (5.46) [21.7;54.7] | 46.0 (5.47) [33.6;57.0] | n.a. | |
| Premorbid weight (kg) | 53.6 (8.98) [28.0;83.0] | 53.0 (8.87) [28.0;83.0] | 58.6 (8.39) [45.0;80.0] | 3 × 10−5 | t(df = 458) = −4.21 |
| Admission BMI (kg/m2) | 15.3 (1.43) [10.9;20.7] | 15.0 (1.24) [10.9;17.9] | 17.3 (1.17) [14.7;20.5] | n.a. | |
| Premorbid BMI (kg/m2) | 19.8 (2.65) [12.9;30.1] | 19.5 (2.55) [12.9;30.1] | 22.1 (2.42) [16.9;27.6] | 9 × 10−11 | t(df = 458) = −6.64 |
| Admission BMI-SDS | −3.0 (1.18) [−7.7;−.1] | −3.2 (1.07) [−7.7;−1.7] | −1.3 (.37) [−1.6;−.1] | n.a. | |
| Premorbid BMI-SDS | −0.3 (.86) [−4.0;1.9] | −0.4 (.8) [−4.0;1.9] | 0.5 (.64) [−1.2;1.6] | 2 × 10−13 | t(df = 458) = −7.61 |
| Admission BMI-for-age centile | 1.9 (4.66) [.0;47.1] | 0.69 (2.08) [0;4.8] | 11.74 (9.22) [5.3;47.1] | n.a. | |
| Premorbid BMI-for-age centile | 40.9 (25.35) [0;96.8] | 37.8 (23.97) [0;96.8] | 67.6 (20.59) [11.8;94.7] | 8 × 10−16 | t(df = 458) = −8.35 |
| Body height at admission | 164 (7.5) [137;183] | 164 (7.6) [137;183] | 163 (7.2) [150;181] | .159 | t(df = 458) = 1.41 |
| Illness duration (weeks)c | 52.9 (46.24) [5.3;270.9] | 54.0 (47.14) [5.3;270.9] | 44.3 (37.21) [5.9;149.0] | .042 | t(df = 458) = 2.04 |
| Weight loss (BMI) | 4.6 (2.25) [0.1;16.8] | 4.5 (2.23) [0.2;16.8] | 4.8 (2.40) [0.1;9.6] | .497 | t(df = 458) = −.68 |
| Weight loss (kg) | 12.3 (6.19) [0.3;45.8] | 12.3 (6.17) [0.4; 45.8] | 12.6 (6.4) [0.3;26.7] | .757 | t(df = 458) = −.31 |
| Weight loss in % of premorbid weight in kg | 22.2 (8.45) [0.6;55.9] | 22.4 (8.34) [1.4; 55.9] | 20.8 (9.21) [0.6;36.1] | .208 | t(df = 458) = 1.26 |
| First inpatient treatment (n, %) | 330 (71.7) | 289 (70.3) | 41 (83.7) | .034 | χ2(df = 1) = 4.45 |
- a t-test or χ2-test for difference between patients with anorexia nervosa vs. atypical anorexia nervosa.
- b If Levene test for variance homogeneity resulted in p < .05, adjusted degree of freedom (df adj.) is reported.
- c For t-test illness duration transformed to ln-logarithm.
3.1 Substantiation of first hypothesis
The regression model with the dependent variable weight loss (kg) and the independent variables admission age, admission height, premorbid weight, illness duration (ln), and eating disorder diagnosis (AN vs. atypical AN) revealed a 5.58 kg higher weight loss for patients with AN vs. atypical AN (standardized beta = −0.28, p < .001, R2 = .80 for model; Table 2). The estimation of the effect of AN versus atypical AN on weight loss using premorbid BMI (model 2) as co-variable (instead of premorbid weight and height) revealed a similar result (b = −5.76 kg, beta = −0.29, p < .001; R2 = .78 for model). Upon use of premorbid BMI-SDS (model 3), the difference in weight loss between AN and atypical AN decreased (b = −4.78 kg, beta = −0.24, p < .001). The variance explained by this model was also lower (R2 = .68; Table 2). Illness duration (ln) was a significant predictor in model 3 only.
| b | BCa 95% CI [lower;upper] | p-value | Standardized beta | |
|---|---|---|---|---|
| Model 1 including premorbid weight and admission height as independent variables | ||||
| Intercept | 50.40 | [43.91;56.75] | <.001 | |
| Admission age | −0.59 | [−0.79;−0.39] | <.001 | −0.15 |
| Premorbid weight | 0.83 | [0.78;0.88] | <.001 | 1.21 |
| Admission body height | −0.42 | [−0.47;−0.36] | <.001 | −0.51 |
| Illness duration (ln) | 0.19 | [−0.18;0.60] | .336 | 0.03 |
| Diagnosis AN vs. atypical AN | −5.58 | [−6.40;−4.73] | <.001 | −0.28 |
| R2 = .80 | ||||
| Model 2 including premorbid BMI as independent variable | ||||
| Intercept | −19.20 | [−22.84;−15.96] | <.001 | |
| Admission age | −0.43 | [−0.62;−0.20] | <.001 | −0.11 |
| Premorbid BMI | 2.22 | [2.07;2.36] | <.001 | 0.95 |
| Illness duration (ln) | 0.12 | [−0.29;0.47] | .543 | 0.02 |
| Diagnosis AN vs. atypical AN | −5.76 | [−6.79;−4.66] | <.001 | −0.29 |
| R2 = .78 | ||||
| Model 3 including premorbid BMI-SDS as independent variable | ||||
| Intercept | 8.54 | [5.05;11.92] | <.001 | |
| Admission age | 0.96 | [0.73;1.20] | <.001 | 0.25 |
| Premorbid BMI-SDS | 6.19 | [5.57;6.93] | <.001 | 0.86 |
| Illness duration (ln) | −0.97 | [−1.51;−0.44] | <.001 | −0.13 |
| Diagnosis AN vs. atypical AN | −4.78 | [−5.89;−3.57] | <.001 | −0.24 |
| R2 = .68 | ||||
- Note: The three models include three different measurements for premorbid weight as independent variable in addition to diagnosis AN vs. atypical AN, admission age and illness duration (ln). Diagnosis for AN is coded as 0, diagnosis for atypical AN is coded as 1. p-value with BCa 95% CI.
- Abbreviations: b, non-standardized beta estimator; BCa 95% CI, 95% confidence intervals calculated with the bias-corrected and accelerated (BCa) bootstrap method.
The additional inclusion of the independent variable ‘first vs. re-hospitalization’ had a negligible effect in all three models; the variable itself was not a significant predictor (data not shown). Replacing the independent variable admission age with age at initial weight loss yielded similar results in terms of R2 (model 1: R2 = .80, model 2: R2 = .78, model 3: R2 = .69) and the respective regression coefficients for independent variables and their significance estimators (data not shown). Because admission age was a significant predictor in all three models, we visualized the relationships between weight loss in kg and premorbid BMI and admission BMI, respectively, and categorized admission age in patients with AN (Figures S1 and S2). The youngest age category (admission age ≤ 15 years) accounted for most of the patients with low premorbid (see BMI < 16 kg/m2) and admission BMI (BMI < 13 kg/m2).
3.2 Substantiation of second hypothesis
Mean premorbid BMI-SDS of patients with both AN (−0.41) and atypical AN (+0.52) deviated significantly from the expected value of 0 for a normal distribution (AN: t(df = 410) = −10.1; p = 2 × 10−21; atypical AN: t(df = 48) = 5.7; p = 7 × 10−7) and by 0.93 from one another. Patients with atypical AN and AN were overrepresented in the upper and lower BMI deciles, respectively (Table 3). Only 123 (30%) patients with AN had a premorbid BMI-decile ≥50, only 8 (16%) patients with atypical AN had a premorbid BMI-decile <50.
| Premorbid BMI-decile-for-age | Total n | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | ≥90 | ||
| AN | 57 13.9 |
62 15.1 |
51 12.4 |
65 15.8 |
53 12.9 |
41 10.0 |
33 8.0 |
25 6.1 |
15 3.6 |
9 2.2 |
411 |
| Atypical AN | 0 0 |
3 6.1 |
1 2.0 |
0 0 |
4 8.2 |
7 14.3 |
7 14.3 |
12 24.5 |
9 18.4 |
6 12.2 |
49 |
3.3 Exploratory analyses
Weight loss in kg (%) ranged from 0.4 (1.4%) to 45.8 kg (55.9%) in patients with AN and from 0.3 (0.6%) to 26.7 kg (36.1%) in patients with atypical AN (Table 1). Irrespective of the diagnosis, all patients with a weight loss <5% had low premorbid BMI (<16.5 and <19 kg/m2) for patients with AN and atypical AN (Figure 2). In contrast, high weight loss exceeding 38% of premorbid body weight occurred only in patients with AN (n = 9; 2%), who all had a premorbid BMI-centile ≥75 (Figures 1 and 2). If a relative weight loss ≥10% quantitatively suffices to meet the DSM-5 criterion of ‘significant weight loss’ in atypical AN, 43 out of the 49 patients fulfilled this criterion (Figures 1 and 2).
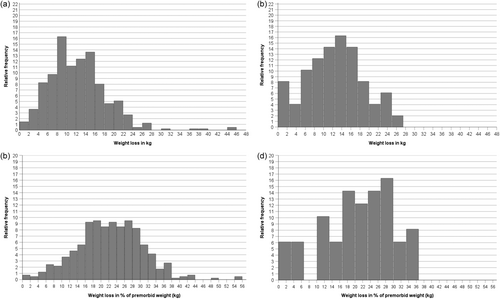
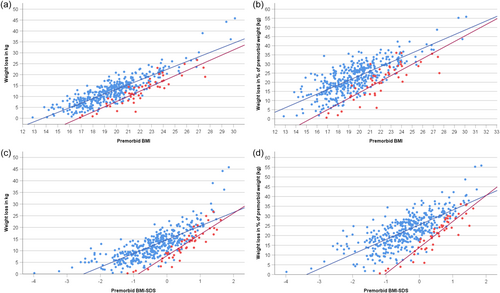
In both eating disorders, the correlations between weight loss in kg or % with premorbid BMI (or BMI-SDS) were all strong to very strong (range: rho = .73 to .92; Table 4; for an extended correlation matrix see Table S2). Scatterplots (Figure 2) depicting weight loss in kg (or %) in relationship to premorbid BMI (or BMI-SDS) graphically illustrate the prediction of high weight loss in kg (or %) by a high premorbid BMI (or BMI-SDS); in accordance with hypothesis 1, the figures also illustrate the clustering of patients with atypical AN in the lower weight loss range.
| Premorbid BMI | Premorbid BMI-SDS | Admission BMI | Admission BMI-SDS | Weight loss in kg | Weight loss in % of premorbid weight (kg) | Age at initial weight loss | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AN | Atypical AN | AN | Atypical AN | AN | Atypical AN | AN | Atypical AN | AN | Atypical AN | AN | Atypical AN | AN | Atypical AN | |
| Premorbid BMI-SDS | .90 | .90 | 1 | 1 | ||||||||||
| 1 × 10−149 | 6 × 10−19 | |||||||||||||
| Admission BMI | .50 | .20 | .37 | −.10 | 1 | 1 | ||||||||
| 3 × 10−27 | .155 | 2 × 10−14 | .501 | |||||||||||
| Admission BMI-SDS | .25 | .06 | .38 | −.01 | .71 | .58 | 1 | 1 | ||||||
| 2 × 10−7 | .698 | 3 × 10−15 | .953 | 2 × 10−64 | 1 × 10−5 | |||||||||
| Weight loss in kg | .84 | .89 | .80 | .92 | .02 | −.17 | −.13 | −.20 | 1 | 1 | ||||
| 8 × 10−109 | 2 × 10−17 | 5 × 10−89 | 2 × 10−20 | .638 | .253 | .006 | .164 | |||||||
| Weight loss in % of premorbid weight (kg) | .73 | .82 | .74 | .92 | −.17 | −.33 | −.29 | −.26 | .96 | .96 | 1 | 1 | ||
| 3 × 10−69 | 1 × 10−12 | 2 × 10−72 | 6 × 10−21 | .001 | .021 | 3 × 10−6 | .073 | 2 × 10−221 | 5 × 10−28 | |||||
| Age at initial weight loss | .36 | .16 | −.03 | −.20 | .36 | .72 | −.25 | .26 | .24 | −.06 | .10 | −.21 | 1 | 1 |
| 6 × 10−14 | .261 | .599 | .171 | 9 × 10–14 | 6 × 10−9 | 4 × 10−7 | .074 | 2 × 10−6 | .706 | .036 | .146 | |||
| Admission age | .33 | .14 | .02 | .20 | .35 | .79 | −.34 | .08 | .21 | −.11 | .09 | −.26 | .82 | .77 |
| 6 × 10−12 | .356 | .698 | .195 | 7 × 10−13 | 2 × 10−11 | 2 × 10−12 | .084 | 2 × 10−5 | .455 | .087 | .070 | 6 × 10−101 | 1 × 10−10 | |
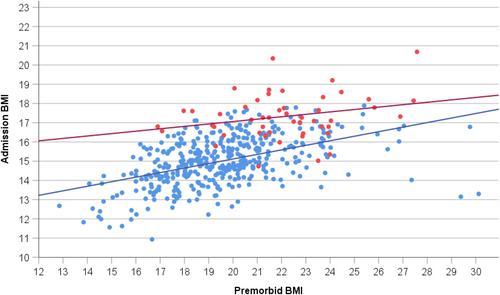
In contrast, correlations between weight loss in kg or % and admission BMI (or BMI-SDS) ranged from approximately zero to weak and negative (range: rho = .02 to −.33). Premorbid and admission BMI (or BMI-SDS) were moderately or weakly correlated only in AN (Table 4; Figure 3). Body height was weakly correlated with both admission age and age at initial weight loss in both AN and atypical AN; height was very weakly correlated with both premorbid and admission BMI in AN only (Table S2).
4 DISCUSSION
Findings from our study substantiate our hypothesis that differentiating AN and atypical AN with the 5th BMI-centile induces disproportionate weight characteristics in AN and atypical AN. First, our principal regression model (model 1) demonstrated a 5.6 kg lower mean weight loss in patients with atypical AN compared to patients with AN upon controlling for age, height, illness duration, and premorbid weight. Further, for any given premorbid BMI, mean weight loss in atypical AN clustered below the mean weight loss in AN. Second, premorbid BMI distributions in AN versus atypical AN were disproportionate, with an overrepresentation of high and low premorbid BMIs in atypical AN and AN, respectively. Our second hypothesis was conditional on the similar mean weight loss (unadjusted) in samples of patients with AN and atypical AN (Garber et al., 2019; Matthews et al., 2024; Peebles et al., 2010; Whitelaw et al., 2014), which we, too, confirmed (Table 1). Because patients with atypical AN have an admission BMI ≥5th centile, high premorbid BMIs are overrepresented, when similar weight loss is demonstrated in both disorders.
Our findings suggest that the preponderance of premorbid overweight/obesity and higher premorbid BMI in patients with atypical AN (Walsh et al., 2023) is related to the diagnostic weight cutoff. Conversely, the epidemiological associations (Leth-Moller et al., 2023; Stice et al., 2017, 2022; Yilmaz et al., 2019) and genetic correlations (Watson et al., 2019) between a low premorbid BMI and AN may partially, if not completely, result from the diagnostic requirement of a ‘significantly low body weight’ in AN (Leth-Moller et al., 2023). In the second part of our study, exploratory analyses compared AN and atypical AN in terms of weight loss, anthropometric variables, age, and illness duration. Herein, considering these findings, we discuss the clinical validity of the DSM-5 diagnostic weight cut-off to differentiate AN and atypical AN.
4.1 Weight loss
Among all patients in our sample, those who lost less than 5% of their premorbid weight had the lowest premorbid BMIs. Further, patients with atypical AN were a mean of 0.8 years younger than patients with AN and in AN, being under age 15 was associated with a lower premorbid BMI (Figure S1). These findings suggest that, being younger and having a lower premorbid BMI are associated with a low magnitude of weight loss in both AN and atypical AN. A weight loss of only 5% has previously been shown to be associated with eating disorder pathology and distress in atypical AN (Forney et al., 2017). Thus, we propose that necessitating ‘significant weight loss’ in atypical AN yields unjustified diagnostic fragmentation, resulting in two subgroups of similar patients with admission BMI ≥5th centile with and without “significant’ weight loss. Given our small sample size of patients with atypical AN (10.7% of our sample), coupled with a low prevalence of patients with atypical AN and a presenting BMI-centile ≥10 (3% of our sample), further research is warranted to assess the distribution of premorbid overweight/obesity in AN versus atypical AN.
4.2 Admission age
Surprisingly, mean admission age differed between AN and atypical AN. In combination with the inherently lower mean weight loss (upon adjustment for age, height, illness duration and premorbid weight), the shorter illness duration and the higher rate of first hospitalizations, we believe that a subset of patients with atypical AN will lose further weight in the future, ultimately converting to an AN diagnosis upon dropping below the 5th BMI-centile. This is especially likely in patients with admission BMIs slightly above the 5th BMI-centile (Figure 2). Because all three regression models revealed an effect of age on weight loss (even upon use of the independent variable premorbid BMI-SDS), the detected age difference between patients with AN and atypical AN may also reflect complex, developmental associations between premorbid weight, weight loss, and admission BMI. The joint occurrence of low admission BMI and low weight loss was predominantly demonstrated in our registry's youngest patients with AN (Figure S2). Similarly, premorbid BMI ≤16 kg/m2 only occurred in our registry's youngest patients with AN, aged ≤15 years, with three exceptions (Figure S1). The association between younger age and lower premorbid BMI is explained by the BMI increments common to boys and girls during childhood and adolescence (see, e.g. Rosario et al., 2010). As delineated in the introduction, mean percent body fat is lower in pre- versus post-pubertal girls (Engelhardt et al., 2020), which may potentially limit the amount of weight loss, in particular among those with the premorbid lowest percent body fat.
Assessing admission BMI in adolescent patients with AN is additionally complex, given the positive correlation between age and admission BMI and the negative correlation between age and admission BMI-SDS (Engelhardt et al., 2020). In our much smaller sample of patients with atypical AN, a large difference in the correlations between age and BMI and BMI-SDS, respectively, was evident. Accordingly, the larger difference in rho-values applied to both eating disorders. Overall, our data highlight the importance of accounting for age in adolescents with an AN-like phenotype. Our finding of a younger mean age in atypical AN requires confirmation.
4.3 Premorbid BMI, but not illness duration, predicts weight loss
Patients with a low weight loss clustered in the low premorbid BMI range, and vice versa, patients with a high weight loss clustered in the high premorbid BMI range irrespective of diagnosis (Figure 2). Weight loss in kg was very strongly correlated with premorbid BMI in both AN and atypical AN; the respective correlations were higher than those based on weight loss in % or premorbid-BMI-SDS. Our three regression models explained a substantial proportion (68%–80%) of the variance in weight loss in kg, mainly due to the strong effect of premorbid weight. Because models 1 and 2 resulted in a somewhat higher proportion of explained variance than model 3, we suggest that the major determinant of weight loss is absolute premorbid body weight adjusted or standardized for body height.
The positive correlation between premorbid BMI and weight loss in kg in AN would seem to intuitively reflect the weight cut-off (Coners et al., 1999): patients with a high premorbid BMI must lose more weight to surpass the weight cut-off than patients with a lower premorbid BMI. However, the consistent strong correlation between premorbid BMI and weight loss in atypical AN cannot be similarly explained. Based on these strong correlations in both disorders, we postulate an underlying regulatory mechanism that accounts for the substantial prediction of weight loss by premorbid BMI (see below).
Similar to previous results with respect to admission BMI (Coners et al., 1999; Föcker et al., 2015; Peters, Kolar, et al., 2021), illness duration was an overall negligible variable in explaining weight loss. Since maximal and minimal weight loss ranged between 10 and 15 kg for any given premorbid BMI - patients with AN and atypical AN being at the upper and lower ends of this range (see Figure 2)—illness duration may vary considerably for patients with the same premorbid BMI. Notably, our simplistic definition of illness duration implying that the illness directly and consistently begins with initial weight loss, represents an oversimplification of the complex onset of AN or atypical AN.
4.4 Diagnostic separation of AN and atypical AN
Overall, our exploratory correlation matrix for weight-related variables revealed no major differences between AN and atypical AN (see similar rho-values in Tables 4 and S2). Patients with atypical AN represent those who develop the eating disorder following less weight loss upon adjustment for age, height, illness duration, and premorbid weight. In combination with the lack of empirical support for clinically relevant differences in psychological and medical characteristics between diagnoses (Billman Miller et al., 2024; Brennan et al., 2023; Johnson-Munguia et al., 2024; Walsh et al., 2023), we interpret our findings as further support for a homogenous illness model. We believe this interpretation represents the most parsimonious explanation for currently available data.
4.5 Usefulness of premorbid weight for weight-based diagnostic categorization
Prior studies have recommended a higher admission BMI-centile to diagnose AN, allowing inclusion of a larger proportion of patients with an underlying AN-like phenotype (Hebebrand & Bulik, 2011). However, empirical support for any admission weight-related diagnostic threshold is lacking. Any cutoff will inherently create a weight-based diagnostic ‘split,’ with one disorder associated with lower and the other associated with higher weight loss in patients matched for age, sex, height, and premorbid weight. The weak prediction of weight prognosis by admission BMI (Hebebrand et al., 1997) may in part reflect the low to moderate correlation between premorbid and admission BMI; weight suppression clearly predicts short and long-term body weight in both healthy subjects and patients with eating disorders (Lowe et al., 2018). Studies in both adolescents and adults with AN indicate that premorbid weight status and weight suppression can be used to clinically predict other relevant psychological and medical variables (Whitelaw et al., 2018). In conclusion, given that premorbid, but not admission BMI strongly predicts weight loss, shifting to use of premorbid BMI and/or weight loss, instead of admission BMI, for clinically relevant subcategorization of patients with an underlying AN-like phenotype appears promising.
4.6 Mechanisms underlying prediction of weight loss by premorbid BMI
We, in accordance with Guisinger (2003) argue that weight loss, in itself, induces the development of an AN-like phenotype or “entrapment,” characterized as the persistent difficulty to overcome preoccupation with food and body weight, fear of eating high calorie foods, weight phobia, and/or the inability to gain weight, despite the occurrence of psychological and somatic consequences of starvation. Entrapment is evidently ‘triggered’ by less weight loss (when adjusted for premorbid BMI) in atypical AN than in AN. ‘Significant weight loss’ is not a necessary requirement; only patients with premorbid overweight or obesity must consistently lose significant weight in order to trigger entrapment.
An etiological concept for the development of an AN-like phenotype would need to explain why (i) entrapment sets in with less weight loss in patients with premorbid leanness versus premorbid overweight/obesity, (ii) the amount of weight loss entailing entrapment shows a rather large range for any given premorbid BMI (10–15 kg according to our data; see Figure 2), (iii) the amount of weight loss for inducing entrapment is below 5% in a subgroup of (mostly young) patients with a low premorbid BMI, (iv) illness duration (defined as the time span between age at initial weight loss and admission age) plays a negligible role in entrapment and (v) age (to a slight extent) co-determines both weight loss and admission BMI. The mean age of onset in restrictive eating disorders—between late childhood and early adulthood—and the greater prevalence among females also require explanation.
The attainment of an absolute or relative deficiency of the circulating level of the adipokine leptin has been hypothesized to trigger entrapment (Hebebrand et al., 2024). In brief, only the free leptin index is relevant for its biological action; a proportion of circulating leptin is bound to the soluble leptin receptor (Hebebrand et al., 2024). Circulating leptin levels are positively correlated with BMI and more so with percent fat mass (Considine et al., 1996). Inter-individual variation of serum leptin levels adjusted for percent body fat increases substantially with percent body fat (Considine et al., 1996). Patients may also differ in the ratio of fat mass to fat free mass lost per kg of body weight. As for sex dependent and developmental aspects, mean percent body fat increases substantially during puberty and adolescence in females only (Loomba-Albrecht & Styne, 2009). Circulating leptin, too, increases throughout all Tanner stages in females only, resulting in two to three time higher serum leptin levels in comparison to adult males (e.g., Ahmed et al., 1999; Blum et al., 1997).
Together, these factors could explain why some patients become entrapped early on during weight loss, whereas in others ‘significant weight loss’ is indeed required. Upon mere consideration of inter-individual variation in percent body fat and circulating leptin level adjusted for fat mass, the greatest degree of weight loss (and concomitant loss of fat mass) would be required in patients with a high premorbid percent body fat and high circulating leptin levels adjusted for percent body weight for their levels to drop into the subnormal range, explaining why ‘significant weight loss’ is indeed a prerequisite for development of entrapment in patients with premorbid overweight/obesity. In contrast, already a minor loss of fat mass would suffice to induce entrapment in those lean patients with both a low premorbid fat mass and a low leptin level adjusted for fat mass. Because BMI is a poor predictor of fat mass in lean females (Goulding et al., 1996), particularly percent body fat may explain variance in the amount of weight loss required for the development of hypoleptinemia in this subgroup. Young female patients, in particular, would be predisposed to low weight loss induced entrapment because of their lower mean premorbid percent body fat and their developmentally low leptin levels. The hypothesis that leptin plays a substantial role in entrapment has been supported by a Mendelian randomization study linking a low leptin level to an increased risk for development of AN (Peters, Antel, et al., 2021). Further, recent case reports of off-label treatment with recombinant human leptin (metreleptin) have entailed clinical improvements and the lessening of entrapment in patients with both AN (Antel et al., 2021; Gradl-Dietsch et al., 2023; Hebebrand, Antel, von Piechowski, et al., 2023; Milos et al., 2020) and atypical AN (Hebebrand, Antel, & Peters, 2023).
4.7 Limitations
We refrained from correcting the p-values for multiple testing. All tests would clearly remain significant upon a correction for five tests (three regression models for the first hypothesis and two t-tests for the second hypothesis (Bonferroni-corrected α = .01)). We used registry-based data and acknowledge inherent limitations including missing or unavailable data and no assessment of reliability data entry and of diagnoses (Thygesen & Ersboll, 2014). Roughly 30% of our patients had received previous inpatient treatment. Not expectedly, re-hospitalized patients differed from those hospitalized for the first time in mean age, illness duration and admission BMI-SDS (Table S1). Importantly, the independent variable ‘first versus re-hospitalization’ in our regression models did not contribute to explaining weight loss. Whereas we consider our registry based AN sample as largely representative of adolescent German inpatients with an admission BMI-centile <5, this is not the case for our much smaller sample of patients with atypical AN. Matthews and coworkers confirmed our results in females and (preliminarily) in males based on clinically ascertained adolescent inpatients with both AN and atypical AN upon first admission (Matthews et al., 2024). Research is warranted to see if results generalize to adolescent outpatients and adults, too.
Notably, in our study, premorbid body weight status was defined via a single estimation of premorbid BMI upon initial weight loss. We nevertheless regard the existent clinical, epidemiological, and genetic data as indirect support in light of short- and medium-term tracking of age-adjusted BMI in children and adolescents (e.g., Marshall et al., 2020). Matthews et al. (2024) report a high correlation between estimated premorbid BMI based on recalled weight at initial onset of weight loss and estimated premorbid BMI based on historical growth data, measured and recorded by providers prior to illness development.
Our use of measured height for calculation of premorbid BMI inherently entails a too low BMI in all patients who experienced an increase in height between the onset of weight loss and admission (see Coners et al., 1999). Because of the younger age of patients with atypical AN, premorbid BMI for this group could be more strongly underestimated; on average, 15-year old German females gain only 2 cm until attainment of adult height (Reinken & van Oost, 1992). On the other hand, overweight entails a temporary increase in height gain in childhood, which is compensated by an earlier pubertal maturity and a subnormal height gain in adolescence (He & Karlberg, 2001). Another factor to consider is a decreased height trajectory in children with AN aged <14 years due to the eating disorder itself (Ayrolles et al., 2023).
We deem it unlikely that both patient groups differ systematically in terms of recalled weight, recalled age at onset of weight loss, and estimated premorbid BMI-centile to an extent that would account for the observed differences. If the correlations reflect underlying regulatory mechanisms, we argue that correlations and explained variances would be higher if measured data were available. We cannot exclude measurement errors (admission weight and height) and incorrect data entry. We did not detect any totally implausible patient data; even the patients with little weight loss fit into the overall pattern (see Figures 2 and 3). Data on race and ethnicity of the patients treated in Germany were not collected.
We realize that the correlation matrix includes inter-correlated phenotypes. Because of our limited knowledge of the variables that best predict clinical parameters, we did not narrow down our choice of variables. We urge other groups to investigate if premorbid BMI is indeed a better predictor of weight loss than premorbid BMI-SDS. The operationalization of premorbid weight status and weight loss requires further research to come up with both a simple and clinically validated (or set of) variable(s) in both adolescents and adults. Our results suggest that premorbid BMI may represent this variable in adolescents.
5 CONCLUSIONS
Our weight related data do not suggest major differences between AN and atypical AN, other than those induced by the DSM-5 weight cutoff. Our findings complement other studies that have not found major differences in psychological and somatic characteristics in AN and atypical AN (Billman Miller et al., 2023; Brennan et al., 2023; Johnson-Munguia et al., 2024; Walsh et al., 2023). The high correlation between premorbid BMI and weight loss suggests a regulatory mechanism underlying entrapment. Subtyping of AN and atypical AN according to premorbid BMI (and/or weight loss), rather than admission BMI, should prove clinically useful.
AUTHOR CONTRIBUTIONS
Johannes Hebebrand: Conceptualization; investigation; methodology; project administration; resources; supervision; writing – original draft; writing – review and editing. Jochen Seitz: Investigation; writing – review and editing. Manuel Föcker: Investigation; writing – review and editing. Hanna Preuss-van Viersen: Investigation; writing – review and editing. Michael Huss: Investigation; resources; writing – review and editing. Katharina Bühren: Investigation; writing – review and editing. Brigitte Dahmen: Investigation; writing – review and editing. Katja Becker: Investigation; resources; writing – review and editing. Linda Weber: Investigation; writing – review and editing. Christoph Correll: Investigation; methodology; resources; writing – review and editing. Charlotte Jaite: Investigation; writing – review and editing. Karin Maria Egberts: Investigation; writing – review and editing. Marcel Romanos: Investigation; resources; writing – review and editing. Stefan Ehrlich: Investigation; resources; writing – review and editing. Maria Seidel: Investigation; writing – review and editing. Veit Roessner: Investigation; resources; writing – review and editing. Christian Fleischhaker: Investigation; resources; writing – review and editing. Eva Möhler: Funding acquisition. Freia Hahn: Investigation; writing – review and editing. Michael Kaess: Investigation; resources; writing – review and editing. Tanja Marina Legenbauer: Investigation; resources; writing – review and editing. Daniela Hagmann: Writing – review and editing. Tobias Johannes Renner: Investigation; resources; writing – review and editing. Ulrike ME Schulze: Investigation; resources; writing – review and editing. Ulf Thiemann: Resources; writing – review and editing. Ida Wessing: Investigation; writing – review and editing. Gisela Antony: Data curation. Beate Herpertz-Dahlmann: Investigation; resources; writing – review and editing. Abigail Matthews: Writing – review and editing. Triinu Peters: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; supervision; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We thank patients and their parents for participation in our registry-based study. We thank three anonymous Reviewers and the handling Editor Tim Walsh for their patience, diligence, and extremely valuable comments. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The German Registry of Children and Adolescents with AN is funded by the participating centers.
CONFLICT OF INTEREST STATEMENT
Johannes Hebebrand has received speech honoraria from Novo Nordisk and Amryt Pharmaceuticals in the past 3 years. He is listed as an inventor in three patent applications of the University of Duisburg-Essen for the use of leptin analogues for treatment of anorexia nervosa and depression. CU Correll has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Tolmar, Vertex, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
The ethics committees of all participating universities (n = 14) and local research centers (n = 2) approved the registry study. A written informed assent/consent of both patient and at least one legal guardian was available.




