Morphological defects in a novel Rdh10 mutant that has reduced retinoic acid biosynthesis and signaling
Abstract
Retinoic acid (RA) signaling is necessary for proper patterning and morphogenesis during embryonic development. Tissue-specific RA signaling requires precise spatial and temporal synthesis of RA from retinal by retinaldehyde dehydrogenases (Raldh) and the conversion of retinol to retinal by retinol dehydrogenases (Rdh) of the short-chain dehydrogenase/reducatase gene family (SDR). The SDR, retinol dehydrogenase 10 (RDH10), is a major contributor to retinal biosynthesis during mid-gestation. We have identified a missense mutation in the Rdh10 gene (Rdh10m366Asp) using an N-ethyl-N-nitrosourea-induced forward genetic screen that result in reduced RA levels and signaling during embryonic development. Rdh10m366Asp mutant embryos have unique phenotypes, such as edema, a massive midline facial cleft, and neurogenesis defects in the forebrain, that will allow the identification of novel RA functions. genesis, 50: 415–423, 2012. © 2011 Wiley-Liss, Inc.
All-trans-retinoic acid (atRA) is a biologically active form of vitamin A, which acts through nuclear RA receptors (RAR) to support diverse aspects of vertebrate life, including embryonic development (Maden,2001; Mark et al.,2006). Retinol is converted to atRA in two distinct steps. The second step involves the oxidation of retinal into RA and is catalyzed by retinaldehyde dehydrogenases (Raldh), of which there are three family members expressed during embryogenesis (Raldh1-3; Napoli,2012). Raldh2 KO mice have been invaluable for uncovering the biological functions of RA during mouse embryo development, such as anterior–posterior axis formation, limb bud initiation, heart formation, craniofacial development, hindbrain patterning, and bilateral symmetry of somite formation. However, Raldh2 KO embryos die around E10, precluding analysis, and identification of the physiological significances of RA at later stages unless RA is provided exogenously (Niederreither et al.,2002).
Retinol dehydrogenases (Rdh) catalyze the first and rate-limiting reaction of atRA biosynthesis, that is, the oxidation of retinol to retinal, and are essential for controlling retinol homeostasis, atRA biogeneration, and vitamin A function (Napoli,1999). Because there are over 20 RDH family members (Lidén and Eriksson,2006), it was unclear whether any one family member would be identified as being essential for embryonic development. Recently, Sandell et al. (2007) identified Rdh10, a member of the short-chain dehydrogenase family (Napoli,2012), as having such an attribute. RDH10 protein primarily oxidizes all-trans retinol to all-trans retinal (Belyaeva et al.,2008; Farjo et al.,2009,2011; Takahashi et al.,2009; Wu et al.,2002). Embryos lacking functional RDH10 protein die around E13 and display limb, craniofacial, and internal organ abnormalities, thereby identifying Rdh10 as a critical enzyme for the synthesis of retinaldehyde during mid-gestation (Rdh10trex/trex: Sandell et al.,2007; Rdh10−/−: Rhinn et al.,2011). The Rdh10trex/trex mutant was generated in an ENU-induced forward genetic screen and carries a cysteine-to-arginine amino acid substitution in the first β-strand that renders the mutant protein unstable (Farjo et al.,2011; Sandell et al.,2007).
ENU is an efficient chemical mutagen that induces single nucleotide substitutions, thereby making it an ideal reagent for performing unbiased forward genetic screens in mice to study specific phenotypes with a corresponding genetic basis for morphological defects. We have reported implementation of a focused ENU-screen that generated multiple mouse mutants with defects in cortical development (Zarbalis et al.,2004). Here, we describe the genetic basis for one of these mutants (mouse mutant line 366) as a missense mutation in the Rdh10 gene (Rdh10m366Asp). The Rdh10m366Asp allele is a strong hypomorph that allows embryos to survive until embryonic day 17.5 (E17.5), providing a model to analyze RA functions throughout mid to late gestation.
RESULTS
Morphological Defects in Line 366 Mutants
To identify novel molecular regulators of cortical development, we carried out a focused ENU-induced forward genetic screen for recessive mutations that disrupt corticogenesis (Zarbalis et al.,2004). One of the mutant lines generated in this screen, line 366, dies around E17.5, although a few embryos were collected near the end of gestation (E18.5), and one live birth (P0) was observed. Numerous morphological defects are easily identifiable. The entire eye is smaller, ventral retina tissue is absent, and eyes are displaced medially (Fig. 1a,b,d,e). There is also a very prominent and consistent midline facial cleft (Fig. 1a–c), signs of which are first evident at E10.5 when the medial nasal prominences (MNP) fail to enlarge. Line 366 mutant embryos display signs of edema as early as E12.5, a condition that continues to worsen over time (Fig. 1f,g). Limb defects are largely restricted to the forelimbs, which are shorter and have missing digits. Remaining digits are frequently fused together (Fig. 1h). Mild interdigital webbing is evident in both fore- and hindlimbs (Fig. 1i). The forebrain has a dome-shaped appearance with a significantly shorter anterior–posterior axis and enlarged ventricles (Fig. 1a,b,j,k). The cerebral cortex is thin and elongated, the ganglionic eminences have a flattened appearance, and the olfactory bulbs are missing, all of which are consistent with defects in neurogenesis (Fig. 1j,k).
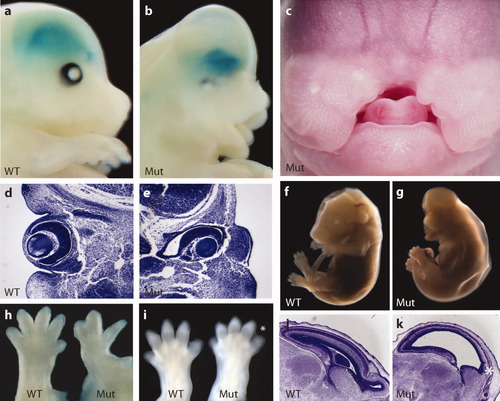
Morphological defects in ENU-induced mutant line 366 embryos. Wild-type (WT) (a) and mutant (Mut) (b) embryos at E14.5 expressing a Dlx-LacZ reporter (Zerucha et al.,2000) that was used in the mutagenesis screen [see Zarbalis et al. (2004)]. (c) Frontal view of a mutant embryo at (E14.5) displaying a very prominent midline facial cleft, while the lower jaw forms normally. (d, e) Transverse sections through the developing eyes at E12.5. (f, g) Whole embryos at E16.75. Note the inflated skin in the mutant that is characteristic of edema (g). (h) Left forelimbs from mutant compared to wild-type limb at E14.5 showing example of limb defects, which are variable. (i) Right hindlimb from mutant and wild-type limb showing mild interdigital webbing (asterisk). (j, k) Sagittal section through the brain at E16.5. Asterisks mark the position of the absent olfactory bulbs in mutant (k).
Cloning of the Line 366 Mutation
We mapped the line 366 mutation to a 100 MB interval on chromosome 1 (D1Mit296-D1Mit169; Zarbalis et al.,2004). Fine mapping identified a missense mutation (C to T) in the Rdh10 gene (Rdh10m366Asp) that causes an alanine-to-valine substitution at amino acid residue 196 in the substrate-binding pocket (A196V; Fig. 2a). Alanine196 is highly conserved, as are the flanking 14–15 amino acids on either side. Deviations in flanking amino acids only occur in zebrafish and Xenopus Rdh10 homologs (Fig. 2b).
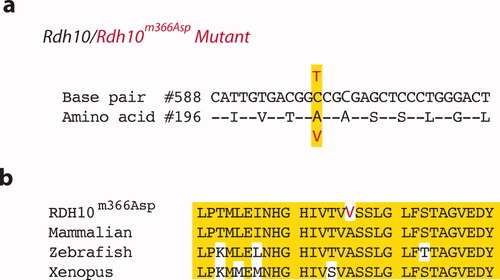
Mutant line 366 carries a mutation in the Rdh10 gene. (a) Base-pair sequence and corresponding amino acid sequence of a region of the Rdh10 gene and RDH10 protein, respectively. Highlighted are the mutated nucleotide and corresponding amino acid. The alanine-to-valine missense mutation is a conservative change, with valine having a pair of methyl groups replacing two hydrogen atoms on the amino acid side chain of the alanine residue. (b) Amino acid #196 of the RDH10 protein is highly conserved amino acid residue. Also highly conserved are the residues surrounding Alanine196. In the segment of RDH10 protein depicted, there is 100% conservation among humans, bovine, rat, and mouse (summarized as mammalian). Some divergence begins to appear in amino acids nearby Alanine196 in zebrafish and Xenopus.
Characterization of the RDH10m366Asp Mutation on RA Biosynthesis In Vitro and In Vivo
Genetic alterations that result in either RA depletion or RA excess have very similar phenotypes (McCaffery et al.,2003); thus, we sought to determine how the RDH10m366Asp mutation affects RA biosynthesis in vitro and in vivo. To do this, wild-type RDH10 or RDH10m366Asp cDNA constructs were co-transfected with retinaldehyde dehydrogenase 2 (RALDH2) into CHO cells. Cell extracts were assayed for atRA production 1 h after the addition of the substrate all-trans-retinol. RDH10m366Asp protein produced fivefold less atRA compared to wild-type RDH10 (Fig. 3a).
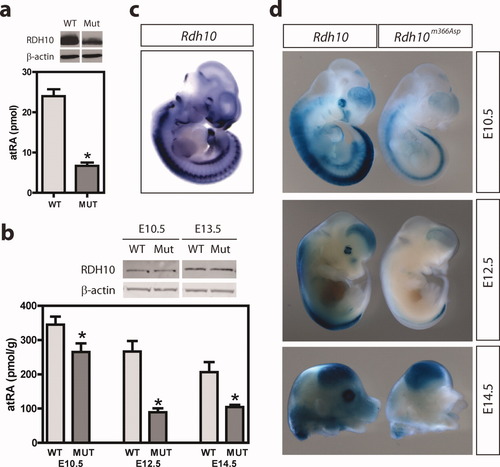
The Rdh10m366Asp mutation reduces RA biosynthesis. (a) On top is a representative western blot of N-terminal-FLAG-tagged RDH10 and N-terminal-FLAG-tagged mutant RDH10m366Asp after transfection of CHO cells. Mutant protein levels were consistently 40% less than wild-type, presumably because of either reduced translation and/or stability. At bottom, CHO cells co-transfected with FLAG-tag WT-RDH10 or FLAG-tagged Mut-RDH10m366Asp and RALDH2 biosynthesize atRA. Retinol was incubated with transfected cells for 1 h (n = 3 wells assayed independently). Background atRA values produced by cells transfected with empty vectors and RALDH2 were subtracted to provide net picomole RA. Net picomole RA values for RDH10m366Asp mutant and wild-type RDH10 proteins were normalized to differences in protein levels. A significant difference between wild-type RDH10 and mutant RDH10m366Asp was found, *P = 0.008. Comparable data were reproduced at least three times under identical conditions. (b) On top is a representative western blot of RDH10 protein from whole wild-type or Rdh10m366Asp embryos at E10.5 and E13.5. The RDH10 protein migrates at ∼ 39 kDa, as expected. Unlike our in vitro studies, we did not detect any appreciable difference in RDH10 protein levels from whole embryos as a result of the A196V mutation. At bottom, RA levels in whole wild-type and Rdh10m366Asp embryos at three embryonic stages expressed as pmol/g protein ± SEM. Because morphological defects in Rdh10m366Asp mutants are first apparent after E10.5, as indicated by hypoplasia of the MNPs, we wanted to assay for differences in RA levels just before seeing these initial defects. We also examined subsequent stages (E12.5 and E14.5) to determine whether changes in RA levels persist or recover. Number of embryos analyzed at each embryonic age (wild-type/mutant): E10.5, 11/13; E12.5, 6/4; E14.5, 5/5; *P < 0.04. (c) Rdh10 expression at E10.5. Note how closely the expression of Rdh10 overlaps with endogenous RA signaling (d) at E10.5. (d) Sagittal view of whole embryos at E10.5 and E12.5, and E14.5 head, stained for β-galactosidase activity after crossing the Rdh10m366Asp mutant to a RA-reporter strain (Rossant et al.,1991). Reduced β-galactosidase staining is observed at all stages. See also Figure 4a,b for anterior view of head at E14.5 after β-galactosidase staining.
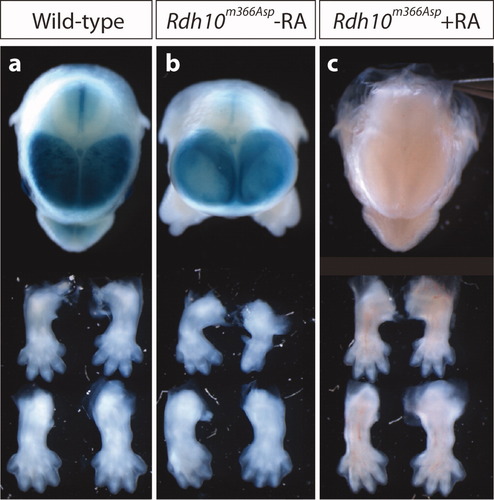
Rdh10m366Asp mutant phenotype is rescued by RA supplementation. (a) Top, anterior view of head shows complete formation of facial midline in wild-type embryos at E14.5. Bottom, forelimbs and hindlimbs. (b) Rdh10m366Asp embryos displaying face, brain, and limb phenotypes. (c) Rdh10m366Asp embryos fed a RA supplemented diet. The severe limb and craniofacial defects were rescued to such an extent that they were indistinguishable from wild-type embryos at E14.5. Limb outgrowth and digit patterning were morphologically normal. The midline facial cleft was nonevident as the snout formed normally. In addition, the eyes were the same size as wild-type embryos, and Rdh10m366Asp mutant embryos no longer had a domed forebrain region.
Our in vitro studies suggest that Rdh10m366Asp mutant embryos have reduced RA levels during development. To test this notion, two independent methods were used to assay for RA levels in vivo. First, RA levels in whole embryos were assayed using a sensitive LC (liquid chromatography)/MS (mass spectrometry)/MS assay (Kane et al.,2005). We found that atRA levels are ∼23, 66, and 50% reduced in mutant embryos compared to wild-type littermates at E10.5, E12.5, and E14.5, respectively (Fig. 3b). Our second approach was to cross Rdh10m366Asp mice to a mouse strain in which lacZ reporter gene expression is controlled by a RA response element (RARE-LacZ mice) to identify changes in bioactive RA in a tissue-specific manner (Rossant et al.,1991). At E10.5, E12.5, and E14.5, there were significant reductions in LacZ staining in Rdh10m366Asp embryos (Fig. 3d; also see Fig 4a,b) and at E10.5 changes in LacZ staining closely overlap with regions of Rdh10 expression (cf. Fig. 3c with Fig. 3d-E10.5). Collectively, these data indicate that the RDH10m366Asp protein has a reduced ability to synthesize all-trans-retinal from all-trans-retinol and that RDH10 has a crucial role in RA biosynthesis during mouse embryo development.
Exogenous RA Rescues Defects in Rdh10m366Asp Mutants
To verify that the morphological defects in developing Rdh10m366Asp mutant embryos result from depleted endogenous RA levels, we attempted to rescue the mutant phenotypes. Pregnant females were switched from a normal chow diet to one supplemented with atRA starting at E7.5 and maintained until E14.5. With RA supplementation, the limb, face, eye, limb, and forebrain defects were all rescued (Fig. 4). The only morphological feature distinguishing Rdh10m366Asp mutants from wild-type embryos was the presence of edema, which was not rescued by the method we used.
DISCUSSION
RDH10 is a primary regulator of the first enzymatic step in RA biosynthesis during embryogenesis despite earlier predictions that this initial rate-limiting step was shared by multiple RDH enzymes, some of which are ubiquitously expressed (Zhang et al.,2001). This discovery, and the generation of two independent Rdh10 mutants, highlights the power of the ENU-induced mutagenesis procedure for identifying critical regulators of mammalian development. The Rdh10m366Asp mutant has a very conservative alanine-to-valine substitution, as both amino acids are hydrophobic, nonpolar, uncharged, and are relatively similar structurally. The detriment of this mutation on RDH10 function appears to be due to its localization in the ligand-binding pocket, which is highly susceptible to even minor perturbations. In vitro studies found that mutating Ser197 in human RDH10 (Takahashi et al.,2009) or other short-chain dehydrogenase/reducatase gene family members (Cols et al.,1997; Filling et al.,2002), a key member of a highly conserved Asn-Ser-Tyr-Lys nonlinear tetrad of residues within the pocket, abolishes enzymatic activity. Specifically, the Ser197 residue is responsible for stabilizing substrate in the catalytic center. Our mutant disrupts an adjacent amino acid, suggesting that the Alanine196 to Valine196 missense mutation also disrupts substrate stability. Mutations at Ser197, however, do not affect protein stability (Takahashi et al.,2009), consistent with our findings for the RDH10m366Asp protein in vivo, indicating that the instability we observed in our in vitro studies is probably related to the N-terminal FLAG tag.
Our RA measurements have provided us with a global view of how RA levels are altered over developmental stages due to significantly depleted RDH10 function. At (early) E10.5, there is a 23% reduction in RA levels in whole embryos. Shortly after, there is a noticeable reduction in RA reporter signal, and initial morphological defects are apparent (truncated MNPs). This data suggests that RA levels are being maintained near threshold levels and modest reductions lead to morphological changes, and presumably, corresponding and underlying molecular changes. At E12.5, there is a 66% reduction in RA levels, indicating that RDH10 provides a substantial portion of total RA at E12.5 (i.e., greater than 66%, because the Rdh10m366Asp mutant is a hypomorphic allele). Others have shown that impaired Rdh10 function leads to embryonic lethality before E13 (Rhinn et al.,2011; Sandell et al.,2007). Here, we show quantitatively that this lethality correlates with a steep, approximately threefold, reduction in RA levels from E10.5 to E12.5 due to RDH10 “absence.” At 14.5, RA levels slightly recover to 50%, indicating that RDH10 continues to generate most of the all-trans-retinal that is used to generate RA at this stage. Rdh10m366Asp mutants maintain survival under such RA deprivation. The reduced RA levels in the Rdh10m366Asp mutant correlate with previous studies, where a 50% reduction in RA levels in the developing cortex and 20% reduction in the meninges of a Foxc1 mutant lead to significant defects in corticogenesis, and a 42–53% decrease in fetal retinoids at E12.5 result in aberrant enteric nervous system precursor cell migration by reducing Pten accumulation (Fu et al.,2010).
Crossing Rdh10trex mutants or an Rdh10 null (Rhinn et al.,2011; Sandell et al.,2007) with a RA-reporter has provided insight into where tissue-specific alterations in RA signaling occur at stages E8.5 and E9.5. The reduction in RA signaling from such a cross is far less severe than that observed in Raldh2−/− embryos and is more on par with Raldh2−/− mice rescued by RA supplementation (Mic et al., 2002). Here, we have performed a similar cross with Rdh10m366Asp mutants, but analysis was performed at stages E10.5–E14.5. Whole embryo staining clearly identifies a global reduction in RA-reporter signal at all stages. Most dramatic changes include the facial mesenchyme and eye tissue. There is also significant signal reduction in the limb and trunk mesenchyme, but some reporter activity persists in the spinal cord and forebrain. Our quantitative RA measurement studies identified a steep drop in RA levels from E10.5 to E12.5 that is clearly evident in our RA-reporter studies. This data identifies tissues to which RDH10 contributes greatest RA signal, and these tissues closely correlate with morphological defects in Rdh10m366Asp mutants. Interestingly, and in contrast to earlier stages (E8.5–E9.5), at E10.5 and E12.5, RA-reporter signal is far more reduced in Rdh10m366Asp mutants than in partially rescued Raldh2−/− embryos (Niederreither et al.,2002), indicating that RDH10 has a far more prevalent role in regulating RA synthesis, and thus RA signaling (between E10.5 and E12.5), than does RALDH2.
The Rdh10m366Asp mutant has many phenotypes that are morphologically very similar to those in the Rdh10trex/trex mutant and Rdh10−/− embryos, such as eye, internal organ, and limb defects (Cunningham et al.,2011; Farjo et al.,2011; Rhinn et al.,2011; Sandell et al.,2007); however, it also has unique defects that will further our understanding of RA biology. For example, the Rdh10m366Asp mutant consistently has a very prominent midline nasomaxillofacial cleft, which is also present in RARα and γ double knockout mice (Lohnes,1994) but is variably seen in Rdh10trex/trex mutants (Farjo et al.,2011; Sandell et al.,2007). Rdh10−/− embryos display severe nasal/facial clefting; however, they rarely survive to E12.5 allowing only limited analysis of the facial skeletal (Rhinn et al.,2011). The edema phenotype suggests a role for RDH10 in cardiac or circulatory function. RA very clearly functions in heart development, for example, cardiac looping, ventricular maturation, atrial septation, and myocardial trabeculation (Niederreither et al.,2001; Rhinn et al.,2011); however, the late-stage survival and commonly seen areas of pooled blood suggest that vascular defects may be the underlying problem in Rdh10m366Asp mutants. Analysis of Raldh2−/− embryos identified a role for RA in vascular morphogenesis (Bohnsack et al.,2004). Malformation of the forebrain is also prevalent, and, to the best of our knowledge, the complete absence of olfactory bulbs is novel to the Rdh10m366Asp mutant. Another phenotype in the forebrain, specifically the observed defects in corticogenesis, was the catalyst for studies that identified RA as a critical inducer of neurogenesis in the cortex via signaling from the meninges (Siegenthaler et al.,2009). Chatzi et al. (2011) have suggested that neurogenesis defects in Rdh10m366Asp mutants are secondary to neural crest-derived craniofacial defects that distort the cranium. The ideal model to clarify this matter is the Rdh10m366Asp mutant, where this defect was first identified by temporarily providing RA supplementation during neural crest cell formation/migration and then analyzing corticogenesis. Finally, the survival of Rdh10m366Asp embryos to later stages in development allows analysis of unique developmental processes, and together with the potential analysis of the phenotypes mentioned, the Rdh10m366Asp mutant will be an invaluable tool for furthering our understanding of retinoid metabolism and the biological functions of retinoids during fetal development.
MATERIALS AND METHODS
Histological Sections
Mouse embryos were dissected in cold PBS and fixed overnight in 4% paraformaldehyde (PFA) at 4°C. The following day, PFA was washed away, and embryos were processed using a graded ethanol series to 100% ethanol. Xylene washes then removed ethanol, and embryos were subsequently embedded in Paraplast wax. Serial sections were collected at 10 μm thickness, mounted onto glass slides, and stained using cresyl violet (Nissl stain).
Cell Transfection
CHO-K1 cells were cotransfected with 1 μg each of either pBSK-N-terminal FLAG-mRDH10 or pBSK-N-terminal FLAG-mRDH10m366Asp and pBSK-RALDH2. Cells were seeded at ∼60% confluency into six-well plates and transfected the next day with Lipofectamine reagent (Invitrogen). Each transfection was done in triplicate wells. A luciferase control vector (Promega) was transfected into all wells to normalize for transfection efficiency. Luciferase activity was measured using the Luciferase assay system (Promega). Medium was replaced with fresh medium without serum 24 h after transfection. HPLC-purified retinol was added at 2.0 μM to the medium under yellow light and incubated at 37°C for 1 h. Medium was removed, and cells were lysed in 1 ml of reporter lysis buffer (Promega). After a freeze-thaw cycle, cells were combined with their medium and extracted. Protein concentrations were determined by the Bradford assay (Bio-Rad).
Western Blotting
For in vitro studies, 10 μg of cell lysate was electrophoresed on 10% SDS–PAGE gels, transferred to nitrocellulose membranes, and blocked with PBS containing 5% nonfat milk. The membrane was then incubated with an anti-FLAG tag antibody (Sigma) diluted 1:2,000 for 1 h at room temperature. After washing, the membrane was incubated with an anti-mouse secondary antibody conjugated to horseradish peroxidase (Sigma) for 1 hr. Staining for β-actin (antibody from Sigma) was used to control the loading. Signals were detected using an enhanced chemiluminescent substrate (Pierce) and quantified by densitometry. A similar approach was used for Western blotting using embryo tissues (Ashique et al.,2009). The anti-RDH10 polyclonal antibody used was raised in a rabbit using the C-terminal peptide (327-341aa) of RDH10.
Tissue Preparation for Retinoid Extraction
Samples were processed under yellow light. Mouse embryos were dissected using a Nikon SMZ-10A dissection microscope equipped with a Volpi (Auburn, NY) NCL 150 light source with a yellow filter. Whole embryo bodies were immediately placed on dry-ice and kept frozen until they were homogenized in 1-mL ice-cold 0.9% NaCl with a ground-glass hand homogenizer. Protein concentrations were determined by the Bradford Assay (Bio-Rad), and retinoid concentrations are expressed per gram total protein.
Retinoid Extraction
Cell and tissue samples were extracted as previously described using a liquid–liquid extraction (Kane et al.,2005). Only glass containers, pipettes, and calibrated syringes were used to handle samples, because retinoids adhere to plastic, which can cause losses up to 40%. Resuspended samples were analyzed immediately.
LC/MS/MS Analysis of RA
We measured atRA in embryo bodies with a highly sensitive and specific liquid chromatography (LC)/mass spectrometry (MS)/MS assay that distinguishes atRA from its isomers and provides definitive mass identification (Kane and Napoli,2010; Kane et al.,2005). An internal standard (4,4-dimethyl-atRA) was used routinely and established recovery of endogenous atRA at 84%. Briefly, resolution of atRA from its isomers was effected with a Supelco ABZ+PLUS 100 × 2.1 mm, 3-μm C-16 alkylamide column.
Data were collected with an Applied Biosystems API-3000 LC/MS/MS system equipped with an atmospheric pressure chemical ionization source operated in positive ion mode (Kane et al.,2005). All data were collected in SRM (selected reaction monitoring) mode, in which the following SRM transitions were monitored (precursor ion to product ion): RA, m/z 301.1–205.0; 4,4-dimethyl RA, m/z 329.4–151.3. RA isomer retentions were verified with authentic standards. Retinoid standards purchased from Sigma-Aldrich (St. Louis, MO) were prepared fresh on the day of use, and concentrations were verified spectophotometrically from ε values (Barua and Furr,1998; Dawson et al.,1981).
RA Rescue
The standard normal chow diet (NCD) of pregnant females was changed to one supplemented with atRA starting at E7.5. Mice were transferred to a fresh cage. The RA-supplemented diet was given sparingly, although in excess of daily requirement, which mice could consume ad libidum. Fresh food was provided daily, and cages were protected from excess light since RA is light-sensitive. RA dosage was based on previous reports (Niederreither et al.,2002). At E7.5, NCD was supplemented with RA at a concentration of 100 μg/g. From E8.5 to E14.5, RA was supplemented at a dose of 250 μg/g.




