A transgenic mouse line expressing cre recombinase in pancreatic β-cells
Abstract
Transgenic mouse lines expressing Cre recombinase in a cell-specific and tissue-specific manner are essential tools for studying gene function and for developing suitable models for human diseases. Here, we used an expression cassette containing the full 5′ untranslated region of the porcine insulin gene to generate a mouse line expressing Cre recombinase specifically in pancreatic β-cells by pronuclear DNA microinjection. We obtained a founder animal that transmitted the construct to its descendants in a Mendelian fashion and whose descendants showed a clear activation of β-galactosidase expression in pancreatic β-cells after crossing into the ROSA26 lacZ reporter mouse line. Cre expression in other organs was negative except for the kidney, intestine, and the cerebral pons where β-galactosidase activity was detected in a small percentage of the cells. This new mouse line is a valuable tool for recombination of floxed alleles in pancreatic β-cells in vivo. genesis 50:437–442, 2012. © 2011 Wiley Periodicals, Inc.
The endocrine compartment of the pancreas is composed of multiple units scattered throughout the organ, the islets of Langerhans. Within each islet, four major types of endocrine cells can be distinguished. The β-cells make up the majority of cells in the islet and their main product is insulin. The α-cells secrete glucagon, the δ-cells secrete somatostatin and the PP cells secrete pancreatic polypeptide (Habener et al.,2005). The availability of transgenic mouse lines expressing the enzyme Cre recombinase (or a 4-hydroxy (OH)-tamoxifen-inducible variant) in pancreatic β-cells contributed decisively to our current understanding of the hierarchy of transcription factors governing islet cell fate as well as the physiology and pathology of the endocrine pancreas.
In currently available lines, Cre expression has been put under the control of the rat Ins2 promoter (RIP; Ahlgren et al.,1998; Dor et al.,2004; Gannon et al.,2000b; Postic et al.,1999), the mouse Neurog3 promoter (Gu et al.,2002), or the mouse Pdx1 promoter (Gannon et al.,2000a; Gu et al.,2002; Zhang et al.,2005). Both the Neurog3 and the Pdx1 promoter drive expression of Cre in pancreatic endocrine progenitor cells and are still active in all endocrine pancreatic cell lineages of adult mice. In contrast, the existing RIP-Cre lines cause recombination specifically in β-cells but not in the other endocrine islet cells. Each of these mouse lines has its own limitations and drawbacks. For example, the RIP also drives ectopic expression in certain areas of the brain, potentially resulting in phenotypes in both β-cells and neural cells (Gannon et al.,2000b; Martin et al.,2003). Such an extra-pancreatic Cre expression is not seen in a mouse line carrying the inducible RIP-CreER system, but very high doses of tamoxifen are necessary to achieve a sufficient recombination efficiency in β-cells (Dor et al.,2004). Finally, Pdx1 promoter-driven Cre expression was reported at several additional extra-pancreatic sites including the kidney, bones, hair follicles, and the intestinal epithelium (Zhang et al.,2005). More recent studies revealed ectopic expression of Cre in specific brain regions, most commonly in the hypothalamus of RIP-Cre, Neurog3-Cre, and Pdx1-Cre mouse lines (Song et al.,2010; Wicksteed et al.,2010). Even more inconvenient is the reported glucose intolerance phenotype in RIP-Cre animals (Lee et al.,2006). Thus, unfortunately, there is no perfect, universally employable Cre mouse line for studying gene function in β-cells. Researchers have to carefully consider the characteristics and drawbacks of each line before deciding which one might be the most appropriate for their specific question. Cre mouse lines with more selective expression characteristics are urgently needed.
We have recently shown that an expression cassette containing 1,500 bp of the 5′ UTR from the porcine INS gene (including the first exon and the first intron) and the 3′ UTR of the bovine growth hormone gene is capable to drive transgene expression in a robust and β-cell specific manner in vitro and in transgenic mice (for details see (Grzech et al.,2010). Here, we used this cassette (porcine insulin promoter; PIP) to create a transgenic mouse line expressing the codon-improved Cre recombinase (iCre) (Shimshek et al.,2002) specifically in pancreatic β-cells (Fig. 1a).
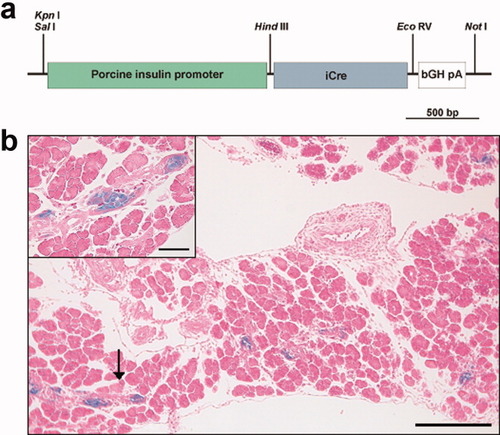
(a) Schematic representation of the construct used to generate transgenic mice including the position of relevant restriction sites. (b) Pancreatic tissue from E16.5 PIP-iCre/R26R double mutant embryos stained with β-gal. Arrows indicate the region showed at higher magnification in the insert. Scale bars represent 200 μm or 50 μm (insert).
After pronuclear microinjection of the PIP-iCre construct, 43 mice were born. Two of them were identified as being transgenic by polymerase chain reaction (PCR) and both transmitted the transgene to their descendants in a Mendelian fashion (data not shown). To determine the pattern of PIP-iCre-mediated DNA recombination, we crossed the two PIP-iCre transgenic mouse lines with ROSA26 lacZ reporter mice (R26R; Soriano,1999) and evaluated β-galactosidase (β-gal) expression in adult (8 weeks old) animals heterozygous for both alleles. One line (#20) showed no β-gal activity at all and was not further analyzed. Pancreatic tissue sections from the second PIP-iCre line (#18) showed a clear β-gal staining of almost every pancreatic islet but not of the exocrine compartment (see below) and was, therefore, characterized in more detail.
Insulin-containing cells can be detected in mouse embryos as early as day 11.5, but their number is extremely low until day 15.5 (Herrera et al.,1991). Typical islets, with centrally located β-cells and the adult hormone expression pattern are only detected by the end of pregnancy, around days 17.5–18.5 (Herrera et al.,1991). We observed several clusters of β-gal-positive cells in the pancreas of PIP-iCre/R26R double transgenic mice fetuses at day 16.5 of pregnancy (Fig. 1b). Also, the same expression cassette used here resulted in a strict β-cell-specific transgene expression at embryonic day 17.5 in a previous study (Grzech et al.,2010), strongly suggesting that the used regulatory sequences mimic the endogenous insulin expression pattern during embryonic development. Closer examination of adult (8 weeks old) pancreas revealed β-gal activity in the vast majority of the islet cells with a few negative cells being localized mostly at the periphery, a pattern compatible with a selective β-gal expression by β-cells as described for this promoter earlier (Grzech et al.,2010). To confirm this impression, we examined colocalization of β-gal activity with glucagon and somatostatin in pancreatic islets in PIP-iCre/R26R double transgenic mice by immunohistochemistry. For both glucagon (Fig. 2a) and somatostatin (Fig. 2b) hormones and β-gal expression were mutually exclusive. To provide additional evidence that this mouse line can be used to selectively target β-cells within pancreatic islets, we sequentially stained pancreatic sections with dithizone, a zinc-chelating agent known to selectively stain pancreatic β-cells because of their high zinc content (Shiroi et al.,2002) and X-gal. As shown in Figures 2c,d, the cells with β-gal activity were also positive for dithizone, indicating that they were indeed insulin-producing cells. Cells with β-gal activity were never observed in the pancreatic islets in control mice (ROSA26 lacZ mice negative for Cre, data not shown).
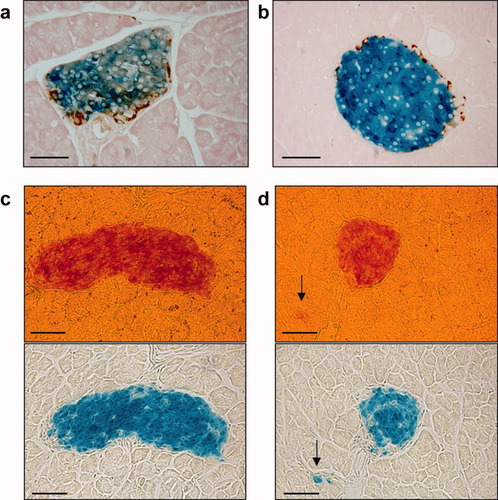
Simultaneous visualization of glucagon (a) and somatostatin (b) by immunohistochemistry on β-gal-stained islets of PIP-iCre/R26R double mutant mice. (c, d) Sequential staining of islets with dithizone and X-gal demonstrating that β-gal-positive cells are also insulin-producing cells. Arrows in (d) indicate a small cluster of β-gal/insulin-positive cells. Scale bars represent 50 μm.
To evaluate potential nonpancreatic expression of Cre recombinase, RNA samples from brain, lung, heart, liver, small and large intestine, kidney, spleen, and muscle were analyzed by reverse transcriptase (RT)-PCR. No RNA expression for Cre recombinase was detected in any of these organs (data not shown). Considering the several reports describing ectopic Cre expression in the brain driven by presumably islet-specific promoters (Gannon et al.,2000b; Martin et al.,2003; Song et al.,2010; Wicksteed et al.,2010), we screened three selected brain regions for iCre expression by RT-PCR. Thereby, as shown in Figure 3a, no transgene expression was detected in the caudate putamen, the hypothalamus and the pons of PIP-iCre transgenic mice. However, histochemical β-gal staining disclosed focal β-gal expression in a small number of cells (putatively in the perikarya of neurons) of the pons (Fig. 3b), in epithelial cells of the small and large intestine (Fig. 3c), and epithelial cells of the renal cortex (Fig. 3d). In the intestine, individual crypts were entirely stained, indicating a stochastic activation of Cre in a small proportion of intestinal crypt founder cells (Fig. 3c). A quantitative evaluation of β-gal-stained tissue sections revealed Cre expression in about 1.5% of crypts (n = 2 mice, 200 large intestine crypts/mouse were evaluated). The incidence of unequivocally stained cells in the kidney was comparably low and staining was restricted to epithelium of cortical proximal tubules. No staining of the pons, intestine, or kidney was observed in nontransgenic control mice, providing substantial evidence that the β-gal staining patterns were actually due to Cre expression and not due to unspecific staining or endogenous galactosidase activity.
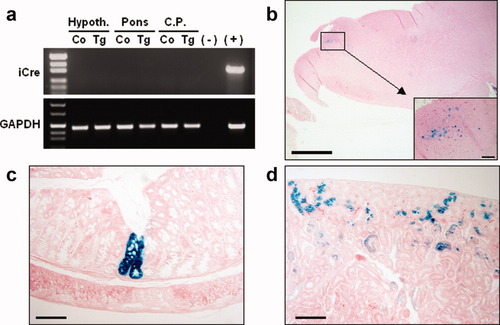
RT-PCR failed to detect expression of Cre recombinase in selected brain regions (hypothalamus, pons, and caudate putamen) (a). β-gal staining revealed ectopic expression of Cre recombinase in the pons (b), large intestine (c), and kidney (d). Scale bars represent 1 mm (b) or 100 μm (c, d, and the insert in b).
Considering the glucose intolerance phenotype reported for RIP-Cre animals (Lee et al.,2006), we investigated whether PIP-iCre animals have a glucose tolerance perturbation on their own. As shown in Figure 4, the response of PIP-iCre to an intraperitoneal glucose tolerance test did not differ from the response of their control littermates.
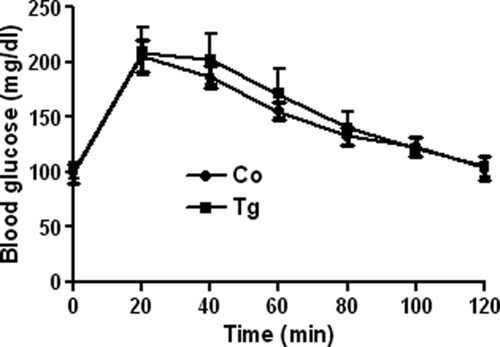
Intraperitoneal glucose tolerance test showing comparable blood glucose levels in fasted PIP-iCre mice and control littermates (4-month-old males) before glucose administration and after the indicated time points (n = 6 mice/group).
In summary, we describe a new transgenic mouse line that shows a consistent and specific expression of Cre recombinase in β-cells of pancreatic islets and a very limited expression in extrapancreatic tissues when compared with other available Cre-expressing mouse lines. Mendelian transmission and maintenance of the described expression pattern of the transgene has been ascertained up to the third generation. We believe that the described PIP-iCre transgenic mouse line, generated from the widely used inbred strain C57BL/6, will be very useful to investigate the function of candidate genes by gene activation or deletion in pancreatic β-cells.
MATERIALS AND METHODS
Construction of the Transgene
The 1,055-bp iCre fragment was amplified by PCR (Expand High FidelityPLUS PCR System, Roche Diagnostics, Mannheim, Germany) using the sense primer (HindIII restriction site underlined) 5′-ACT AAG CTT CAC CCC CCG CCA TGG TGC CCA AGA AGA AGA G-3′ and the antisense primer (EcoRV restriction site underlined) 5′-TAG GAT ATC TCA GTT TCA GTC CCC ATC CTC-3′. The template plasmid was a courtesy of Dr. Markus Gerhard. The PCR product was cloned into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA) and Sp6/T7-sequenced to confirm amplification fidelity. The final PIP-iCre construct was generated by replacing the human betacellulin (hBTC) sequence of the previously reported PIP-hBTC construct (Grzech et al.,2010) with the iCre sequence using a HindIII/EcoRV digest (Fig. 1a). Correct positioning of each element was confirmed by restriction enzyme digests and sequencing from the PIP through the iCre sequence using the primer 5′-CAT CTC GGC AGG AGG ACG T-3′.
Generation of Transgenic Mice
The PIP-iCre expression cassette was released from the vector backbone by SalI/NotI double digestion, purified by agarose gel electrophoresis, diluted to 2 ng/μl in injection buffer and used for pronuclear microinjection into fertilized oocytes from the inbred strain C57BL/6. The injected zygotes were transplanted into the oviducts of pseudopregnant females and potential founder animals were screened by PCR. The animals had free access to a standard rodent diet (V1534; Ssniff, Soest, Germany) and water. All experiments were approved by the author's institutional committee on animal care and carried out in accordance with the German Animal Welfare Act with permission from the responsible veterinary authority.
Glucose Tolerance Test
Mice fasted for 6 hours were injected intraperitoneally with glucose (1.5 g/kg body weight) and blood samples were obtained by puncture of the tail vein immediately before glucose administration and at the indicated time points after injection. A glucometer (Precision, Abbott Diabetes Care, Wiesbaden, Germany) was used to determine glucose levels.
Histochemical Detection of β-Gal Activity
The organs were fixed in phosphate buffered saline (PBS) containing 0.02% NP-40, 1% formaldehyde, and 0.2% glutaraldehyde at 4°C for 2 hours, washed two times with PBS for 20 min (room temperature), and incubated overnight at 37°C under careful shaking in staining solution (0.02% NP-40, 2 mM MgCl2, 5 mM K3[Fe(CN)6], and 5 mM K4[Fe(CN)6] × 6H2O, 0.01% sodium deoxycholate, and 1 mg/ml X-gal in PBS, pH 7.4). After staining, samples were washed in PBS as above and postfixed in PBS with 4% paraformaldehyde at 4°C, routinely processed and embedded in paraffin blocks. Histological sections were costained with eosin.
Dithizone and β-Gal Double Staining
The pancreas was fixed for 1 hour in 4% paraformaldehyde (in PBS, pH 7.4) at 4°C, washed in PBS, and equilibrated for 4 hours in 15% sucrose solution (in PBS) and overnight in 30% sucrose solution (in PBS) at 4°C. Cryostat sections were done at −25°C and dried for 1 hour at room temperature. Sections were stained with dithizone (Sigma, Taufkirchen, Germany) solution (10 mg diphenylthiocarbazone, 1 ml dimethyl sulfoxide, and 9 ml PBS) and photos of islets were taken immediately. Next, slides were washed three times in PBS, fixed in 4% paraformaldehyde for 10 min, and stained overnight at 37°C in X-gal substrate as described above. Sections were dehydrated with ethanol and mounted with Permount (Sigma).
Immunohistochemistry
For the immunolocalization of glucagon and somatostatin on β-gal-stained pancreas, 5 μm thick sections were deparaffinized and the slides were boiled for 20 min in 10 mM sodium citrate buffer. The primary antibodies (rabbit anti-glucagon and rabbit anti-somatostatin, Dako, Hamburg, Germany, 1:300 dilution) were incubated over night at 4°C. An appropriate horseradish conjugated secondary antibody (Dako, Hamburg, Germany, 1:100 dilution) was used. Diaminobenzidine (Sigma) was used as the chromogen and sections were counterstained with eosin.
Reverse Transcriptase PCR
Isolation of total RNA, first strand cDNA synthesis, and semiquantitative RT-PCR were performed as described earlier (Schneider et al.,2001). The primers iCreRTs (5′-GGC AGG CCT TCT CTG AAC A-3′) and iCreRTas (5′-GGA AGG CCA GGT TCC TGA T-3′) were used for the detection of transgene-derived iCre expression. The integrity of the template cDNA was confirmed by amplifying a sequence of the β-actin gene (sense, 5′-GGCATCGTGATGGACTCC-3′; antisense, 5′-GTCGGAAGGTGGACAGGG-3′).
Acknowledgements
We thank Dr. Ingrid Renner-Müller and Petra Renner for animal care, Tamara Holy for pronuclear microinjection, and Josef Millauer for routine mouse genotyping. Rosa26lacZ reporter mice were kindly donated by Prof. Dr. A.J.M. Berns (The Netherlands Cancer Institute).




