Exploring the Role of NLRP3 in Neurodegeneration: Cutting-Edge Therapeutic Strategies and Inhibitors
Funding: The authors received no specific funding for this work.
ABSTRACT
Inflammasomes, particularly the NLRP3 inflammasome, play a pivotal role in mediating neuroinflammation in neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Huntington's disease (HD). Recent findings indicate that the activation of the NLRP3 inflammasome in microglia and astrocytes triggers the release of pro-inflammatory cytokines, including IL-1β and IL-18, which contribute to chronic inflammation and neuronal damage. This process accelerates neurodegeneration and exacerbates disease progression. Misfolded protein aggregates, mitochondrial dysfunction, and oxidative stress are key factors in the pathological activation of the NLRP3 inflammasome in these diseases. Recent studies have highlighted that targeting the NLRP3 inflammasome, either through direct inhibitors like MCC950 or natural compounds such as oridonin and β-hydroxybutyrate, shows promise in mitigating neuroinflammation and protecting neuronal integrity. These inhibitors have demonstrated neuroprotective effects in animal models of AD, PD, and MS, presenting a new therapeutic approach for halting disease progression. However, the complexity of NLRP3 regulation requires further investigation to balance its inflammatory and protective roles. This review examines the recent advancements in NLRP3 inflammasome research and discusses potential strategies for modulating inflammasome activity to slow or prevent the progression of neurodegenerative diseases.
1 Introduction
More than 1% of the world's population is currently affected by neurodegenerative conditions—a figure projected to rise sharply due to global population aging (Fernández-Cruz and Reynaud 2021). Disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Huntington disease (HD) all fall under this umbrella, and they share a common thread: gradual deterioration of the nervous system. This leads to a profound decline in cognitive and motor functions, disruption of internal balance, and an overall decrease in life quality (Bae et al. 2020). Unfortunately, existing treatments mainly alleviate symptoms without halting or reversing disease progression, underscoring the need for innovative therapeutic approaches (Bae et al. 2020).
One of the defining features of neurodegenerative diseases is persistent inflammation in the brain, coupled with neuron loss and the accumulation of misfolded proteins (Ikram et al. 2020; Katsuno et al. 2018). These misfolded proteins form toxic aggregates that trigger immune responses, causing gliosis and other forms of central nervous system (CNS) damage (Guzman-Martinez et al. 2019). At the heart of this inflammatory cascade is the NLRP3 inflammasome—a multiprotein complex within microglia (the brain's immune cells) that detects cellular stress and triggers the release of inflammatory molecules like IL-1β and IL-18 (Ferrara et al. 2023). Although these cytokines are part of the body's defense mechanism, their sustained release contributes to chronic inflammation and accelerates neurodegeneration (Swanson et al. 2019; Hung et al. 2020).
Recent findings have linked specific protein clumps, such as amyloid-beta (Aβ) in AD, to the activation of the NLRP3 inflammasome (Hulse and Bhaskar 2022). When activated, NLRP3 not only amplifies inflammation but also worsens neuronal injury. Animal studies have shown that blocking this inflammasome can reduce harmful inflammation, promote the clearance of Aβ plaques, and even restore cognitive abilities (Heneka et al. 2013). Moreover, age-related changes in the regulation of NLRP3—particularly through altered acetylation—appear to fuel chronic inflammation, adding another layer to its involvement in neurodegenerative processes (He et al. 2020).
Given these insights, the NLRP3 inflammasome emerges as a powerful target for new therapies. Modulating its signaling network, both upstream and downstream, offers a potential path toward altering disease progression rather than merely managing symptoms. This review delves into the current landscape of NLRP3 research, its mechanistic role in brain diseases, and emerging strategies aimed at neutralizing its harmful impact.
2 NLRP3 Inflammasome
The innate immune system, which acts as the body's first line of defense against pathogens, is intricately regulated by the peripheral nervous system (PNS), the neuroendocrine network, and the CNS. This coordination ensures both robust immune responses to infection and the preservation of immune balance (Sternberg 2006). Key immune cells—including monocytes, macrophages, and neutrophils—play a pivotal role by expressing pattern recognition receptors (PRRs). These receptors are specialized to sense molecular indicators of danger: pathogen-associated molecular patterns (PAMPs) from infectious agents and damage-associated molecular patterns (DAMPs), which arise from internal cellular stress such as misfolded proteins, peptide aggregates, or misplaced genetic material (Walsh et al. 2014; Singhal et al. 2014).
Although PRRs serve as essential surveillance tools, their overactivation or dysregulation can drive persistent inflammation, contributing to chronic disease states (Duan et al. 2020; Banjara and Ghosh 2017). The PRR family encompasses a range of sensor types, including cytosolic RIG-I-like receptors (RLRs), surface-bound Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), each tailored to recognize distinct molecular patterns and initiate specific immune pathways (Moretti and Blander 2021; Saresella et al. 2016).
2.1 NLRP3 Inflammasome Formation
The NOD-like receptor (NLR) family in humans includes 22 known proteins (Chou et al. 2023), whereas mice express at least 34 variants. Among these are NLRP3, NLRP1b, NLRP2, NLRP6, NLRP7, NLRP9b, NLRP12, NLRP14, NLRC4, and NLRC5, along with AIM2 (Absent in Melanoma 2), a related receptor that functions similarly but does not belong to the NLR family (Freeman and Ting 2016; Shadab et al. 2023). Although NLRP3 has received the most attention in inflammasome research, the roles of many other NLRs in inflammation—especially within the brain—remain underexplored, partly due to the limited structural data available on their assembly mechanisms. Preliminary studies suggest they may also influence inflammatory signaling in adaptive immunity (Yang et al. 2019).
When activated, NLRs initiate the formation of inflammasomes—cytoplasmic protein complexes that kickstart immune signaling cascades (Singhal et al. 2014). This activation engages the nuclear factor kappa B (NF-κB) pathway, which in turn drives the production of pro-inflammatory mediators like cytokines and chemokines (Banjara and Ghosh 2017). NLRP3 serves as the archetypal inflammasome-forming receptor and plays a central role in both classical (canonical) and alternative (noncanonical) signaling pathways (Diamond et al. 2015). Structurally, all NLR proteins—including NLRP3—share a common architecture made up of three domains: an N-terminal pyrin domain (or occasionally a CARD), a C-terminal leucine-rich repeat (LRR) domain, and a central nucleotide-binding NACHT domain (Hong et al. 2019; Haque et al. 2020).
Recent advances in cryo-electron microscopy have unveiled that the inactive form of NLRP3 exists as a decamer, composed of two pentameric rings formed by intertwined LRR domains. These rings are held together by a transitional LRR segment that supports the overall structural stability. Along with the LRRs, the NACHT and pyrin domain dimerization are critical for the structural integrity of the complex. Insights into the binding interactions of specific NLRP3 inhibitors, such as CRID3, which stabilizes the NACHT domain, are paving the way for targeted therapeutic interventions (Hochheiser et al. 2022).
The inflammasome itself consists of three main components: a sensor protein from the PRR family (like NLRP3), an adaptor protein known as ASC (apoptosis-associated speck-like protein containing a CARD), and the effector enzyme caspase-1 (Duan et al. 2020). When danger signals activate NLRP3, it interacts with ASC through pyrin domain interactions, causing ASC molecules to cluster and form a visible aggregate, or “speck.” This aggregate recruits procaspase-1 via CARD-CARD interactions, leading to its oligomerization and activation. Once activated, caspase-1 processes and releases key inflammatory cytokines, thereby amplifying the body's immune defense mechanisms (Xiao et al. 2020; Bulté et al. 2023) (Figure 1).
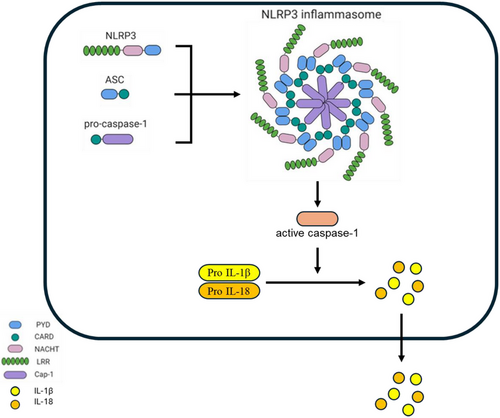
2.2 NLRP3 Inflammasome Activation
Within the CNS, the activation of the NLRP3 primarily occurs in microglia and astrocytes. Microglia serve as the brain's innate immune sentinels, whereas astrocytes support neuronal function and maintain homeostasis. These glial cells become reactive in response to pathological triggers such as misfolded proteins, neuronal stress, or cellular debris. Once activated, the NLRP3 drives the release of inflammatory mediators—cytokines and chemokines—that fuel neuroinflammatory responses. Persistent or uncontrolled inflammasome activity in these cells has been strongly associated with the progression of neurodegenerative diseases, where chronic inflammation leads to progressive neuronal damage. Both cell culture studies and animal models have shown that NLRP3 activation contributes to heightened microgliosis and astrogliosis (Scholz and Eder 2017; Freeman et al. 2017).
In AD, astrocyte-driven inflammation through the NLRP3 pathway plays a detrimental role in disease progression, whereas blocking this inflammasome has demonstrated protective effects (Nassar et al. 2022). Similarly, in a mouse model of chronic mild stress (CMS), NLRP3 activation in microglia was linked to synaptic dysfunction, behavioral abnormalities, and the emergence of neurotoxic astrocytes exhibiting A1-like phenotype (Li et al. 2022). Moreover, overactivation of dynamin-related protein 1 (Drp1) in mature oligodendrocytes has been shown to impair glycolysis and trigger NLRP3 activation in Alzheimer's models. Interestingly, genetically removing Drp1 not only normalized metabolic function but also inhibited NLRP3 activity and improved cognitive outcomes (Zhang et al. 2020).
Beyond its role in disease, inflammasome-driven inflammation can also affect brain development by interfering with neurogenesis, synaptic formation, and the shaping of neural circuits. The exact mechanisms behind NLRP3 activation during developmental stages remain an area of active investigation. Importantly, mutations in the NLRP3 gene are linked to a variety of autoinflammatory syndromes, such as familial cold autoinflammatory syndrome, Muckle–Wells syndrome, neonatal-onset multisystem inflammatory disease (NOMID), and chronic infantile neurologic cutaneous and articular (CINCA) syndrome (Eren and Özören 2019). Point mutations like R262W, L307P, and V200M in the gene (also referred to as CIAS1 or PYPAF1) have been directly tied to familial cold urticaria (Gattorno et al. 2007; Aganna et al. 2002).
To date, more than 90 NLRP3 mutations—mainly autosomal dominant missense changes in exon 3—have been implicated in cryopyrin-associated periodic syndromes (CAPS) (Masters et al. 2009; Touitou et al. 2004). Mouse models carrying R258W and A350V mutations, homologous to human R260W and A352V, mimic symptoms of Muckle–Wells syndrome (Naz Villalba et al. 2016). These mutations typically disrupt normal inflammasome assembly and result in excessive IL-1 production, even without typical activators (Conforti-Andreoni et al. 2011). In addition, mutations in the LRR domain, such as Y859C (in exon 6), are associated with unique CAPS presentations, including late-onset Muckle–Wells syndrome (Jéru et al. 2010). Another variant, Q703K, has also been implicated in the broader spectrum of autoinflammatory diseases (Theodoropoulou et al. 2020).
3 NLRP3 in Neurodegenerative Diseases
The NLRP3 inflammasome is a key player in initiating and sustaining inflammation in the brain and has emerged as a significant factor in the development of several neurological disorders, including AD, PD, ALS, and MS. Functioning as a sensor within the innate immune system, NLRP3 becomes activated in the presence of cellular disturbances—ranging from protein misfolding and oxidative stress to mitochondrial instability. Upon activation, NLRP3 drives the production and release of inflammatory mediators like interleukin-1β and interleukin-18, fueling a state of persistent neuroinflammation. In diseases like AD and PD, pathological proteins—Aβ and α-syn, respectively—can directly engage NLRP3, setting off a feedback loop that intensifies inflammation and heightens neuronal vulnerability. This sustained inflammatory activity disrupts critical brain functions, contributing to synaptic failure, degradation of the blood-brain barrier, and gradual loss of neurons (Heneka et al. 2018; Brahadeeswaran et al. 2022). In this section, we will delve into the underlying biological processes through which NLRP3 exacerbates neurological damage, emphasizing its pivotal role in driving disease progression and impairing neural function.
3.1 AD
AD is a slowly advancing neurodegenerative condition most frequently seen in older adults. It is marked by the buildup of misfolded proteins in the brain—specifically, Aβ plaques and tangles of hyperphosphorylated tau protein (Abyadeh et al. 2024). These abnormalities typically first appear in the neocortex and gradually extend into key memory-related areas such as the hippocampus and entorhinal cortex. The presence of these toxic protein aggregates triggers the brain's immune response, particularly involving microglia and astrocytes (Zhang et al. 2024; Darricau et al. 2024). Although initially aimed at containing damage and clearing debris, prolonged activation of these cells leads to sustained inflammation, primarily through elevated levels of cytokines like IL-1β (Simard et al. 2006). This persistent immune activation, including the detection of heightened IL-1β and IL-18 in the CSF of AD patients, has been strongly linked to cognitive decline, especially impairments in memory (Feng et al. 2020).
A central driver of this inflammatory cascade is the NLRP3 inflammasome—a molecular complex in the innate immune system responsible for activating caspase-1 and facilitating the release of pro-inflammatory cytokines (Saresella et al. 2016). In AD, cells from both the brain and the peripheral immune system display signs of increased NLRP3 activity. Monocytes isolated from patients show elevated levels of NLRP3, ASC (apoptosis-associated speck-like protein containing a CARD), caspase-1, and related cytokines, indicating systemic upregulation of this inflammasome pathway (Saresella et al. 2016). Intriguingly, peripheral monocytes are also able to infiltrate the brain in AD, homing in on areas already burdened by pathological changes (Shi et al. 2024).
In experimental systems, fibrillar Aβ has been shown to activate NLRP3 in microglia, triggering cellular damage and prompting the release of inflammatory molecules like TNF, IL-1β, and nitric oxide (Halle et al. 2008). Consistent with this, human and animal studies reveal heightened levels of active caspase-1 in brain regions affected by AD, particularly in individuals with mild cognitive impairment—a stage often considered a precursor to full-blown AD (McManus and Latz 2024). Animal models have further highlighted the significance of NLRP3. In transgenic mice mimicking AD pathology, knocking out NLRP3 or caspase-1 leads to notable improvements: reduced amyloid buildup, better microglial function, and improved cognitive performance. These improvements are partly due to a shift in microglia toward an anti-inflammatory state (M2 phenotype), which enhances their ability to clear Aβ deposits (Heneka et al. 2013).
Another key element in this process is ASC specks, which are released during inflammatory cell death (pyroptosis) and can directly interact with Aβ to encourage the formation of harmful oligomers. Blocking ASC in mouse models—either genetically or using anti-ASC antibodies—has been shown to reduce the spread of amyloid pathology (Venegas et al. 2017). From a therapeutic perspective, targeting the NLRP3 pathway has shown considerable promise. Small-molecule inhibitors like MCC950 (CRID3) have improved memory and reduced amyloid levels in mouse models. Similarly, drugs like mefenamic acid, which inhibits inflammasome activation by interfering with ion flux, and the caspase-1 blocker VX-765 have shown the ability to reverse cognitive decline and reduce amyloid burden in experimental settings (Dempsey et al. 2017).
Pharmacological inhibition of the NLRP3 inflammasome has shown therapeutic promise. For instance, MCC950 (CRID3), a small-molecule inhibitor, improved memory performance, reduced Aβ load, and enhanced Aβ clearance in AD mouse models (Daniels et al. 2016). Similarly, the NSAID mefenamic acid, which prevents NLRP3 activation by blocking chloride efflux, reversed neuroinflammation and improved microglial function in 3 × Tg AD mice (Flores et al. 2018). Another compound, VX-765, a caspase-1 inhibitor, reversed cognitive impairments and lowered Aβ deposition in J20 mouse models of AD (Tzeng et al. 2018).
Despite these encouraging results, targeting components of the NLRP3 pathway has proven to be complex. Although deleting NLRP3 or inhibiting caspase-1 leads to neuroprotection, eliminating IL-18 does not produce the same effect in APP/PS1 mice (Azargoonjahromi 2024). In fact, IL-18 deficiency appeared to increase neuronal vulnerability, indicating a more nuanced role for this cytokine in disease progression. IL-1β, too, has shown dual behavior: Although it may help clear Aβ in early stages, sustained IL-1β expression can worsen tau pathology (Li et al. 2025). These findings suggest that therapeutic targeting of inflammasome-related pathways must be approached with precision to avoid unintended outcomes (Stancu et al. 2019). Finally, attention has also turned to the role of tau in activating the NLRP3 inflammasome. Although most prior research focused on Aβ, emerging evidence indicates that tau seeds can also stimulate inflammasome activity in microglia, thereby contributing to tau propagation (Ising et al. 2019).
3.2 PD
PD is a progressive neurological condition marked by the gradual loss of dopamine-producing neurons within the substantia nigra region of the brain. This neuronal degeneration results in characteristic motor symptoms, including tremors, muscle stiffness, and slowed movement (bradykinesia). Recent research has increasingly highlighted the role of neuroinflammation—particularly that driven by the NLRP3 inflammasome—throughout the onset and progression of PD. This inflammasome, a central component of the innate immune system, facilitates the release of inflammatory molecules like IL-1β and IL-18, both of which are implicated in neuronal injury seen in PD (Wang et al. 2019; Li et al. 2021).
Among the various inflammasomes, NLRP3 has emerged as especially significant in PD pathology. Studies utilizing the MPTP model—a widely used experimental setup for simulating PD in animals—have demonstrated that NLRP3 is a key contributor to MPTP-induced neuronal damage. In contrast to other inflammasome complexes like NLRP1, NLRP2, NLRC4, or AIM2, NLRP3 uniquely amplifies MPTP's neurotoxic effects. Pharmacological inhibition of NLRP3 using the compound MCC950 has been shown to mitigate the damage to the nigrostriatal pathway in these models, reinforcing the inflammasome's central role in disease development (Huang et al. 2021). The pathological accumulation of alpha-synuclein (α-syn), a defining characteristic of PD, is closely linked to NLRP3 activation. Misfolded α-syn can stimulate immune responses in microglia, the brain's resident immune cells, initiating NLRP3 signaling. This activation sequence includes the engagement of caspase-1, which subsequently drives the production of IL-1β and IL-18 (Si et al. 2021; Guan and Han 2020). Elevated levels of modified α-syn and IL-1β in the peripheral circulation of PD patients further underscore the systemic impact of this inflammatory axis (Wang et al. 2020).
Activation of the NLRP3 inflammasome involves a two-phase mechanism. The initial stage, known as priming, begins with the recognition of DAMPs like α-syn by receptors such as TLR2, TNFR, and IL-1R. These receptors activate the NF-κB signaling cascade, which enhances the transcription of genes encoding NLRP3 and its pro-inflammatory cytokine targets (de Araújo et al. 2022; McKee and Coll 2020). Notably, α-syn can serve as a DAMP itself, interacting with TLR1/2 on microglia and promoting NF-κB activity and NLRP3 upregulation (Daniele et al. 2015). This sets the stage for full inflammasome activation and a sustained inflammatory response that contributes to PD pathophysiology (de Araújo et al. 2022).
The second phase entails the actual formation of the inflammasome structure. Once inside microglia, α-syn aggregates stimulate mitochondrial production of reactive oxygen species (ROS), a key signal that promotes the activation of NLRP3 (Panicker et al. 2019). The enzyme BRCC3 assists by removing inhibitory ubiquitin tags from NLRP3, allowing it to oligomerize and associate with ASC and caspase-1 to form the active inflammasome complex (de Araújo et al. 2022; Cheng et al. 2020). This complex catalyzes the cleavage of pro-IL-1β and pro-IL-18 into their active forms, perpetuating neuroinflammatory damage (Bai et al. 2020; Rashidi et al. 2020). Mitochondrial dysfunction is deeply integrated into this process, as damaged mitochondria release additional DAMPs—such as mtDNA and ROS—that further potentiate inflammasome activity. In addition, when microglia engulf α-syn aggregates, they release cathepsin B, which further stimulates NLRP3 (Codolo et al. 2013).
Interestingly, dopaminergic neurons can exert anti-inflammatory effects by modulating NLRP3 activity. These neurons, through dopamine receptors such as DRD1 and DRD2, can suppress inflammasome components. Activation of DRD1 has been shown to downregulate the expression of NLRP3, caspase-1, and IL-1β while also reducing microglial activation (Jiang et al. 2016; Wang et al. 2018). Furthermore, dopamine signaling via cAMP promotes the degradation of NLRP3 by enhancing its ubiquitination, thereby limiting its ability to form active inflammasome complexes (Yan et al. 2015). This interplay suggests a feedback loop in which dopamine not only suffers the consequences of inflammation but also attempts to restrain it. Unfortunately, as PD progresses and dopaminergic neurons deteriorate, this regulatory mechanism fails, exacerbating the inflammatory milieu (Zheng et al. 2022).
Mitochondrial health is another key factor influencing NLRP3 activity in PD. Mitochondria are crucial for maintaining cellular energy and metabolic stability, but in PD, their function is often impaired (Henrich et al. 2023). Damaged mitochondria release mtDAMPs like ROS and mtDNA, which act as potent activators of the NLRP3 inflammasome in microglia. Experimental PD models involving toxins such as MPTP or rotenone confirm that mitochondrial dysfunction intensifies inflammasome activation, thus fueling neuroinflammation and further neuronal loss (Zhang et al. 2020; Sarkar et al. 2017; Lee et al. 2019). The process of mitophagy, which removes damaged mitochondria, is vital for suppressing this inflammatory pathway. When mitophagy is disrupted—as often observed in PD—NLRP3 activation becomes unchecked, increasing neurotoxicity and cell death (Qiu et al. 2022).
Therefore, the NLRP3 inflammasome is a central mediator of neuroinflammation in PD. Its activation—driven primarily by α-syn pathology and mitochondrial damage—triggers inflammatory cascades that harm dopaminergic neurons and drive disease progression. The complex relationship between dopamine signaling and inflammasome activity adds another layer of intricacy to PD pathology, revealing potential therapeutic targets aimed at modulating this inflammation-centric pathway.
3.3 MS
The NLRP3 inflammasome plays a central role in the innate immune response and has gained recognition for its involvement in the pathogenesis of MS. Its effects in MS are context-dependent, influenced by both the nature and intensity of the initiating trigger. In certain settings, NLRP3 activation can offer protective benefits, particularly by enhancing the immune system's ability to respond to pathogens that might otherwise initiate or worsen MS. This defense mechanism operates through the secretion of pro-inflammatory cytokines, which enhance immune responses. These cytokines also activate microglia—key immune cells within the CNS—enabling them to act as antigen-presenting cells, support adaptive immunity, and clear myelin debris from damaged regions (Seok et al. 2021). Furthermore, the NLRP3 inflammasome activates pyroptosis, an intensely inflammatory type of apoptosis, which helps remove infected or damaged cells and thereby limits viral replication. Despite these protective aspects, prolonged or excessive NLRP3 activation can fuel chronic inflammation and neurodegeneration in MS (Cui et al. 2022).
A defining feature of MS is the chronic infiltration of immune cells into the CNS, leading to persistent inflammation, demyelination, and neural damage. NLRP3 inflammasome activity contributes significantly to this process by promoting the production of IL-1β and IL-18, which support the proliferation and activity of T Helper 17 (Th17) cells (Li and Jiang 2023; Hutton et al. 2016). Th17 cells are a subset of CD4+ T lymphocytes that release IL-17 and granulocyte-macrophage colony-stimulating factor (GM-CSF), cytokines that facilitate immune cell invasion of the CNS and promote damage to the protective myelin sheath surrounding neurons. This attack compromises oligodendrocytes—the cells responsible for producing myelin—leading to progressive neurological impairment (Imitola et al. 2018).
In addition to cell clearance, pyroptosis contributes to a vicious cycle of inflammation by releasing cellular components into the extracellular environment. These contents can further stimulate NLRP3 or other PRRs, reinforcing and perpetuating the inflammatory loop (Zhao and Zhao 2020). This ongoing immune activation exacerbates MS pathology. IL-1β and IL-18, for example, are instrumental in promoting differentiation of both Th1 and Th17 cell populations. Th1 cells secrete interferon-gamma (IFN-γ), which activates microglia and macrophages, triggering additional inflammatory responses and tissue injury (Balasa et al. 2020). Simultaneously, Th17 cells intensify inflammation by recruiting neutrophils and monocytes to the CNS. They also upregulate the expression of chemokines and adhesion molecules on endothelial cells, facilitating immune cell migration from the bloodstream into the CNS (Haghmorad et al. 2024).
At the intracellular level, NLRP3-driven inflammation activates key signaling cascades, including the NF-κB, MAPK, and JAK-STAT pathways (Haftcheshmeh et al. 2022; Castejón et al. 2022). These molecular pathways modulate the expression of genes involved in inflammatory signaling, apoptosis, and stress responses. Their activation influences numerous factors impacting neuronal integrity, such as oxidative stress, autophagic dysfunction, excitotoxic damage from glutamate, and changes in neurotrophic support. Together, these effects amplify neuroinflammation and promote the progression of MS (Manzoor and Koh 2012).
The NLRP3 inflammasome itself is made up of three core components: the NLRP3 sensor protein, the adaptor ASC, and the effector enzyme pro-caspase-1. NLRP3 resides in the cytoplasm and acts as a sentinel for both PAMPs and DAMPs. When triggered, NLRP3 undergoes a conformational transformation, shedding its auto-inhibited state caused by internal domain interactions (Zhang et al. 2023). This structural change allows it to assemble into an active inflammasome complex by oligomerizing with ASC, which in turn recruits pro-caspase-1. Once activated, caspase-1 processes the pro-forms of IL-1β and IL-18 into their mature, biologically active forms, leading to their secretion and the amplification of inflammatory responses within the CNS (Shadab et al. 2023).
Support for the role of NLRP3 in MS comes from both experimental and clinical studies. In preclinical models like experimental autoimmune encephalomyelitis (EAE), researchers have observed heightened activation of the NLRP3 inflammasome in microglia and astrocytes, accompanied by increased levels of IL-1β and IL-18 in both CSF and blood serum (Hou et al. 2020). Complementary findings in human subjects further confirm this role, with MS patients exhibiting elevated activation of NLRP3 components and related cytokines in brain tissue, CSF, and peripheral blood mononuclear cells (PBMCs) in comparison to controls (Zhu et al. 2019). Altogether, the NLRP3 inflammasome acts as a double-edged sword in MS. Although its role in pathogen defense and tissue clearance can be beneficial, its sustained or dysregulated activation drives chronic inflammation, demyelination, and neurodegeneration. The involvement of Th17 cells, pro-inflammatory cytokines like IL-1β and IL-18, and key signaling pathways illustrates the complex and multifaceted influence of NLRP3 inflammasome activity in shaping the immune landscape of MS (Adamu et al. 2024).
3.4 HD
HD is a hereditary neurodegenerative disorder passed down through an autosomal dominant pattern. It results from an abnormal expansion of CAG trinucleotide repeats in the Huntington (HTT or IT15) gene, leading to the production of a mutant huntingtin protein (mHTT) that contains an elongated polyglutamine sequence (Bower and Disease 2014). This misfolded protein disrupts normal neuronal function and triggers widespread neurological dysfunction, which manifests in progressive motor abnormalities like chorea and dystonia, as well as cognitive deterioration and psychiatric symptoms (Keller et al. 2022). A central pathological mechanism in HD is chronic neuroinflammation, where the NLRP3 inflammasome has emerged as a significant contributor to ongoing neuronal injury. This inflammasome, an essential component of the innate immune response, orchestrates the activation of caspase-1, which in turn drives the maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18. Notably, in HD, this immune complex becomes increasingly active—especially within the striatum, a brain region critical for motor function and severely affected in HD (Paldino and Fusco 2022).
In well-established mouse models of HD—such as the R6/2 transgenic mice expressing the human HTT gene with approximately 120 CAG repeats—elevated expression of inflammasome-related genes, including caspase-1, has been reported as early as 13 weeks of age. The activation of NLRP3 in these mice leads to pyroptosis, a highly inflammatory form of programmed cell death, particularly within vulnerable neuron populations such as striatal parvalbumin-positive interneurons and GABAergic spiny projection neurons (Paldino et al. 2020). Importantly, pharmacological blockade of NLRP3 with compounds like MCC950 has been shown to suppress IL-1β release and reduce ROS levels, ultimately mitigating inflammation and improving motor performance in HD models (Chen et al. 2022; Paldino et al. 2020).
One upstream regulator of NLRP3 activity in HD is galectin-3 (Gal-3), a lectin known for its role in modulating immunity and autophagy. Elevated levels of Gal-3 have been identified in both the blood of HD patients and in the microglial cells of HD mouse models (Barake et al. 2020; Siew et al. 2019). Intriguingly, Gal-3 expression begins to rise in microglia even before the onset of physical symptoms and continues to escalate with disease progression. Mechanistically, Gal-3 amplifies inflammatory signaling through the NF-κB–NLRP3 axis, contributing to sustained immune activation. Furthermore, Gal-3 hampers the autophagic degradation of damaged endolysosomes, resulting in the accumulation of cellular waste and subsequent stimulation of the inflammasome. Targeted deletion of Gal-3 in HD mice improves survival and reduces brain pathology, pointing to Gal-3 as a promising therapeutic target for dampening NLRP3-driven inflammation (Siew et al. 2019). In contrast, other galectins like Gal-1 and Gal-8 appear to play a counterbalancing, neuroprotective role by attenuating immune responses (Barake et al. 2020).
Another major factor in NLRP3 inflammasome activation in HD is mitochondrial dysfunction, a hallmark of the disease. Mitochondria in HD-affected neurons become damaged, leading to the leakage of mtDAMPs—including ROS and mitochondrial DNA—that serve as potent signals for inflammasome activation (Fusco and Paldino 2017). This mitochondrial stress not only fuels NLRP3 activity but also contributes to other forms of inflammatory cell death such as necroptosis, accelerating the loss of neurons. Therapeutic strategies aimed at restoring mitochondrial health show promise in modulating this inflammatory cascade. For example, PARP-1 inhibitors like olaparib have been shown to blunt inflammasome activation, reduce pyroptotic cell death, and improve neurological symptoms in HD-model mice (Paldino et al. 2020). These findings emphasize that controlling NLRP3 inflammasome activity—either directly or via upstream pathways such as Gal-3 expression and mitochondrial function—could be key to altering disease progression.
3.5 ALS
ALS is a relentlessly progressive neurodegenerative condition that primarily targets motor neurons in the cerebral cortex, brainstem, and spinal cord. The loss of these neurons results in muscle weakness, atrophy, and ultimately paralysis (Morris 2015). Although the precise etiology of ALS remains elusive, approximately 10% of cases are linked to hereditary mutations, with the SOD1 (superoxide dismutase 1) gene being the most extensively investigated (Wang et al. 2024). The pathological hallmark of ALS includes the misfolding and aggregation of proteins, notably mutant SOD1, which significantly contributes to neuronal dysfunction and disease progression (Duranti and Villa 2022).
A growing body of research has highlighted the NLRP3 inflammasome as a key mediator in the neuroinflammatory processes associated with ALS. Elevated expression of NLRP3 and its adaptor protein ASC has been documented in astrocytes of SOD1^G93A mouse models, as well as in postmortem spinal cord tissues from ALS patients (Johann et al. 2015). In these models, microglia internalize aggregated or soluble mutant SOD1, triggering the production of ROS that, in turn, activate caspase-1 and induce cleavage of IL-1β, a critical inflammatory cytokine. Interestingly, aggregated forms of SOD1 have a stronger effect in activating this pathway, highlighting their role in driving NLRP3 inflammasome activation. Therapeutically, the specific NLRP3 inhibitor MCC950 has demonstrated efficacy in blocking IL-1β release, underscoring the inflammasome's central role in mediating inflammatory responses in ALS (Deora et al. 2020).
Beyond SOD1-related cases, NLRP3 inflammasome activation has also been linked to other forms of ALS. Transcriptomic analyses in SOD1^G93A mice reveal increased expression of caspase-1, IL-1β, IL-18, and NF-κB pathway genes in the spinal cord, indicating widespread inflammasome activation (Bellezza et al. 2018; Gugliandolo et al. 2018). This inflammatory cascade involves both astrocytes and microglia, the latter of which are observed to express NLRP3 and contribute to ongoing neuronal damage (Johann et al. 2015). Activated microglia and reactive astrocytes are typically found in regions undergoing motor neuron degeneration, further implicating neuroinflammation as a central player in ALS pathophysiology (Liu and Wang 2017; Liu et al. 2021). However, the roles of microglia and astrocytes in ALS are not exclusively detrimental. Under certain conditions, microglia may exhibit protective behaviors, such as engulfing debris and releasing supportive cytokines. Yet in other contexts, these same cells exacerbate neural injury through the release of pro-inflammatory mediators like IL-1β and IL-18 (Pehar et al. 2017; Clarke and Patani 2020). In the SOD1^G93A mouse model, disease progression and the onset of paralysis appear tightly linked to the level of microglial activation and the extent of the neuroinflammatory response (Liu and Wang 2017; Herskovits et al. 2018).
Despite substantial evidence implicating the NLRP3 inflammasome in ALS, the specific roles of its associated cytokines remain under investigation. For instance, some studies report elevated IL-18—but not IL-1β—in the blood serum of ALS patients (Italiani et al. 2014). Meanwhile, both NLRP3 and caspase-1 have been detected in brain tissues from individuals with ALS (Kadhim et al. 2016). Yet, clinical trials using Anakinra, an interleukin-1 receptor antagonist, have failed to curb neuroinflammation in ALS, hinting that IL-18 might play a more pivotal role in the disease than IL-1β (Maier et al. 2015). Moreover, emerging research suggests that inflammasomes other than NLRP3 may also contribute to ALS pathogenesis, further complicating the inflammatory landscape of the disease (Debye et al. 2018; Meissner et al. 2010).
In light of these findings, therapeutic approaches aimed at modulating inflammasome activity have shown promise in preclinical ALS models. Agents such as the cyclic dipeptide His-Pro and the selenium compound diphenyl diselenide have demonstrated neuroprotective effects by suppressing nitrative stress and inhibiting NLRP3 activation via reductions in NO and ROS levels (Zhang et al. 2021; Grottelli et al. 2019). In addition, the hormone 17β-estradiol has been found to enhance motor neuron survival and dampen inflammasome activation in a humanized ALS mouse model, offering yet another avenue for therapeutic intervention (Heitzer et al. 2017). Therefore, although the exact role of the NLRP3 inflammasome in ALS remains complex and not fully understood, there is substantial evidence linking its activation to neuroinflammation and motor neuron degeneration.
3.6 Epilepsy
Epilepsy is a chronic neurological condition marked by recurring, spontaneous seizures that arise from abnormal bursts of electrical activity in the brain. These seizures vary widely in presentation—from subtle lapses in awareness to full-body convulsions—depending on the areas of the brain affected (Huff and Huff 2020). Increasingly, research points to neuroinflammation as a major contributor to both the onset and progression of epileptic seizures. Among the central players in this inflammatory landscape is the NLRP3 inflammasome, which has been associated with both experimental models of epilepsy and in clinical cases involving human patients (Haque et al. 2024).
Experimental investigations have shed considerable light on how NLRP3 contributes to epilepsy. In vitro studies, including those using microglial cells derived from epileptic mice, have shown that exposure to seizure-inducing agents like kainic acid or picrotoxin leads to the activation of NLRP3 inflammasome components. These include caspase-1, IL-1β, and IL-18, key mediators of inflammatory signaling (Sun et al. 2019; Rong et al. 2019). Importantly, these inflammatory responses can be significantly blunted by MCC950, a selective NLRP3 inhibitor, which blocks IL-1β secretion and dampens neuroinflammation. This finding strongly supports NLRP3's role as a mediator of inflammation in epileptic conditions (Meissner et al. 2010). Another informative platform is the organotypic hippocampal slice culture (OHSC) model, which preserves essential features of brain architecture and neuron-glia communication. Within this model, heightened expression of NLRP3 is associated with increased neuronal excitability, cell death, and elevated levels of pro-inflammatory cytokines such as IL-1β, IL-6, and TNFα during seizure-like activity (Magalhães et al. 2018). Notably, the natural anti-inflammatory agent curcumin has been found to suppress NLRP3 activation and reduce epileptiform discharges in OHSCs, underscoring the therapeutic potential of targeting this inflammasome in epilepsy (Drion et al. 2019; Gong et al. 2015).
In vivo studies using seizure-inducing agents like pilocarpine and kainic acid further support the pivotal involvement of the NLRP3 inflammasome in epilepsy. These models consistently show increased levels of NLRP3 and its downstream effectors in the hippocampus, particularly during status epilepticus (SE)—a prolonged seizure state that causes extensive neuronal damage and glial activation. Experimental interventions that block NLRP3 activation—either through genetic deletion or pharmacological inhibition—have been shown to reduce seizure severity and protect against neuron loss, providing compelling evidence of NLRP3's involvement in the progression of epileptic pathology (Hu et al. 2020; Yang et al. 2023). For instance, in the pilocarpine model, NLRP3-deficient mice displayed reduced IL-1β production and less neuronal necrosis compared to wild-type controls, pointing to NLRP3's role in refractory temporal lobe epilepsy (TLE) (Wu et al. 2020).
Human studies reinforce these experimental findings. Patients with TLE, particularly those with drug-resistant forms, exhibit elevated levels of NLRP3 and IL-1β in the hippocampus and temporal neocortex. These proteins are predominantly localized in pyramidal neurons and glial cells, indicating a localized inflammatory response contributing to neuronal dysfunction (de Brito Toscano et al. 2021; Yue et al. 2020). Moreover, serum IL-1β levels have been positively associated with seizure severity in patients, offering a potential biomarker for disease activity (Wu et al. 2020). Another molecule of interest is the P2X7 receptor, a purinergic receptor known to activate the NLRP3 inflammasome. This receptor has been found to be overexpressed in the temporal lobes of TLE patients, linking extracellular ATP signaling with inflammasome-mediated inflammation and seizure propagation (Song et al. 2019). Collectively, the evidence paints a clear picture: Activation of the NLRP3 inflammasome has a central role in the neuroinflammatory processes that underlie both the initiation and persistence of epilepsy.
4 Therapeutic Potential of NLRP3 Inflammasome Inhibitors
A number of therapeutic agents targeting the NLRP3 inflammasome have been developed, with several progressing into clinical trials. These inhibitors are designed to either directly block NLRP3 activation or intervene in downstream inflammatory processes initiated by the inflammasome. When the inflammasome itself is a central contributor to disease pathology, such as in many neurodegenerative disorders, direct NLRP3 inhibition may provide the greatest therapeutic benefit. However, in conditions where inflammation is driven primarily by downstream cytokine cascades, targeting later stages in the pathway may be more effective. Given the established role of chronic inflammation in neurodegeneration, these inhibitors are emerging as promising candidates for new treatment strategies.
4.1 BAY 11-7082
BAY 11-7082 is a small molecule vinylsulfone initially characterized as an inhibitor of the NF-κB pathway via blockade of IKKβ kinase activity. It suppresses the phosphorylation of IκBα, thereby preventing NF-κB nuclear translocation and subsequently inhibiting NLRP3 inflammasome activation (Kumar et al. 2012). BAY 11-7082 further lowers NLRP3 components and diminishes the activation of caspase-1 in BV2 microglial cells subjected to oxygen-glucose deprivation followed by reoxygenation, highlighting its therapeutic potential in stroke (Chen et al. 2022). It effectively mitigates neuroinflammation caused by chronic cold stress through inhibition of GABA-induced NLRP3 activation in microglia (Lang et al. 2020). Mechanistically, BAY 11-7082 acts as a Michael acceptor, alkylating essential cysteine residues in the ATPase domain of NLRP3, which blocks ASC pyroptosome formation and NLRP3 inflammasome assembly, with greater specificity toward NLRP3 compared to NLRP1, NLRC4, and AIM2 (Juliana et al. 2010). Furthermore, in a model of burn-induced acute lung injury, BAY 11-7082 reduced IL-1β and IL-18 levels and alleviated histopathological damage when administered shortly after injury (Han et al. 2015). In systemic diseases like lupus nephritis and neuropathic pain, BAY 11-7082 demonstrated multifaceted therapeutic effects by concurrently inhibiting NF-κB and NLRP3 signaling, reducing inflammation, and improving clinical outcomes (Zhang et al. 2017; Zhao et al. 2013). Its favorable pharmacokinetic profile and tolerability make BAY 11-7082 a promising candidate for future therapeutic applications (Juliana et al. 2010).
4.2 MCC950
MCC950 (also known as CP-456,773 or CRID3) is a highly selective inhibitor of the NLRP3 inflammasome, a protein complex implicated in several inflammatory diseases. Its therapeutic potential has been extensively studied in various disease models. MCC950 operates by directly binding to the NACHT domain of the NLRP3 protein, blocking ATP hydrolysis and preventing inflammasome assembly, which in turn inhibits the activation of the pro-inflammatory cytokine IL-1β (Wu et al. 2020). Notably, MCC950's inhibitory effects are independent of several common inflammasome activation pathways, such as calcium signaling, potassium efflux, and mitochondrial dysfunction (Coll et al. 2015). MCC950 has shown promise in treating a wide range of inflammatory conditions. In atherosclerosis, it prevents macrophage inflammation and pyroptosis, reducing plaque sizes in hyperlipidemic mouse models (Zeng et al. 2021; van der Heijden et al. 2017). Furthermore, MCC950 has been effective in attenuating the severity of spontaneous chronic colitis in mice (Perera et al. 2018) and mitigating diabetic retinopathy in high glucose-induced conditions by inhibiting dysfunction in human retinal endothelial cells (Ye et al. 2022). It also holds potential in neuroinflammatory diseases: MCC950 reduces neuroinflammation in ischemic stroke by preserving the blood–brain barrier (Franke et al. 2021) and improves insulin sensitivity in diabetic encephalopathy (Zhai et al. 2018). In addition, it protects against diabetic kidney injury by lowering the expression of fibrosis markers in mesangial cells exposed to high glucose levels (Zhang et al. 2019). Moreover, MCC950 has shown significant therapeutic effects in models of neurodegenerative diseases. In PD, oral administration of MCC950 alleviates dopaminergic degeneration (Gordon et al. 2018), and in ALS, it reduces microglial neuroinflammation, potentially slowing disease progression (Deora et al. 2020). The compound has also demonstrated efficacy in reducing inflammation in models of MS (Coll et al. 2015) and cystic fibrosis (Brahadeeswaran et al. 2022).
4.3 Oridonin
Oridonin (Ori) is a bioactive ent-kaurane diterpenoid and the main active compound in Rabdosia rubescens, widely used in traditional Chinese medicine (Kuo et al. 2014). It possesses notable anticancer activities such as inducing apoptosis, suppressing angiogenesis, and causing cell cycle arrest (Kadota et al. 1997; Fujita et al. 1976). Oridonin exerts anti-inflammatory effects by inhibiting NF-κB and MAPK pathways and repressing the release of inflammasome-independent proinflammatory cytokines like TNF-α and IL-6 (Zhao et al. 2017; Xu et al. 2009). It has demonstrated therapeutic potential in models of neuroinflammation, sepsis, and colitis (Wang et al. 2014, 2015). Mechanistically, oridonin covalently binds to cysteine 279 within the NACHT domain of NLRP3, blocking its interaction with NEK7 and preventing inflammasome activation without affecting AIM2 or NLRC4 inflammasomes. It selectively inhibits NLRP3 inflammasome assembly, not impacting upstream events like potassium efflux or mitochondrial damage (He et al. 2018). In vivo, oridonin reduced myocardial infarct size and improved cardiac function following myocardial infarction (Gao et al. 2021), suppressed neuroinflammation and preserved BBB integrity in traumatic brain injury (Yan et al. 2020), and alleviated insulin resistance by decreasing macrophage infiltration and NLRP3 activation (Liang et al. 2021). In addition, oridonin significantly lowered circulating IL-1β levels and neutrophil infiltration in gouty arthritis and peritonitis models (He et al. 2018).
4.4 OLT1177
OLT1177 is an orally active β-sulfonyl nitrile compound that selectively inhibits the NLRP3 inflammasome by binding to NLRP3 and suppressing its ATPase activity (Paik et al. 2021). It blocks ASC oligomerization and inflammasome assembly without altering upstream signals like K⁺ efflux (Marchetti et al. 2018). In animal models, OLT1177 reduced neuroinflammation, colitis, and myocardial injury (Toldo et al. 2019; Oizumi et al. 2022; Sánchez-Fernández et al. 2019). Clinical trials in healthy volunteers showed good oral bioavailability, a long half-life, and no toxicity (Marchetti et al. 2018). Phase 2 studies revealed its potential in treating acute gout (Jansen et al. 2019). Due to its strong safety profile and effective NLRP3 inhibition, OLT1177 is under further investigation for inflammatory diseases such as heart failure, gout, and Schnitzler's syndrome (Marchetti 2019).
4.5 Tranilast
Tranilast is a synthetic compound structurally related to tryptophan metabolites initially identified as an anti-allergic compound preventing release of histamine from mast cells (Darakhshan and Pour 2015). It is a fairly safe compound, with high doses showing good tolerance in patients (Zahid et al. 2019; Platten et al. 2005). Tranilast selectively inhibits the NLRP3 inflammasome while leaving NLRC4 and AIM2 inflammasomes unaffected (Huang et al. 2018). It binds directly to the NACHT domain of NLRP3, disrupting NLRP3-NLRP3 interactions and preventing inflammasome assembly, IL-1β production, and caspase-1 activation, without interfering with upstream events like K⁺ efflux, mitochondrial damage, or ROS generation (Jiang et al. 2017; Saeedi-Boroujeni et al. 2021). Therapeutically, tranilast has demonstrated significant benefits in murine model of gout and type 2 diabetes mellitus (Paik et al. 2021; Huang et al. 2018). It also ameliorated gestational diabetes symptoms by suppressing NLRP3 activation and reducing inflammatory responses (Cao and Peng 2022). Moreover, tranilast improved NLRP3 ubiquitination, thereby attenuating vascular inflammation and atherosclerosis in animal models (Chen et al. 2020). Its favorable safety profile and broad anti-inflammatory actions are currently being further evaluated in clinical trials (Das et al. 2021).
4.6 CY-09
CY-09 is a potent and direct inhibitor of the NLRP3 inflammasome, identified by Jiang et al. through structure–activity relationship studies of the CFTR (inh)-172 analog, C172 (Jiang et al. 2017). Unlike C172, CY-09 lacks CFTR-inhibitory actions (Ma et al. 2002; Sonawane and Verkman 2008). In LPS-primed bone marrow-derived macrophages, CY-09 dose-dependently blocks ATP and nigericin-induced caspase-1 activation and IL-1β secretion, independently of signal 1 or post-translational modifications like ubiquitination (Jiang et al. 2017). Mechanistically, CY-09 binds directly to the Walker A motif of NLRP3, preventing ATP binding without affecting NLRP1, NLRC4, RIG-1, or NOD2. In vivo, CY-09 exhibits excellent pharmacokinetics with good oral bioavailability and safety (Jiang et al. 2017).
4.7 3,4-Methylenedioxy-β-Nitrostyrene (MNS)
MNS is a potent NLRP3 inflammasome inhibitor identified through kinase inhibitory library screening (Gan et al. 2018). MNS interacts with the LRR and NACHT regions of the NLRP3 protein, suppressing its ATPase activity, while having no impact on AIM2 or NLRC4 inflammasomes (He et al. 2014). It specifically inhibits NLRP3 by impairing ATPase function without altering K⁺ efflux (He et al. 2014). MNS functions as a Michael acceptor, relying on its nitrovinyl side chain for biological activity. Furthermore, MNS abrogates IL-1β and IL-18 secretion and caspase-1 activation without changing gene expression levels of inflammasome components (He et al. 2014). In vivo, MNS has demonstrated therapeutic potential in enhancing wound healing following burn injury (Xiao et al. 2016).
4.8 JC124
JC124 is a selective NLRP3 inflammasome inhibitor that targets ASC oligomerization without affecting NLRP3 ATPase activity (Marchetti et al. 2015). It directly binds the NLRP3 inflammasome complex and reduces ASC aggregation, caspase-1 activation, and IL-1β secretion. In models of TBI, JC124 suppresses the expression of TNF-α, NLRP3, IL-1β, inducible NOS, ASC, and caspase-1, offering significant neuroprotection. It also decreased cortical lesion volume and neuronal degeneration (Kuwar et al. 2019). In APP/PS1 mice, JC124 reduced Aβ buildup and improved cognitive performance (Yin et al. 2018). Furthermore, JC124 demonstrated protective effects in acute myocardial infarction and AD models, enhancing its potential as a therapeutic agent (Yin et al. 2018; Fulp et al. 2018).
4.9 FC11A-2
FC11A-2, a benzimidazole compound, has shown potent inhibition of the NLRP3 inflammasome by targeting caspase-1 activation without affecting NF-κB signaling (Liu et al. 2013). It suppresses IL-1β and IL-18 release in LPS-primed THP-1 cells activated with ATP, with an IC50, although complete cytokine suppression was not achieved even at 30 µM. FC11A-2 inhibits procaspase-1 autocleavage, reducing active caspase-1 and subsequent cytokine maturation. In addition, FC11A-2 improved disease symptoms, such as body weight, colon length, and inflammation markers (Liu et al. 2013). Moreover, FC11A-2 markedly reduced the severity of experimentally induced colitis in mice by suppressing caspase-1 activation (Liu et al. 2013).
4.10 Natural Products
Natural products have been recognized for their ability to inhibit the NLRP3 inflammasome, and their effects on neurodegenerative diseases are becoming increasingly recognized. Compounds like crocin, ginsenoside Rg1, hyperoside, kaempferol, luteolin, parthenolide, and silibinin have demonstrated neuroprotective and anti-inflammatory effects through various mechanisms. These natural products represent a valuable area for future research and clinical trials, potentially transforming the treatment of neurodegenerative diseases. For a detailed overview of these natural products and their role as NLRP3 inflammasome inhibitors, refer to Table 1, which summarizes the key findings, disease models, and mechanisms of action of these compounds.
| Natural compounds | Model | Mechanism of action | Ref. |
|---|---|---|---|
| Crocin | AD, PD | Suppresses inflammation, activates PI3 K/AKT signaling, reduces apoptosis, enhances memory and neuronal survival, inhibits NLRP1 and AIM2 gene expression | Su et al. (2024), Alizadehmoghaddam et al. (2024), Ahmed et al. (2020) |
| Ginsenoside Rg1 | Depression, AD | Modulates HPA axis, regulates synaptic plasticity, restores mitophagy, reduces calcium overload, enhances glucocorticosteroid receptors, inhibits NLRP1, ASC, caspase-1, IL-1β, IL-18 | Yang et al. (2023), Wang et al. (2023) |
| Hyperoside | AD | Reduces plaque deposition, ameliorates neuronal cell death, inhibits apoptosis via calcium signaling, mitigates neuroinflammation, inhibits NLRP1 inflammasome | Wang et al. (2023), Song et al. (2023), Chen et al. (2021) |
| Kaempferol | PD, AD, ischemic stroke, epilepsy | Inhibits caspase cascade, reduces apoptosis, inhibits NLRP1/NLRP3 inflammasomes, modulates ROS generation, MAPK pathways, NF-κB, and JNK signaling | Rahul (2021), Zhang et al. (2020), Lin et al. (2019), Hong et al. (2009) |
| Luteolin | AD, PD | Inhibits ER stress, reduces neuroinflammation, modulates apoptotic proteins, decreases NLRP1, NLRP3, NOX4, and TXNIP proteins, PPARγ-dependent mechanism | Goyal et al. (2024), Zhu et al. (2024), Kou et al. (2022) |
| Parthenolide | AD | Reduces ROS, restores mitochondrial function, improves cognitive function, inhibits NLRP1, NLRP3 inflammasomes, modulates AMPK/GSK3β/Nrf2 and MAPK/TRIM31/NLRP3 axis | Sun et al. (2023), Fan et al. (2023) |
| Silibinin | AD | Reduces neuroinflammation, activates ROS-BDNF-TrkB pathway, reduces apoptosis, downregulates NLRP1/NLRP3 inflammasome, inhibits NF-κB signaling | Song et al. (2016), Matias et al. (2019) |
| Sulforaphane (SFN) | Cerebral ischemia | Inhibits NLRP3 inflammasome activation, downregulates cleaved caspase-1, reduces IL-1β and IL-18 expression | Yu et al. (2017) |
| Rutin | Spinal cord injury | Inhibits NLRP3 inflammasome activation, reduces TNF-α, IL-1β, IL-18, ROS production | Wu et al. (2016) |
| Resveratrol (RSV) | Brain injury, cerebral ischemia | Inhibits NLRP3 inflammasome activation, reduces IL-1β, IL-18, and TNF-α, protects against neuronal apoptosis | Zhang et al. (2017), Ma et al. (2014) |
| Mangiferin | Brain injury, chronic mild stress | Inhibits NLRP3 inflammasome activation, reduces IL-1β, IL-18, and oxidative stress | Wang et al. (2017), Fan et al. (2017) |
- Abbreviations: AD, Alzheimer's disease; AIM2, Absent in Melanoma 2; NF-κB, nuclear factor kappa B; PD, Parkinson's disease; ROS, reactive oxygen species.
5 Conclusion
Evidence from neurodegenerative diseases consistently underscores the inflammasome's detrimental role, activated by pathological protein aggregates, oxidative stress, and mitochondrial dysfunction. Although the inflammasome initially serves a protective immune function, chronic activation contributes to persistent inflammation, neurotoxicity, and disease exacerbation. The development of inhibitors targeting NLRP3 inflammasome activation and downstream signaling pathways represents a promising therapeutic frontier. Agents such as MCC950, CY-09, oridonin, and natural products like β-hydroxybutyrate have demonstrated efficacy in preclinical models by suppressing inflammasome-mediated inflammation and protecting neuronal integrity. However, targeting the inflammasome involves careful modulation of inflammatory processes to avoid disrupting beneficial immune responses. Future therapeutic approaches should focus on precisely balancing inflammasome inhibition, enhancing the clearance of pathological protein aggregates, and preserving neuronal health. Continued investigation into the molecular regulation of NLRP3 inflammasome activity, combined with clinical trials of promising inhibitors, could significantly advance treatment strategies and improve outcomes for patients with neurodegenerative disorders.
Author Contributions
All authors contributed to writing of the original draft. The manuscript was reviewed and edited by Mohammed Ahmed Mustafa. All authors read and approved the final manuscript.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The author confirms that the data supporting the findings of this study are available within the article.