Myelin plasticity, neural activity, and traumatic neural injury
ABSTRACT
The possibility that adult organisms exhibit myelin plasticity has recently become a topic of great interest. Many researchers are exploring the role of myelin growth and adaptation in daily functions such as memory and motor learning. Here we consider evidence for three different potential categories of myelin plasticity: the myelination of previously bare axons, remodeling of existing sheaths, and the removal of a sheath with replacement by a new internode. We also review evidence that points to the importance of neural activity as a mechanism by which oligodendrocyte precursor cells (OPCs) are cued to differentiate into myelinating oligodendrocytes, which may potentially be an important component of myelin plasticity. Finally, we discuss demyelination in the context of traumatic neural injury and present an argument for altering neural activity as a potential therapeutic target for remyelination following injury. © 2017 Wiley Periodicals, Inc. Develop Neurobiol 78: 108–122, 2018.
INTRODUCTION
Oligodendrocytes form myelin sheaths by extending long processes that wrap around axons to affect action potential conduction. Pio del Rio Hortega first described these cell types in 1928 (Pérez-Cerdá et al., 2015). For decades, myelin and oligodendrocytes were considered a static player in the nervous system. In 1966, Yakovlev and Lecours described the “maturation” and formation of myelination in the human nervous system over decades of life, concluding once myelin was formed it was static (Yakovlev and Lecours, 1966). Work in the 1980s examined the possibility of oligodendrocyte turnover in adult organisms in both health and disease, though oligodendrocytes were thought incapable of full remyelination following demyelination (reviewed by Debbage, 1986). Only recently have scientists begun to consider that the formation of myelin is a dynamic and ongoing process throughout an organism's lifetime. This review summarizes recent studies that show that myelin growth and refinement occurs during development, but also that myelin plasticity is required for motor or cognitive learning, memory, and recovery from injury or disease. Following insult, pathological demyelination and aberrant spared myelin structures can lead to abnormal signal conduction, nerve fiber vulnerability and even degeneration. As a result many investigators are interested in improving remyelination as a potential therapeutic target to improve recoveries and outcomes.
The term “myelin plasticity” can encompass a number of different cellular processes. As researchers continue to probe this recently discovered form of central nervous system (CNS) plasticity, new questions continue to emerge with each new finding. What are the proper methods to empirically measure myelin plasticity? What is the role of neural activity in modulating myelin sheath adaptation? How do discrete changes in myelin morphology contribute to better signal conduction following injury or disease? This review focuses on the state and limitations of our understanding of CNS myelin plasticity in a variety of circumstances from development to recovery from traumatic injury. Further, we review current myelin measurement techniques for what they can and cannot tell us about myelin plasticity. For the purposes of limiting the focus of this review, we omit oligodendrocyte precursor biology and inflammatory demyelination models. For further consideration on myelin precursor biology the reader is referred to one of the many excellent reviews on the topic (e.g., Levine et al., 2001; Nishiyama et al., 2016). For an excellent review of the regulation of myelin regeneration in inflammatory disease, please see Fancy et al. (2011). In addition, we discuss the exciting opportunities for experimentation around the role of neural activity and myelin growth and present an argument that influencing myelin plasticity through neural activity may be a target for restoration of the function of injured neural circuits.
DEFINING MYELIN PLASTICITY
Role of Myelin in the CNS
In the CNS of vertebrates, action potential speed is controlled in part by the myelination of axons. Myelination increases conduction velocity by reducing the capacitance and increasing membrane resistance of axons, such that action potentials regenerate at Nodes of Ranvier via saltatory conduction. Many features of myelin can affect its function, and different measures used to describe myelin are listed in Table 1. Longer myelin sheaths, or internodes, and thicker myelin increase signal velocity (Waxman, 1980). Modeling has suggested that axon diameter and internode length are of greater importance than myelin thickness in affecting conduction velocity (Waxman, 1980; Lasiene et al., 2008). The size of the node is another important determinant in conjunction with internode length, and thus can affect conduction velocity, as well (Ford et al., 2015; Arancibia-Carcamo et al., 2017). Other features reflect more subtle myelin characteristics, such as the level of compaction between layers, the number of wraps, and its attachment to the axon. Myelin adheres to proteins in the axon at the paranodes, regions flanking the node on each side (for a review on nodal architecture, please see Chang and Rasband, 2013). More subtle features of myelination are more rarely measured because they require tedious, unbiased analysis of electron microscopy (EM). Nonetheless, decompaction, aberrant laminar structure, and blebbing of myelin membranes has been described, particularly as a function of normal aging process (reviewed by Peters, 2002). The effect of these abnormalities on conduction velocities has not been fully modeled. However, Guiterez et al. (1995) have taken advantage of a genetic model of myelin decompaction to show that loss of laminar structure leads to a significantly reduced conduction velocity of the optic nerve.
| Change in | Observed via | Evidence for Model of Plasticity |
|---|---|---|
| G-ratio | Cross-sectional EM; Immunohistochemistry (IHC) (less accurate) | Remodeling; Replacement |
| Myelin Compaction | Longitudinal or horizontal EM | Remodeling |
| Number of Myelin Wraps | Cross-sectional EM (combined with metabolic or membrane label and immune-EM) | Remodeling; Replacement |
| Number of Myelinated Axons | EM; IHC; live imaging | New Internodes; Remodeling |
| OPC/Mature Oligodendrocyte Number | IHC; live imaging combined with a genetic/viral reporter system | New Internodes; Replacement |
| Myelin or Axon Protein Expression | IHC; Western blotting | New Internodes; Remodeling; Replacement |
| Myelin Sheath Length | IHC; live imaging and genetic/viral membrane reporter | Remodeling; Replacement |
- Measures of myelin morphology and requisite experimental and/or imaging techniques used to demonstrate myelin changes. Each measure may add evidence to one of the three models of myelin plasticity. Advances in in vivo live imaging have allowed scientists to observe many of these measures in single cells and myelin over time and in response to stimuli.
| Pathology | Observed via | Potentially repaired via |
|---|---|---|
| Blebbing/Loss of Compaction | EM, genetic/viral labeling | Remodeling; Replacement |
| Demyelination | IHC; EM | Replacement |
| Channel Spreading | IHC | Replacement |
| Paranodal Attachment Abnormalities | EM | Remodeling; Replacement |
- Commonly occurring myelin abnormalities observed following SCI, and potential mechanisms that may contribute to re-establishment of proper myelination and signal conduction.
In the CNS myelin sheaths are formed during the maturation of oligodendrocytes from glial progenitor cells (for a review on cues of progenitor maturation and differentiation, see Rivera et al., 2010). A recent model of zebrafish and mouse developmental myelination proposes that oligodendrocytes wrap via a leading cellular process that makes initial contact with the axon (Snaidero et al., 2014). Each new wrap evolves from this internal lip as new membrane is generated. In the mouse, this process maintains cytoplasmic continuity with the soma until post-natal day 60 (P60), even after compaction has begun. The continuity may be re-established, potentially allowing for some degree of refinement (Snaidero et al., 2014). In an alternate model formed from an examination of developing bovine and avian tissue, myelin is proposed to form via fusion of membranes and proteins around the axon to be ensheathed (Szuchet et al., 2015). These exemplars are seemingly incompatible and future work is needed to determine whether one model is correct or even if a hybrid of these two mechanisms occurs. Furthermore, it has not been shown which, if either, of these models contributes to myelin plasticity and development in the adult.
Although many CNS axons are empirically found to be myelinated for maximal conduction velocity, many are myelinated for sub-maximal conduction, implying the appropriate conduction velocity for a given axon is not necessarily the fastest, but rather the velocity that allows for action potentials to arrive at axon terminals at the appropriate time (Waxman, 1980). For example, the auditory system has emerged as a prime model for studying variable signal timing (reviewed by Seidl, 2014). Signals from each ear must arrive in auditory processing nuclei with temporal precision in order to localize sound. Auditory axons display a spectrum of myelination patterns in order to accomplish this precision. Indeed, a recent study has shown that temporal precision of the auditory system in rodents is established in part via myelination differences, and that neural activity plays a role (Stange-marten et al., 2017). Furthermore, axons in the mouse cortex have been observed to be irregularly myelinated along their lengths (Tomassy et al., 2014), and in the zebrafish different CNS tracts are reliant to differing degrees on neuronal activity to dictate internode lengths (Koudelka et al., 2016). These studies all point to the need for precise signal timing, and suggest an inherent capacity of new oligodendrocytes to modify sheath morphology and thereby conduction times throughout life.
In addition to affecting action potential conduction velocity, recent reviews have highlighted how myelination may also provide metabolic (Beirowski et al., 2016), structural, and functional (Nave, 2010) support that contributes to axon survival. Thus, it is important to consider that myelin plasticity likely plays a role in multiple axonal functions beyond conduction speed.
Three Models of Myelin Plasticity
As oligodendrocytes and myelin serve such important and varied functions, it is logical that changes in neural circuitry would dictate an alteration of myelination. For the purpose of this review, we distinguish between three possible cellular processes that structurally alter myelin, described in Figure 1: (1) formation of new myelin sheaths around previously unmyelinated axons, (2) remodeling of an existing myelin sheath, and (3) the removal and replacement of a myelin sheath with a structurally different internode. In Table 1 we classify some measures of myelin changes, the category of myelin plasticity each may represent, and the appropriate research techniques to demonstrate these processes. However, it is important to recognize the limits and assumptions of each technique. For example, each histological approach reveals only a part of the morphological features of myelin and cannot be confidently interpreted as affecting the function of conduction velocity. Furthermore, many of these techniques are mutually exclusive, making the assessment of a full panel of myelin characteristics a great challenge that has slowed progress in the field. Here we consider the uses and limitations of current methods but also consider emerging technologies that will help investigators probe some of the most exciting models of myelin plasticity.
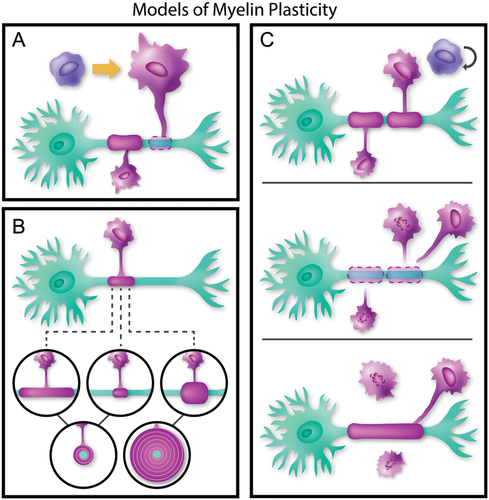
Three models of myelin plasticity: (A) myelination of bare axons by new internodes: an OPC differentiates to form a new oligodendrocyte in order to myelinate an unmyelinated section of axon (B) remodeling or refinement of existing myelin: an existing sheath may lengthen, shorten, or thicken. In cross section, thickening of an internode reflects an increase in the number of myelin wraps (C) replacement of myelin sheaths by new internodes: two existing, short internodes retract from the axon, while an OPC leaves the cell cycle to differentiate and form a single longer sheath. Currently, no data exist regarding the fate of oligodendrocytes with retracted sheaths.
Myelination of Bare Axons by New Internodes. In the adult CNS, an axon may not be uniformly myelinated along its length (Ibrahim et al., 1995; Tomassy et al., 2014), providing a potential site for future myelination and signal refinement. Thus, one source of myelin plasticity in adults may be the coverage of completely or partially unmyelinated axon segments; this concept is illustrated in Figure 1A. The logical question then follows: are mature oligodendrocytes capable of division to form new internodes, or are progenitor cells responsible for forming new myelin sheaths?
In the 1980s some studies suggested that mature oligodendrocytes were capable of dividing (Sturrock, 1981) often based on little more than the morphology of nuclei which Ramon y Cajal had suggested implied mitosis (reviewed by Debbage, 1986). A few studies did suggest long-term non-pathological turnover of oligodendrocytes and myelin (e.g. Korr, 1982; Sturrock, 1981; reviewed by Debbage, 1986) but many studies focused on demylination models to determine the source of new myelin sheaths (again, see Debbage, 1986). Distinguishing between previously developed and newly generated oligodendrocytes or myelin was made possible with the advent of proliferation reporters, such as bromo-deoxyuridine. For example, tritiated thymidine was used in a study that concluded oligodendrocytes proliferate in response to injury (Ludwin 1984), with the determination of oligodendrocytes based solely on cellular morphology. More recently, genetic or viral reporters of the oligodendrocyte lineage and membrane-targeting reporters that visualize myelin have been developed (Powers et al., 2013; Deng et al., 2014; Hill et al., 2014). Though these reporters need not be restricted to injury, they have proved useful to isolate the source of newly generated oligodendrocytes and myelin following injury (e.g. Powers et al., 2013). Indeed, studies show that oligodendrocyte precursor cells (OPCs), not oligodendrocytes, are the source of remyelination, and that OPCs divide and differentiate in response to injury (Blakemore and Keirstead, 1999). Recent studies have shown more directly that, at least in injury, mature oligodendrocytes do not contribute to remyelination (Crawford et al., 2016). To our knowledge, researchers have not yet conducted similar studies in non-injured models to show that mature oligodendrocyte do not proliferate or form new myelin. Such studies are needed to definitively show that OPCs are the source of new sheaths in non-pathological conditions in a manner similar to the post-insult environment.
If the majority of new myelin is formed by newly differentiated oligodendrocytes, forming new myelin is ultimately about signaling OPC populations to differentiate into myelinating oligodendrocytes. A number of studies have shown that OPC populations are sensitive to their surroundings and respond to multiple migration, proliferation, and differentiation cues in a variety of species, locations, and conditions (for just a few examples and reviews, see Dawson, 2003; Kirby et al., 2006; Hill et al., 2013; Hughes et al., 2013). Consequently, myelin plasticity may ultimately require the initiation of signals that affect the OPC cell cycle to induce migration, division, or differentiation. In Figure 1A, a previously bare segment of axon is myelinated by a newly matured oligodendrocyte. An intriguing possibility is that this “plasticity” may actually represent late developing myelination, which has been suggested to occur in humans in cortical white matter (Yakovlev and Lecours, 1966). The distinguishing characteristic between late developing myelin and adult myelin plasticity would be the degree to which these myelination patterns are pre-determined, in contrast with a reliance on external influences.
Remodeling or Refinement of Existing Myelin. There are several potential mechanisms by which existing myelin sheaths may be remodeled, as illustrated in Figure 1B. Remodeling may involve altering the length of the myelin sheath, which affects conduction velocity via altering the number of “jumps” action potentials make down the length of an axon. Adding or subtracting wraps of myelin affects the sheath thickness, which directly relates to the resistance and capacitance of the axonal membrane, thus affecting conduction velocity. There is as yet little direct evidence regarding the potential for remodeling of existing myelin, although the model proposed by Snaidero et al. (2014) allows for the re-establishment of cytoplasmic channels, potentially allowing for refinement. Many of the commonly used techniques for measuring myelin are listed in Table 1; unfortunately, EM and immuno-labeling are temporally limited and can only describe the current state of a myelin sheath. Immuno-labeling and EM cannot describe the history of a sheath (e.g., established or regenerating) and cannot confirm that an observed change in myelin morphology is the result of remodeling or degeneration. More recently developed tools, such as genetic or viral reporters and live imaging, provide greater insight into OPC and myelin dynamics (for a review, please see Rassul et al., 2016). The further development of these tools, particularly in mammalian systems, will allow researchers to discriminate between old and new myelin sheaths and track the fates of oligodendrocytes over time. Furthermore, these techniques will allow scientists to address which of the types of remodeling occur and probe into the mechanisms by which these changes take place. Tracking the history and changes of individual sheaths and cells will definitively prove or rule out the possibility of remodeling, and this line of inquiry will likely yield exciting findings.
Replacement of Myelin Sheaths by New Internodes. Due to the compacted layers of mature myelin sheaths, instead of remodeling it may be more energetically and structurally feasible to replace non-optimal or dysfunctional sheaths. New sheaths would be more structurally suited to new signaling requirements. Thus, one final mechanism of myelin plasticity may be myelin replacement, which is shown in Figure 1C: two existing sheaths are broken down, and an OPC exits the proliferative cycle to differentiate into a myelinating oligodendrocyte. This mechanism would necessitate a complex pathway, wherein the oligodendrocyte detects the inadequacy of a myelin sheath and subsequently removes it. A new OPC then matures and extends a process to form a myelin sheath structurally different from the previous one. The fate of the oligodendrocyte once a sheath is retracted is another intriguing component of this pathway, as the oligodendrocyte may myelinate multiple other axons. Furthermore, studies in adult mice show that a sudden loss of oligodendrocytes leads to axonal deterioration and long-term disruption of myelination (Pohl et al., 2011; Schulz et al., 2011; Traka et al., 2016). Therefore, if oligodendrocytes purposefully retract myelin sheaths, an axon must be able to survive the transient demyelinated period. These studies indicate that myelin sheath removal in the healthy nervous system would rely on molecular stabilization of the axon through a process that is not yet understood.
THE CASE FOR NEURAL ACTIVITY AS A MODIFIER OF MYELINATION
Neural Activity Modulates Myelin Development
Myelination occurs both during prenatal and postnatal development (reviewed by Baumann and Pham-Dinh, 2001). Although individual myelin profiles are variable both within a fiber tract and even within a single axon, there is an evolving literature that suggests myelin growth and adaptation is strongly influenced by the activity of discrete axonal pathways. Indeed, a number of studies both in vitro and in vivo illustrate that neural activity may be a key regulator of myelin morphology, in addition to its known effects on neural and axon morphology.
To determine the extent to which neuronal activity changes myelin structure, researchers used pharmacological agents to increase (via scorpion toxin, a sodium channel agonist) or decrease (via tetrodotoxin [TTX]) neuronal firing in an in vitro prep from dissociated embryonic mouse cerebral neurons (Demerens et al., 1996). Blocking action potentials with TTX halted myelination, as measured by the number of myelinated axons. The most significant effect occurred when TTX was added transiently just before myelination began. Inducing higher firing rates with scorpion toxin caused more fibers to be myelinated than in control cultures without affecting the number of oligodendrocytes. This finding implies that increased neural activity didn't cause OPC proliferation, but did encourage individual oligodendrocytes to myelinate more axons. Downregulation of neural activity in mouse optic nerves in vivo by TTX decreased the number of myelin sheaths when administered in the post-natal critical period (Demerens et al., 1996). These data were further confirmed in developing zebrafish, where blocking synaptic vesicle release decreased the number of myelin sheaths, while increasing vesicle release increased sheath number (Mensch et al., 2015). Taken together, these studies imply that neuronal activity affects myelination not by increasing proliferation, but by influencing the number of sheaths produced. Further in vivo work has suggested that while neuronal firing may induce preferential wrapping of active axons over neighboring axons, neuronal firing is also important to maintain and encourage continued development of the myelin sheath (Hines et al., 2015), implying again that activity affects the behavior of already myelinating cells, rather than proliferation. Finally, the studies discussed above do not show a direct effect of neuronal activity on oligodendrocyte sheath production. Its important to note that neural activity also influences the location and size of excitatory segments of the axon (Kuba et al., 2010), which may indirectly affect the location and attachment of myelin sheaths.
If neural activity does signal certain axons for myelination or myelin plasticity, what is the mechanism by which neuronal activity induces changes in oligodendrocytes and myelin? The in vitro study above examined oligodendrocyte numbers (Demerens et al., 1996), but what is the effect of increases in neuronal firing or a change in rate on OPC populations and subsequent differentiation into myelinating oligodendrocytes? A study in developing rat optic nerves found that blocking neuronal firing via TTX decreased the number of new OPCs, an effect that could be rescued by applying platelet derived growth factor (Barres and Raff, 1993). Wake et al. (2011) showed that in cultured dorsal root ganglion neurons, glutamate vesicle release along axons increased myelination and also caused an increase in oligodendrocyte protein production, though electrical activity did not seem to influence OPC proliferation. A later study showed that non-synaptic neurotransmitter released from axonal varicosities increased OPC internal calcium levels, which led to myelin protein production and preferential myelination of electrically active axons (Wake et al., 2015). Together, these studies suggest that OPCs are capable of detecting changes in neural activity, and respond by differentiation and the initiation of myelination, but they failed to definitively and quantitatively measure new myelin sheaths.
Most of the studies reviewed above utilized pharmacological approaches, but there is an increasing interest in how changes in the environment or exposure to a physical or cognitive challenge can modulate myelination. For example, two laboratories have recently shown the effect of developmental deprivations on myelin formation. Surprisingly, monocular deprivation during mouse development led to an increase in oligodendrogenesis without increasing OPC numbers, though myelin sheaths are shorter (Etxeberria et al., 2016). These data imply that, contrary to the findings outlined earlier, decreased neuronal activity causes more new myelinating oligodendrocytes, though variations between the experiments make direct comparisons difficult. For example, it is possible that the pharmacological approaches used in the studies above caused a greater overall decrease in activity. On the contrary, the 2 weeks of deprivation used in this study might have initiated a compensatory mechanism not seen in shorter term experiments. The authors also showed that glutamate might be a key signal involved in regulating developmental myelination, as knocking glutamate transmission down caused similar effects (Etxeberria et al., 2016). A study published in 2012 showed that depriving mice of social interactions during a critical period from P21 to P35 reduced oligodendrocyte complexity and decreased oligodendrocyte protein levels in the prefrontal cortex without affecting axon structure. Furthermore, these animals showed decreased performance on cognitive tasks (Makinodan et al., 2012). Together, these studies point to the requirement of neuronal activity, rates of neural firing and circuit afferent activity for formation of myelin in development. Although the studies reviewed above focus on the critical periods when a distinct axonal tract is formed and develops activity, more recent work has begun to examine modifiers of myelin growth and plasticity in the mature nervous system.
Although these studies provide strong evidence both in vitro and in vivo for the importance of activity as a modifier of myelination, we must also consider in vitro work that shows OPCs differentiating and myelinating in the absence of axons. As early as 1990, cultures showed the initiation of wrapping on glass fibers (Bullock and Rome, 1990). Further work in vitro work showed the addition of factors deposited on the fibers affected myelination in culture (Howe, 2006). In 2012, the Chan group further explored the importance of nanofiber type and diameter in differentiation and myelination by cultured rat OPCs (Lee et al., 2012). More recently, work has shown that oligodendrocytes cultured with microfibers may have intrinsic properties, which dictate their myelinating capacity to some degree, regardless of axonal cues (Bechler et al., 2015). Though evidence strongly suggests neural activity is an important regulator, these culture systems provide useful tools for exploring additional cues that may affect myelination.
Myelin Plasticity in the Adult
As we have detailed, for many years the question of myelin plasticity only arose in the context of injury or disease. Recently scientists began to explore the possibility that under non-pathological conditions, myelin and white matter remodeling are essential to normal function. White matter plasticity in adult humans and animal models is reviewed in great detail elsewhere (Young et al., 2013; O'Rourke et al., 2014; Wang and Young, 2014; Fields, 2015; Purger et al., 2016). Here we highlight select studies and relevant concepts and begin to consider where the opportunities and remaining questions lay for the role of myelin plasticity in the function of the adult nervous system.
Much of the literature regarding adult white matter remodeling revolves around learning induced changes. Many of these studies use diffusion tensor imaging (DTI) and fractional anisotropy (FA) to visualize changes in the flow of water in CNS tracts over time. These metrics can serve as an indirect measure of myelination changes through the mathematical assessment of white matter volume and direction. Learning induced changes in white matter is reviewed elsewhere (Zatorre et al., 2012). For example, DTI has shown that participants that learn a new motor skill (in this case, juggling) exhibited increased FA in the intraparietal sulcus not seen in those that did not learn a new task (Scholz et al., 2009). Similar work in rodents revealed an increase in FA in the sub-sensorimotor cortex white matter following motor skill learning, which correlated with an increase in myelin basic protein staining (Sampaio-Baptista et al., 2013). Although FA is not a direct measurement of myelin changes, it does show an effect of learning on CNS tracts in living organisms. Further refinement of MRI scans and processing may provide more in-depth and exciting information regarding myelin-specific changes.
If learning requires (or causes) changes in white matter in the CNS, it then becomes necessary to ask what role OPCs and oligodendrocytes may play, and how myelination may change. If new oligodendrocytes are the source of new myelination, what would the effect on learning be if new oligodendrocytes could not form? McKenzie et al. (2014) asked this question by conditionally knocking out a transcription factor, MyrF, essential for oligodendrocyte differentiation. The loss of MyrF did not cause demyelination when it was knocked out in OPCs at P60. However, the mature animals had deficits in learning a skilled motor task (complex wheel running) despite no other observed behavioral deficits. The authors concluded that the production of new oligodendrocytes and new myelin was essential to learn a new motor task (McKenzie et al., 2014). More recent data suggests that the maturation of new oligodendrocytes is essential to learning on a time scale of mere hours (Xiao et al., 2016), providing support for the idea that oligodendrocytes may have developed mechanisms of rapid deployment or adaptation of myelin structure.
Several recent studies contribute evidence that new myelin in the adult CNS must be formed by new oligodendrocytes, a recent study has shown that knocking out new oligodendrocyte formation in the adult brain leads to motor deficits and conduction deficits, which can be rescued by transplanting OPCs (Schneider et al., 2016). As social interaction may be necessary for the development of appropriate myelination (Makinodan et al., 2012), so too may isolating adult animals cause thinner myelin in the prefrontal cortex, which can be rescued by reintroduction of social interaction (Liu et al., 2012). When enriched environments are combined with skilled reaching training, more oligodendrocytes are generated in the adult rat cortex (Keiner et al., 2017).
These studies demonstrate myelin plasticity occurs in the adult CNS as a response to non-pathological fluctuations in nerve or organism activity. They also provide strong evidence for the formation of new myelinating oligodendrocytes as the source of rapid and long-lasting sheath plasticity, though they have not completely ruled out the possibility of remodeling of existing myelin sheaths. It is noteworthy that most of these studies fail to directly measure changes in myelin morphology, but instead rely on indirect measures such as increases in OPC differentiation. Technological advances will allow scientists to probe more directly into the precise morphological changes that accompany myelin changes associated with learning or daily functioning. The effect of comparable changes of myelin morhpology on conduction velocity have been mathematically modeled (Brill et al., 1977). However, given the challenge of measuring multiple myelin and axon indices at once, we have incomplete data to mathematically model the physiological impact of myelin plasticity. Hence, further quantiative studies of myelin and axon parameters that definitivety delineate new from old myelin are needed. A final question with an equal shortage of empirical data is how newly matured oligodendrocytes are integrated into existing or remodeled circuits to affect function and behavior.
Dependence of Myelin Plasticity on Neural Activity
Whether myelin plasticity relies on the myelination of bare axons, the remodeling of existing sheaths, or sheath replacement, researchers need to determine the signaling mechanisms to prompt these changes. Studies have pointed to the possibility that neural activity may influence adult myelin plasticity, potentially in a manner comparable to developmental myelination.
In 2014, Gibson and colleagues published a study regarding the effects of short-term optogenetic stimulation on myelination (Gibson et al., 2014). Stimulating cortical areas induced circular running behavior and subsequently increased OPC proliferation. Weeks later, there were more oligodendrocytes and thicker myelin sheaths in the cortical and white matter areas of stimulated mice than in non-stimulated animals. The implication of this finding is that new oligodendrocytes are responsible for these thicker myelin sheaths, though this was not directly tested. Future work may elucidate whether such axons were previously unmyelinated and definitively show that thicker myelin was formed exclusively by new oligodendrocytes. One final component of Gibson et al.'s (2014) work worth noting is that stimulated animals also showed an altered gait, showing a functional consequence of altered myelination.
Another recent study has shown the potential importance of physical activity on brain myelination. Alvarez-Saavedra et al. (2016) showed that voluntary running induced OPC and oligodendrocyte proliferation and myelination of the cerebellum. Though the direct effect of neuronal activity on myelin changes was not measured, this study provides compelling evidence for increasing activity as a way to affect myelin plasticity. Indeed, it has been known for some time (as reviewed by Cotman and Berchtold, 2002) that physical activity may increase plasticity, likely through increased expression of brain-derived neurotrophic factor (BDNF). In support of the idea that BDNF and exercise alter oligodendrocyte behavior, studies have found that exercise after traumatic injury affects the levels of oligodendrocyte proteins associated with myelination and repelling neural growth (Ghiani et al., 2007; Chytrova et al., 2008). However, other studies have shown that exercise in and of itself is not sufficient to increase OPC differentiation (Xiao et al., 2016). One other important consideration is changes in neuronal activity induced through increased physical activity may actually reflect a significant alteration in the pattern of neural activity, rather than the overall amount of firing. Teasing out the global effects of increased physical activity and individual effects of an alteration of an axon's signaling remains an important question.
Many researchers have begun to probe into the mechanisms by which active axons signal OPC differentiation and myelination. A full commentary on the exciting work being conducted on the signaling cascades and molecules between axons and OPCs/oligodendrocytes is beyond the scope of this review. Several recent reviews have touched on this evolving field (e.g., Wang and Young, 2014; Fields, 2015), including the potential for signaling via non-synaptic neurotransmitter release. Future exciting work will further elucidate how this complex signaling cascade targets axons for myelination.
TRAUMATIC NEURAL INJURY, DEMYELINATION, AND REMYELINATION
Acute injury cascades have been characterized in a number of species and follow a similar pattern across many models, including rodents, cats, and nonhuman primates (for just a few examples, see Bresnahan, 1978; Griffiths and McCulloch, 1983; Cao et al., 2005; Smith and Jeffery, 2006, for a review see Silva et al., 2014). Following injury, oligodendrocytes and their myelin sheaths are susceptible to damage and cell death, which leads to morphological and functional consequences. Types of myelin abnormalities and the experimental methods used to detect them are listed in Table 2. Typical morphological changes of the spared tissue in the chronic spinal cord injury (SCI) environment include the retraction of axons from their post-synaptic contacts, axonal degeneration and death, and the formation of a physical and chemical barrier to encapsulate the injury, the glial scar (reviewed by Horner and Gage, 2000). Demyelination and myelin abnormalities can occur at both the internode and the paranodal attachments (Griffiths and McCulloch, 1983), and abnormal myelination is predicted to affect conduction (Babbs and Shi, 2013) and also affects behavior (Poggi et al., 2016). Channel spreading is a phenomenon wherein sodium and potassium channels “leak out” of the normally highly constrained nodal area (Hunanyan et al., 2010; Karimi-Abdolrezaee et al., 2004), which is related to demyelination and subsequent changes in action potential conduction velocity. Blast injuries have been found to decrease axon initial segment size which may affect excitability (Baalman et al., 2013). All of these factors combine to yield a disruption of neuronal signaling which can lead to loss of motor and sensory function below the injury.
Even in the absence of an intervention after neural injury, spared nerve fibers undergo remyelination. Older studies showed very thin myelin observed by EM (<20% of normal thickness) following a compressive injury (Gledhill et al., 1973; Gledhill and McDonald, 1977), which was thought to represent failed remyelination. More recent studies have also suggested chronic demyelination in a rat contusion SCI model (Totoiu and Keirstead, 2005). However, rigorous new methods showed that in rodents with chronic SCI spared axons are remyelinated, and regenerated myelin is nearly indistinguishable from pre-injury myelin (Lasiene et al., 2008; Powers et al., 2012, 2013). Surprisingly, this is a recent epiphany in the field that formerly associated very thin myelin sheaths with new myelin. New rigorous methods indicate abnormally thin profiles are likely myelin on degenerating or dystrophic axons (Lasiene et al., 2008). It may be that a dystrophic axon creates a relationship of aberrant communication that causes a series of myelin sheath retractions and replacements, but this is a new and important area of future research. Work by Lasiene et al. (2008) was the first to definitely discriminate between cut fibers and spared fibers while simultaneously quantifying myelin regeneration, showing that on spared axons, myelin is near normal thickness. Further studies in rats confirmed these findings and also demonstrated that regenerating myelin sheaths are near normal thickness but initially shorter than normal (<50 µm) (Powers et al., 2012).
Future studies need to employ complementary techniques to establish if these findings hold true in other models, but the implication is that myelin regeneration is more efficient than previously thought. More recently, Powers et al. used a retroviral-driven membrane-bound reporter to isolate new from old myelin sheaths after neural injury (Powers et al., 2013). These data show that regenerating myelin sheaths are short and thick at 1-month post-injury, but the sheath expands to near normal lengths over several months. These data indicate that myelin sheath plasticity exists in the adult injured nervous system and is largely reliant on the formation of new myelin sheaths. These findings were corroborated by Hesp et al. (2015) using a membrane reporter to visualize new myelin and oligodendrocytes, which showed that new oligodendrocyte maturation continues for several months post-SCI. These data indicate a surprisingly long window of myelin replacement and refinement after neural injury. Extended periods of oligodendrocyte death shown to occur in SCI (Crowe et al., 1997) may be related to the removal of damaged sheaths and oligodendrocytes, which would correlate with late oligodendrocyte regeneration.
Though these data support a replacement model of myelin plasticity, they do not rule out the remodeling model. Thus, each of the forms of myelin plasticity outlined in Figure 1 may occur following traumatic neural injury: demyelinated or previously unmyelinated axons may be myelinated, spared or damaged myelin may be remodeled; and damaged or old myelin may be replaced. It will be important to identify the signals that call for myelin regeneration or refinement after injury and more important to define the functional effects of this protracted myelin plasticity.
Two main issues arise when considering evidence of efficient long-term remyelination following injury. The first is to reconcile these findings with many years of contradicting work. Since remyelination of an injured CNS was first described in 1961 (Bunge et al., 1961), many studies have been conducted in a variety of animal models of various types of injury. Frequently, these studies were limited by experimental design and insufficient techniques, as well as biases towards assumptions of chronic demyelination. An early study of weight-drop induced injury in cats described thin regenerated myelin, but only had one animal in the 3- and 16-month time points (Blight, 1983). Our intention is not to refute seminal and pioneering work in remyelination, but merely to call attention to the models and methods available at the time. In a long term model of cuprizone induced demyelination, Ludwin and Maitland (1984) described thin myelin following injury but they observed a trend towards normal myelin at 6 months. This result could reflect only a slight variation that caused slower remyelination, which was ultimately observed by Lasiene et al. and Powers et al. Thus arises another important point when reconciling multiple studies: the need to consider the specifics of the demyelinating insult. The extent to which remyelination in a multiple sclerosis (MS) model, such as cuprizone induced demyelination, can be directly compared with a compression or contusion SCI model has not been established. In some MS models, regenerated myelin can re-establish function, but in human patients there is variable regeneration (reviewed by Plemel et al., 2017). Further work is necessary to determine the factors which dictate poor remyelination in these patients, and what, if any, correlates can be made to the SCI literature for the betterment of both patient populations.
The topic of patient populations leads to the second matter, the necessity of establishing the relevance of rodent models of post-SCI remyelination to human patients. Studies detailing the cellular changes in human SCI patients are exclusively post-mortem studies with multiple changing variables (e.g., Kakulas, 1999; Norenberg et al., 2004; Guest et al., 2005). The differences in methods make it difficult to directly compare rodent and human studies. Future research techniques may describe the patterns of myelination in chronic SCI patients with more precision and controls, which may determine whether findings in rodents are relevant in humans. Larger models, such as porcine and non-human primates present another potential avenue for describing post-injury remyelination as it may be relevant to humans.
ACTIVITY BASED THERAPIES FOR REMYELINATION
The importance of myelin for structural support, trophic support, and maintenance of axonal electrical profiles all indicate the importance of re-establishing sufficient myelination following SCI. Indeed, studies have shown that encouraging remyelination in disease models contributes to better axonal survival as well as functional recovery (e.g., see Mei et al., 2016). Researchers and clinicans have proposed a variety of approaches aimed at myelin preservation or remyelination after SCI; for a review please see Mekhail et al. (2012). Proposed pharmacological approaches target multiple pathways including the inflammatory response, decreasing oligodendrocyte apoptosis, and increasing OPC proliferation and differentiation. Biomaterials have been proposed to bridge glial scars and improve regeneration. Cell transplants of neural precursors and OPCs have been proposed as well.
Activity-based therapies for SCI are used to improve neural regeneration and promote synaptic plasticity. Physical therapy for SCI increases neural activity in descending and ascending tracts. Increasing neural activity via electrical stimulation has also been used following SCI. For example, certain patterns of intraspinal microstimulation have been shown to improve functional outcomes in rodent SCI models (Kasten et al., 2013; McPherson et al., 2015) epidural stimulation may raise neuronal excitability below the lesion (reviewed by Mayr et al., 2016), and cortical stimulation of the uninjured hemisphere may lead to better behavioral recoveries and axonal growth (Carmel et al., 2010, 2014). Furthermore, many stimulation paradigms also involve a degree of retraining on a physical task, similar to physical therapy. As outlined previously, retraining may be important for remyelination as training on a task affects oligodendrocyte development (Keiner et al., 2017) and white matter tracts (Zatorre et al., 2012). The mechanisms of these stimulation paradigms rely on directly causing or increasing the likelihood of action potential firing. However, until recently the effect of altering neural activity on glia following SCI had not been explored. A recent paper has shown that following SCI inducing neuronal activity via cortical stimulation causes an increase in OPCs, mature oligodendrocytes, and myelin protein expression (Li et al., 2017). This exciting finding, in conjunction with the evidence for activity based myelination, and the evidence in support of the use of activity therapies for peripheral nerve injuries (Gordon et al., 2010), provides a strong argument for a possible new target for treating demyelination following SCI which may also help induce neuronal recovery. Furthermore, though the demyelination process in MS is different than SCI, both conditions require remyelination efforts, and recent work has shown that in a model of MS, neuronal activity may induce OPC populations to differentiate via glutamate signaling (Gautier et al., 2015).
There is reason to believe that comparable to developmental and non-pathological conditions, neural activity may also regulate myelin plasticity in the post-SCI environment. The model of myelination proposed by Snaidero et al. (2014) would allow for extant internodes to elongate for some time following their initial formation. Findings that sheaths are initially shorter may actually be indicative of the phases of sheath remodeling and the need for axonal or local signals to refine axonal coverage and myelin sheath morphology (Lasiene et al., 2008; Powers et al., 2012, 2013). Axo-myelinic synapses provide a mechanism for neuronal activity based signaling directly from axons to myelin sheaths (Micu et al., 2016); they may be capable of signaling for alterations in internode characteristics.
Although refinement of surviving myelin is a possibility, the majority of research thus far points to newly generated myelinating cells as the main source of myelin plasticity after SCI. Given the importance of neuronal activity in developmental myelination, it is logical to look to activity as a driver for post-injury remyelination. The ability to fire action potentials is an important signal that an axon is still viable; remyelination of axons that are cut off from their cell bodies and are no longer able to conduct signals would serve no functional purpose. Following demyelination in the adult mouse brain, newly born OPCs form temporary synapses with corpus callosum neurons as they are remyelinated (Etxeberria et al., 2010). Axon-OPC synapses are seen in mouse development (Wake et al., 2015). It is possible that a similar mechanism of communication occurs in the remyelinating spinal cord. Indeed, the remyelination of surviving axons that has been shown to occur in chronic rodent SCI models (Powers et al., 2013; Hesp et al., 2015) may be a result of neural activity in those axons. Further research will elucidate the effect of increasing or decreasing activity levels in individual axons or tracts to determine the effect on remyelination.
A new frontier for researchers is to rigorously model and assess how the amount and patterns of neural activity encode changes in axonal and myelin structure. Not all neural activity is created equal. Research has shown that stimulation frequencies that mimic developmental oscillations are most effective at inducing myelination in vitro (Malone et al., 2013). Higher activity levels are not necessarily better at inducing myelination, as stimulating neurons to seizure-like levels did not induce myelination changes (Gibson et al., 2014). Similarly, physiologically relevant stimulation paradigms may induce better functional recoveries in SCI (McPherson et al., 2015). An approach that addresses both neural and glial recovery will be necessary to improve outcomes after SCI; neither component in isolation is sufficient. Acutely, the lack of trophic and metabolic support caused by demyelination may contribute to neuronal death. By the chronic phase, when remyelination can occur, meaningful neural connections may be beyond repair. Future research will elucidate the patterns of activity most conducive to both neural and myelin recovery, which will ultimately lead to better functional outcomes following SCI.
CONCLUSIONS AND FUTURE QUESTIONS
Future work will determine which of the three potential forms of myelin plasticity outlined in Figure 1 occurs in both the intact and injured nervous system. One form of plasticity may dominate or multiple mechanisms may coincide. In turn, there may be mechanisms of plasticity that are induced by distinct insults such as pathology. It bears mentioning that the Nodes of Ranvier are also capable of undergoing structural changes, and the relative location and size of the nodal regions also contribute to the conduction of action potentials. Recent studies showed that these parameters may also be adjusted to alter signal timing (Ford et al., 2015; Arancibia-Carcamo et al., 2017).
For example, if a partially myelinated axon requires longer internodes, all three plasticity forms may co-occur as some existing internodes lengthen, newly matured oligodendrocytes simultaneously myelinate previously bare portions of the axon and replace older shorter internodes. Many questions arise from such a hypothetical. The complexity of signaling necessary to govern such elaborate processes across axons, OPCs, and oligodendrocytes must be great. An oligodendrocyte can produce a 300 μm sheath which lies 30 μm away from its cell body (Ransom et al., 1991); what are the energetic constraints of remodeling versus replacement over such large spatial domains? The advent of long-term live imaging via cranial and spinal windows, high-resolution microscopy, and reporter strains will help to answer these and other exciting questions.
Our awareness and appreciation of myelin plasticity has undergone a renaissance driven by exciting new discoveries of remarkable myelin regeneration and plasticity. Scientists are applying new research techniques to explore myelination as a vital component of adult neural plasticity. Many fundamental questions regarding myelin plasticity remain, especially in the context of neural injuries. New technology, such as live imaging and novel genetic/viral reporters, will continue to allow researchers to establish the extent to which existing myelin degenerates, regenerates, or remodels as a function of aging or injury. A key question is which cellular populations signal myelin growth and which models best represent the biology of myelin segment replacement, regrowth and adaptation. If the theory that increasing or altering neural activity alters myelin morphology continues to gain momentum, it will be necessary to empirically establish the patterns and levels of axonal activity that drive glia to myelin sheath growth, remodeling, or replacement. Further, while MRI and DTI are powerful imaging tools for evaluating myelin in humans that have led to exciting hypotheses, there is much work to be done. A reconciliation of MRI signals with those of rigorous genetic models is needed to develop a deeper understanding of how myelin plasticity is manifest in humans compared with animal models. As future studies answer many of the exciting questions outlined in this review, we are likely to see new treatments that induce both glial and neural recovery to provide better outcomes for individuals with neural injuries.
The authors would like to thank Dr. Lesley Chaboub and Dr. Matt Hogan for their critical review of this article. They also acknowledge John Boom for his invaluable assistance in manuscript preparation. B.R.K. is funded in part by the National Science Foundation Graduate Research Fellowship. P.J.H. and B.R.K. are funded in part by grants from the Craig H. Neilsen Foundation and Wings for Life.