Different effects of pioglitazone and rosiglitazone on lipid metabolism in mouse cultured liver explants
Abstract
Background
Pioglitazone (PIO) and rosiglitazone (ROSI) are widely used as oral antidiabetic agents for treatment of type 2 diabetes. Although these medications exert similar effects on blood glucose, recent clinical studies indicated that PIO has a more pronounced beneficial effect on lipid parameters than ROSI. In order to get further insight into the lipid effects of both drugs, we tested whether PIO, compared to ROSI, could exert direct effects on lipid liver metabolism in relation with plasma lipids.
Methods
We performed in vitro studies using mice liver slices incubated 21 h either with ROSI (1 µmol/L) or PIO (7.5 µmol/L).
Results
We showed that both glitazones slightly reduced HMG-CoA reductase mRNA levels at the same degree but only PIO reduced intracellular cholesterol content, suggesting an alteration of cholesterol uptake rather than an inhibition of cholesterol biosynthesis. This concept was supported by the reduction of scavenger receptor class B type I expression, hepatic lipase activity and high-density lipoprotein cholesterol uptake in PIO-treated liver explants. Conversely, hepatic lipase mRNA levels were increased 3.5-fold. ROSI, but not PIO, induced acetyl-CoA carboxylase and fatty acid synthase gene expression and increased apoB secretion suggesting a stimulation of lipogenesis. Concurrently, peroxisome proliferator-activated receptor-γ mRNA levels were induced by ROSI and not significantly changed by PIO. Besides, PIO appeared to be a more potent activator of AMP-Activated Protein Kinase than ROSI.
Conclusions
PIO and ROSI exert specific direct effects on liver and extrapolating these data to humans could explain the significant improvements in plasma lipids observed in diabetic patients treated with PIO. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
Pioglitazone (PIO) and rosiglitazone (ROSI), two members of the thiazolidinediones (TZDs) family, are widely used as oral antidiabetic agents for treatment of type 2 diabetes 1, 2. These medications have both a glucose-lowering effect and the potential to modify lipid metabolism. Despite the widespread clinical use of these drugs, the precise molecular mechanisms by which TZDs exert their effects remain largely unknown. TZDs are known to be specific ligands for the gamma isoform of the peroxisome proliferator-activated receptor (PPAR), a family of nuclear receptors controlling the transcription of genes involved in preadipocyte differentiation 3, 4, fatty acid (FA) transport, synthesis and storage 5, 6. The stimulation of adipogenesis may promote the incorporation of free FAs from the circulation into adipose tissue 7 lowering plasma free FA levels 8 and providing a putative mechanism for increased insulin sensitivity 9-11. Indeed, it has been reported that treatment with TZDs decreases ectopic lipid deposition in diabetic rats 12-14 and humans with non-alcoholic fatty liver disease 15, 16. More recently, evidence has accumulated that TZDs treatment also increases the production of fat-derived signalling molecules including leptin 17, 18 and adiponectin 19, 20, which may affect metabolic function of liver and skeletal muscle and improve FA and glucose metabolism 21.
Thus, TZDs appear to exert their insulin sensitizing effects by acting as selective ligands of the PPARγ receptors primarily in adipose tissue 22. Nevertheless, recent data suggested that TZDs treatment could improve systemic parameters of insulin sensitization independently of PPAR 23, 24 supporting the concept of a direct effect of TZDs.
As far as lipid effects are concerned, clinical studies have shown differences between PIO and ROSI. Indeed, PIO decreases triglycerides when ROSI does not and the increase in plasma high-density lipoprotein cholesterol (HDL-C) is significantly greater with PIO than with ROSI 25. In addition, plasma low-density lipoprotein cholesterol is increased with ROSI but not with PIO 25. The mechanisms by which PIO and ROSI exert different effects on lipid parameters are not clearly understood. Furthermore, the direct action of TZDs on hepatocytes has been poorly studied and should be considered in regard to the central role of liver on lipid homeostasis.
Thus, the aim of this study was to determine, using cultured liver explants, a model retaining intact cell structure, whether PIO and ROSI could exert direct effects on liver lipid metabolism independently of factors related to adipocytes. We further comparatively examined the impact of PIO and ROSI with regard to the mechanisms by which the two medications differently affect plasma lipids in humans.
Materials and methods
Animal experiment and treatments
Official French regulations (n° 87 848) for the use and care of laboratory animals were followed throughout, and experimental protocol was approved by the local ethic committee for animal experimentation. C57BL/6 male mice (Janvier, Le Genest Saint Isle, France) were housed in individual plastic cages and fed with standard diet (AO4, UAR, Epinay-sur-Orge, France) until the preparation of liver slices.
Cultured liver explants
C57BL/6 male mice (13 week old) were anaesthetized with intraperitoneal injection of ketamine/xylazine (7.5 mg/1 mg for 100 g body weight). The liver was perfused with cold and oxygenated Hanks' balanced salt solution (HBS) to clear the organ of blood before slicing using a Brendel/Vitron slicer (Tucson, AZ) in the same medium. Thin slices (about 200 µm) from each liver were rinsed and pre-incubated 30 min at 37 °C in HBS before being randomly distributed in 50-mL culture tubes containing 10 mL of oxygenated William's Medium E (WME) supplemented with heat-inactivated calf serum (10%), antibiotic–antifongic cocktail (1%). Concentrated suspensions of glitazones in WME were prepared from commercial pills (Avandia® for ROSI, Glaxo-SmithKline, Marly-le-Roi, France and Actos® for PIO, Laboratoires Takeda, Puteaux, France) using a mini-beadbeater homogenizer (BioSpec Products, Bartlesville, OK) and added under a small volume to the culture tubes to reach a final concentration of 1 µmol/L for ROSI or 7.5 µmol/L for PIO. As glitazones bind serum proteins with high affinity 26, the compounds were made soluble binding calf serum proteins in the medium. Tubes were then installed horizontally on a rocking shaker, pierced on the top to allow gas exchange and incubated for 21 h in a 5% CO2 and 37 °C atmosphere under slight agitation. At the end of the incubation period, slices were randomly allocated to the different experiments described thereafter. Chemicals and mediums used in this procedure were supplied by Sigma (Saint-Quentin-Fallavier, France).
Lipid parameters
At the end of the incubation period, 4–5 explants from each tube were submitted to lipid extraction according to the method of Folch et al. 27. One mL of organic phase was transferred to a clean tube containing 1 mL of 1% Triton X-100 in chloroform and dried down under nitrogen. The residue was re-solubilized in 0.25 mL distilled water and used for determination of triglyceride and cholesterol contents using a commercial kit (BioMérieux, Marcy l'Etoile, France).
FA incorporation, FA oxidation and lipoprotein secretion
After the 21-h incubation period, three other slices from each tube were rinsed with WME and placed in 3.5 mL of serum-free WME oxygenated and supplemented with the different treatments and L-carnitine (0.5 mmol/L). 0.2 mmol/L of [1-14C] palmitic acid (55.5 GBq/mol, PerkinElmer, Courtaboeuf, France) complexed to albumin [FA/bovine serum albumin (BSA) molar ratio 2.5/1] was added to the medium. After 4 h of incubation at 37 °C under slight agitation, slices were rinsed with cold WME and immediately submitted to lipid extraction 27. Lipid classes were then separated by thin-layer chromatography on silica gel and radioactivity was measured with an AR-2000 imaging scanner (BioScan, Washington, DC). Oxidation rates were estimated measuring labelled CO2 and acid-soluble products contents recovered in the incubation medium 28. Apolipoprotein B and A1 secreted in the medium were determined with commercial kits from Orion Diagnostica (Espoo, Finland). Protein concentrations in liver explants were estimated by the bicinchoninic acid procedure using BSA as a standard (Sigma).
Hepatic lipase activity
Hepatic lipase (HL) activity was measured from fresh liver slices following the procedure described by Iverius and Ostlund-Lindquist 29 using 1.67 mmol/L triolein emulsified in WME with tri-9,10[3H]oleoyl-glycerol (22 KBq/assay; PerkinElmer) as substrate. After a 2-h incubation at 37 °C, HL activity was estimated from the radioactivity of [3H]oleate released in the medium and incorporated into liver explants.
[3H]-cholesteryl ether-HDL uptake
First, a HDL fraction was isolated from human plasma by sequential flotation ultracentrifugations as described previously 30. HDL was then radiolabelled with [3H]-cholesteryl ether (CE) as following. Briefly, [3H]cholesteryl hedacyl ether was combined with L-a-phosphatidylcholine and butylhydroxytoluene in a 500 : 1 : 6 molar ratio and sonicated to form liposomes. HDL-[3H]CE was obtained by addition of liposomes to the HDL fraction in presence of lipoprotein-free plasma as cholesteryl ester transfer protein (CETP) source after an overnight incubation at 37 °C under light agitation. Labelled HDL were separated from remaining liposomes by another sequential flotation ultracentrifugation and washed twice in KBr (density 1.21). Finally, HDL-[3H]CE were aliquoted and stored at − 80 °C until used. Measurement of the uptake was carried out at 37 °C by incubating two liver slices in 1 mL of WME containing 40 µg of proteins (0.3 mCi of HDL-[3H]CE), under slight agitation. After 3 h, slices were removed from medium, washed three times, and homogenized in 400 mL of phosphate saline buffer (PBS) with a mini-beadbeater (BioSpec Products). Then, the radioactivity recovered in the homogenate was estimated, representing the amount of HDL-C uptaken by the liver cells.
Immunoblotting
Fresh liver slices allocated for immuno-detection of phosphorylated AMP-Activated Protein Kinase (AMPK) were placed in oxygenated WME supplemented with ROSI (1 µmol/L) or PIO (7.5 µmol/L) or vehicle as described above. After incubation from 0 to 1 h, slices were homogenized in a Potter-Elvehjem homogenizer in saline phosphate buffer containing protease and phosphatase inhibitor cocktails (Roche diagnostic) and diluted into 2× sample buffer (125 mmol/L Tris–HCl, pH 6.8, 20% glycerol, 4% sodium dodecyl sulfate (SDS), 5 mmol/L dithiothreitol (DTT) and 5% ß-mercaptoethanol). Equal amounts of proteins were separated by electrophoresis on 8% polyacrylamide gels containing 0.1% SDS and transferred onto Hybond-ECL nitrocellulose membranes (GE Healthcare, Saclay, France). Membranes were then blocked for 1 h at room temperature with 5% BSA in Tris/tween buffered saline solution (TBST), 20 mmol/L Tris–HCl, pH 7.4, 500 mmol/L NaCl and 0.1% tween 20. Membranes were incubated either overnight at 4 °C with a phospho(p)-AMPKα (Thr172) antibody (dilution 1 : 1000; Cell Signalling, Danvers, MA), or 1 h at room temperature with a β-actin antibody (dilution 1 : 10 000; Sigma). Blots were washed with TBST for 1 h and incubated with secondary antibody, horseradish peroxidase linked to anti-IgG (dilution 1 : 10 000) for 1 h. After incubation, blots were washed with TBST and visualized with ECL reagents (GE Healthcare).
Gene expression
Total mRNA from cultured liver explants were extracted with Tri-Reagent (Euromedex, Souffelweyersheim, France). Total mRNA was reverse-transcripted using the Iscript cDNA kit (Bio-Rad, Marnes-La Coquette, France). Real-time polymerase chain reaction was performed as described previously 31 in a 96-well plate using an iCycler iQ (Bio-Rad). The sequences of the forward and reverse primers used are described in Table 1. The relative gene expression was calculated from standard curves for each gene established using four dilutions (1/10 to 1/10 000) of cDNA positive controls and normalized with 18S and TATA box binding protein.
| Gene | 5′-Sense primer-3′ | 5′-Antisense primer-3′ | Length of polymerase chain reaction products (bp) |
|---|---|---|---|
| 18S | GTGTGGGGAGTGAATGGTG | GCGAGACAGTCAAACCACG | 60 |
| ACC1 | ACACCATGTTGGGAGTTGTG | GCTGTTCCTCAGGCTCACAT | 64 |
| ACC2 | CATGGTAGTGGCTTTGAAGGA | CGTGTCGATATCGTTGTTCTG | 114 |
| ACO | ATTTACGTCACGTTTACCCCG | ATACCACCCACCAGCTTCC | 127 |
| CPT-I | GGATCTACAATTCCCCTCTGC | ATCTTAACTGCCGGATCCAC | 110 |
| FAS | GGCTGCAGTGAATGAATTTG | TTCGTACCTCCTTGGCAAAC | 55 |
| FAT/CD36 | AATTAGTAGAACCGGGCCAC | CCAACTCCCAGGTACAATCA | 67 |
| HMG-CoAred | GCCTGGATGGGAAGGAGTA | CTTATGGCTCTGCAGCCTCT | 90 |
| HL | GTGAATGTGGGGTTAGTGGAC | ACTTCGCAGATTCCTCCAGC | 129 |
| LDLR | CATAGGCTATCTGCTCTTCACC | TTGGGGAGCAGACTGGTGTA | 89 |
| PPARα | GTTTTCACAAGTGCCTGTCTGT | GTATGAACAAAAGGCGGGTTG | 87 |
| PPARγ | ATCTTAACTGCCGGATCCAC | AGGCACTTCTGAAACCGACA | 69 |
| SR-BI | TCCCTTCGTGCATTTTCTCA | GTTCATCCCAACAAACAGGC | 86 |
| TPB | ACGGCACAGGACTTACTCCA | GCTGTCTTTGTTGCTCTTCCAA | 77 |
- Primer pairs were designed using Primers! software and were synthesized by MWG-Biotech AG (Ebersberg, Germany). 18S, 18S ribosomal RNA; ACC, acetyl-CoA carboxylase; ACO, acyl-CoA oxidase; CPT-I, carnitine palmitoyltransferase I; FAS, fatty acid synthase; FAT/CD36, fatty acid translocase; HMG-CoAred, hydroxymethylglutaryl-CoA reductase; HL, hepatic lipase; LDLR, low-density lipoprotein receptor; PPAR, peroxisome proliferator-activated receptor; SR-BI, scavenger receptor class B type I; TPB, TATA box binding protein.
Statistical analysis
Results are expressed as means ± SEM. Data were subjected to one-way analysis of variance followed by Tukey–Kramer post hoc test. Differences were considered significant at p < 0.05.
Results
PIO reduced intracellular triglyceride and cholesterol content in liver explants
Consistent with the notion of a direct effect of TZDs on lipid metabolism, we investigated the measure of some parameters related to FA oxidation and esterification. First, we measured the consequences of a 21-h treatment with PIO or ROSI on triglyceride and cholesterol content in liver slices. Incubation with PIO caused a significant reduction in triglyceride and cholesterol contents compared with ROSI and control (Table 2). We concomitantly observed that treatments did not alter the capacity of liver slices to oxidize palmitic acid (Figure 1A) nor to esterify it into phospholipids (Figure 1B) and triglycerides (Figure 1C). As lipid and cholesterol synthesis are closely related to lipoprotein secretion, we also explored the possibility that treatment with glitazones may modify the capacity of liver explants to produce apolipoprotein B and apolipoprotein A1 (Figure 1D and E). In our conditions, neither PIO nor ROSI significantly changed these parameters except for apolipoprotein B secretion that was slightly stimulated by ROSI when compared to PIO.
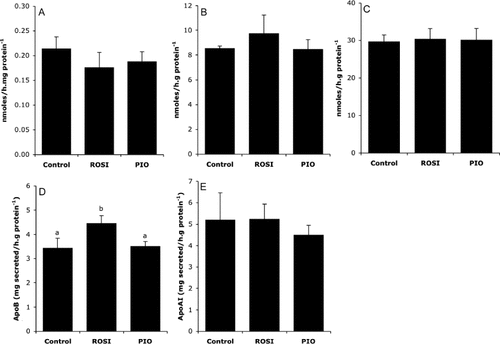
Metabolic fate of palmitic acid and apolipoprotein secretion by liver explants. Liver explants were first treated 21 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle and incubated for four supplementary hours in the presence of 0.2 mmol/L [1-14C] palmitic acid/BSA (2.5/1) as described in Materials and Methods section. (A) Fatty acid oxidation activity calculated from catabolism of [1-14C] palmitic acid in 14CO2 and 14C-acid-soluble products recovered in the incubation medium. (B) Incorporation of palmitic acid into phospholipids and (C) into triglycerides. (D) Apolipoprotein B and (E) apolipoprotein A1 secretion rates by liver slices. Values are means ± SEM (n = 6 per series). Columns that do not share a superscript letter differ (p < 0.05)
| Control | Rosiglitazone | Pioglitazone | |
|---|---|---|---|
| Liver explants | |||
| Triglycerides (mg/g protein) | 132.4 ± 4.3a | 139.6 ± 12.3a | 105.5 ± 8.4b |
| Cholesterol (mg/g protein) | 17.0 ± 1.8a | 21.0 ± 4.2a | 13.5 ± 1.5b |
- Liver explants were treated 21 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle and submitted to lipid extraction as described in Materials and Methods section. Values are means ± SEM (n = 6).
- a, b Values in each row that do not share a superscript letter differ (p < 0.05).
PIO reduced HL activity and HDL-CE uptake in liver explants
When measured on liver explants with triglycerides as substrate, the result of HL activity is the hydrolysis of FA that may be released in the medium or successively incorporated into hepatocytes. So both parameters were investigated and presented in Figure 2A and B, respectively. PIO treatment led to a lesser FA hydrolysis and release in the medium than ROSI and control indicating a reduction in HL activity, whereas FA recovered into tissues were not significantly modified whatever the treatment.
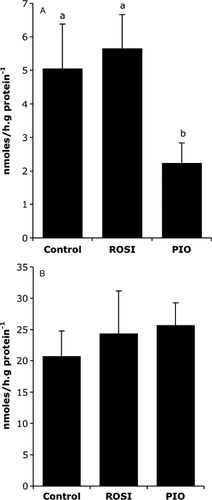
Hepatic lipase activity. Liver explants were first treated 21 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle and incubated for two supplementary hours in the presence of 9,10[3H]oleoyl-glycerol (1.67 mmol/L) as described in Materials and Methods section. Hepatic lipase activity was estimated from the radioactivity of [3H]oleate released in the medium (A) and further incorporated in liver explants (B). Values are means ± SEM (n = 6 per series). Columns that do not share a superscript letter differ (p < 0.05)
We also wanted to determine whether ROSI and PIO affected HDL-CE uptake in our model. We observed that PIO decreased HDL-CE recovery in liver explants when compared with ROSI and control (Figure 3).
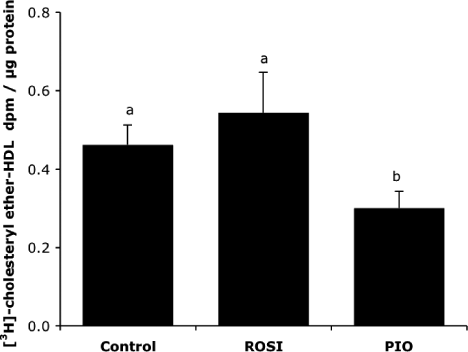
High-density lipoprotein-cholesteryl ether uptake. Liver explants were first treated 21 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle and incubated for three supplementary hours in the presence of [3H]-cholesteryl ether-high-density lipoprotein (40 µg/mL) as described in Materials and Methods section. High-density lipoprotein-cholesteryl ether uptaken by the liver cells was represented by the radioactivity recovered in tissues. Values are means ± SEM (n = 3 per series). Columns that do not share a superscript letter differ (p < 0.05)
PIO was a more potent activator of AMPK than ROSI
The central role of AMPK in the regulation of carbohydrate and lipid metabolism prompted us to investigate whether PIO and ROSI could differently activate AMPK in liver explants. In a recent study, it has been demonstrated that the activation process of AMPK by TZDs is direct, rapid (5–15 min) and gives rise to metabolic effects such as increased glucose disposal within 30 min 32. Thus, in the present work, we analyzed the effects of PIO and ROSI on AMPK activation from 0 to 1 h. Kinetics data indicated that PIO increased levels of phosphorylated AMPK more rapidly and more intensively than ROSI (Figure 4).
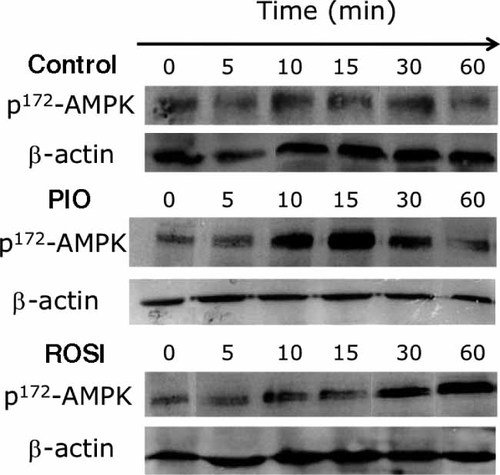
Immunoblot of phospho(p)-AMPKα. Liver explants were incubated from 0 to 1 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle. Representative blots of phospho(p)-AMPKα and ß-actin from two independent experiments
PIO and ROSI specifically modified expression of genes involved in lipid metabolism
In accordance with the reduction of intracellular cholesterol content observed after PIO treatment, we measured the expression of genes involved in cholesterol metabolism (Figure 5). We showed that both treatments decreased hydroxymethylglutaryl-CoA reductase (HMG-CoA red) expression suggesting a similar impact of both glitazones on the regulation of endogenous cholesterol production. Some data relative to gene expression also supported that cholesterol supply to hepatocytes was modified by treatments. Thus, PIO specifically inhibited scavenger receptor class B type I (SR-BI) and highly induced the expression of HL, whereas no difference was detected with ROSI. In our conditions, both glitazones were ineffective on low-density lipoprotein receptor mRNA levels.
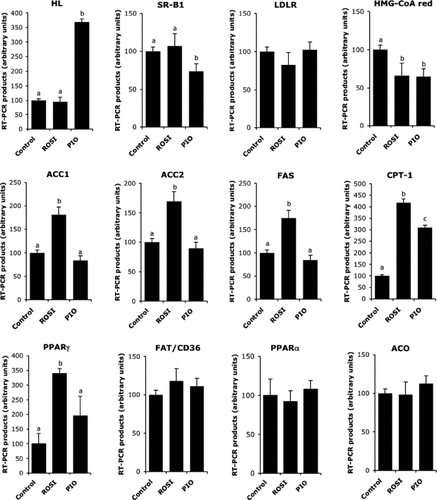
mRNA expression of genes involved in liver lipid metabolism. Liver explants were treated 21 h with either rosiglitazone (1 µmol/L), pioglitazone (7.5 µmol/L) or vehicle and used for real-time polymerase chain reaction analysis as described in Materials and Methods section. Values are means ± SEM (n = 4 per series). Columns that do not share a superscript letter differ (p < 0.05)
The current data also indicated that ROSI coordinately induced the expression of PPARγ, acetyl-CoA carboxylase isoforms (ACC1 and 2) and FA synthase (FAS) mRNA levels suggesting a PPARγ-dependent activation of lipogenesis. On the other hand, glitazones increased carnitine palmitoyltransferase I (CPT-I) gene expression, with a more marked effect for ROSI and did not alter FA translocase (FAT/CD36) gene expression.
Interestingly, mRNA levels of PPARα and of acyl-CoA oxidase, a direct target of PPARα, were modified neither by ROSI nor PIO.
Discussion
PIO and ROSI are the two members of the TZDs class currently used for the treatment of type 2 diabetes. Although PIO and ROSI induced similar effects on glycemic control, it has been reported that PIO is associated with significant improvements in lipid parameters versus ROSI 25, 33. A body of evidence suggests that both medications primarily act on adipose tissue through PPARγ activation where they induce the release of adiponectin and limit that of free FA. Thus, it has been proposed that adiponectin could mediate part of the beneficial effects of TZDs 19. Nevertheless, a direct action of these compounds on tissues other than adipose cannot be excluded. Thus, this study was undertaken to explore the possibility that PIO and ROSI treatments induce direct and specific effects on liver lipid metabolism. In this way, we comparatively tested the effects of PIO and ROSI on lipid metabolism in mouse liver explants. In Human, PIO and ROSI are administered daily respectively at 30 and 4 mg for type 2 diabetes therapy and clinical studies. In our experiments, tissue explants were treated with 1 µmol/L of ROSI and 7.5 µmol/L of PIO to mimic therapeutic doses and physiological concentration ranges 34, 35.
In the present study, we showed that PIO decreased cholesterol content in liver explants. Hepatic cellular cholesterol levels are controlled by coordinated regulation of biosynthesis, uptake and secretion. The ability of TZDs to inhibit cholesterol synthesis in different cell types has been previously demonstrated by Wang et al. 36. The authors reported that PIO and ROSI were only weak inhibitors of cholesterol synthesis and that the mechanisms of inhibition were independent of PPARγ. In accordance, in our model of liver explants, both glitazones slightly reduced HMG-CoA reductase mRNA levels at the same degree suggesting that mechanisms underlying the reduction of cholesterol content in explants treated with PIO are not related to an inhibition of cholesterol biosynthesis but rather an alteration of cholesterol uptake. This concept is supported both by the reduction of HDL-CE uptake and by the reduction of HL activity observed after PIO treatment. HL is involved at different steps of lipoprotein metabolism since it exerts both triglyceride lipase and phospholipase activities 37, 38. Thus, HL can catalyze the hydrolysis of phospholipids from HDL 37, 38 and give rise to a remodelling of HDL favorable to cholesterol uptake by hepatocytes 39, 40. Thus, we may think that the alteration of HL activity induced by PIO may reduce cell cholesterol delivery, decrease cholesterol content and generate a compensatory effect on HL mRNA transcription. This consideration is in accordance with other works demonstrating the existence of an inverse relationship between HL expression and cellular cholesterol content 41. In addition to its lipolytic activity, HL has been shown to exert a ligand-binding function towards HDL which may enhance the interaction of the lipoprotein with SR-BI, thus facilitating cholesterol uptake 42, 43. In our study, we show a reduction of SR-BI transcript levels induced by PIO which may also account for the decrease in cholesterol uptake by hepatocytes. The mechanism underlying the alteration of HL activity by PIO remains to be explored. As compared with ROSI, PIO reduces HL activity, decreases SR-BI expression and hepatic cholesterol uptake. Extrapolating these data to humans, these differences could explain the higher increase in plasma HDL-C in patients treated with PIO compared with those treated with ROSI.
Our data also support the notion of a distinct effect of these drugs on lipogenesis since only ROSI increased ACC1, ACC2 and FAS expression, cellular triglyceride content and apolipoprotein B secretion. In line with this, a recent pilot study indicated that PIO but not ROSI reduces hepatic de novo lipogenesis in subjects with type 2 diabetes mellitus 44.
As TZDs are potent PPARγ activators in adipose tissue, it was reasonable to hypothesize that some of the effects induced by PIO and ROSI on liver may be mediated through PPARγ activation. PPARγ is expressed at low levels in the liver under physiological conditions 45. Nevertheless, some studies have demonstrated that PPARγ expression was induced in animal models of steatotic liver and associated with the transcriptional activation of lipogenic and adipogenic genes important for lipid accumulation 46-48. In line with this, concomitant inductions of PPARγ and lipogenic genes (ACC and FAS) by ROSI are consistent with a stimulation of de novo lipogenesis involving a PPARγ-dependent regulation process. The fact that FAT/CD36 was not modified by ROSI likely concurs with its dramatically low expression level in the liver of normal mice. Ectopic induction of FAT/CD36 occurs in the liver when cells are supplied with a high FA flux causing TG accumulation 31 suggesting a specific PPARγ activation pathway that did not concern our model. Conversely, as PIO treatment did not modify the expression of PPARγ and related genes, it can be reasoned that the effects of PIO on lipid metabolism are PPARγ-independent. Our findings also suggest that the effects induced by both glitazones in liver explants were PPARα-independent since the expression of PPARα and acyl-CoA oxidase, a well-known PPARα-responsive-gene 49 were unchanged.
In the same way as metformin, another antidiabetic drug, TZDs, may act at the junction of carbohydrate and lipid pathway, by activating the AMPK pathway 50. Thus, TZDs have been shown to activate AMPK in mammalian tissues 32, 51 inducing an activation of FA oxidation and a decrease in glycerolipid synthesis in association with a change in cellular energy state 52, 53. In accordance with these previous data, the present work indicates that both PIO and ROSI treatments gave rise to an increase in protein level of p-AMPK favourable to an inhibition of ACC activity resulting in a decrease in the concentration of malonyl-CoA, an allosteric inhibitor of the rate-limiting enzyme of mitochondrial beta-oxidation, CPT-I. PIO and ROSI also concomitantly induced CPT-I mRNA levels suggesting an increase in FA oxidation capacities. Nevertheless, it is not clear why activation of AMPK and induction of CPT-I gene expression were not associated with increased palmitate oxidation rates in liver explants. However, the capacity of PIO and ROSI to regulate carbohydrate-lipid metabolism pathways through AMPK appeared to be different. Thus, part of the molecular and metabolic changes induced by PIO might be consecutive to the stronger and faster activation of AMPK by this drug.
In summary, in addition to provide supporting evidence that TZDs exert direct effects on liver lipid metabolism, our findings demonstrate that PIO and ROSI differ in their actions. Of particular note is the impact of PIO on HL activity, SR-BI expression and HDL-C uptake which would result to an increase in HDL-C levels in vivo. Keeping in mind that the transposition of data from murine to human species remains questionable, we suggest that, compared to ROSI, PIO involves more direct hepatocellular mechanisms which could explain, at least in part, the significant improvements in triglyceridemia and HDL-C observed in diabetic patients treated with PIO.
Acknowledgements
The authors thank Prof Laurence Perségol for her helpful assistance with HDL preparation, Monique Baudoin for figure construction and typing of the manuscript and Dr Chad Stroud for proofreading.
Conflict of interest
None declared.




