Chimerism evaluation in measurable residual disease-suspected cells isolated by flow cell sorting as a reliable tool for measurable residual disease verification in acute leukemia patients after allogeneic hematopoietic stem cell transplantation
Abstract
Background
The presence of minimal/measurable residual disease (MRD) before or after hematopoietic stem cell transplantation (HSCT) is known as a predictor of poor outcome in patients with acute myeloid (AML) or lymphoblastic (ALL) leukemia. When performed with multiparameter flow cytometry (MFC), assessment of residual leukemic cells after HSCT may be limited by therapy-induced shifts in the immunophenotype (e.g., loss of surface molecules used for therapeutic targeting). However, in such cases, questionable cells can be isolated and tested for hematopoietic chimerism to clarify their origin.
Methods
Questionable cell populations were detected during the MFC-based MRD monitoring of 52 follow-up bone marrow samples from 37 patients diagnosed with T cell neoplasms (n =14), B cell precursor ALL (n = 16), AML (n = 7). These cells (suspected leukemic or normal) were isolated by flow cell sorting and tested for hematopoietic chimerism by RTQ-PCR.
Results
The origin of cells was successfully identified in 96.15% of cases (n = 50), which helped to validate the results of MFC-based MRD monitoring.
Conclusions
We believe that a combination of MFC, cell sorting, and chimerism testing may help confirm or disprove MRD presence in complicated cases after HSCT.
1 INTRODUCTION
The level of minimal/measurable residual disease (MRD) prior to and following hematopoietic stem cell transplantation (HSCT) is an independent prognostic factor for outcome in patients with acute leukemia (Czyz & Nagler, 2019). Furthermore, MRD status improves risk stratification and guides antileukemic therapy after HSCT (Giebel et al., 2016; Shah et al., 2018). Residual leukemic cells can be detected by either multiparameter flow cytometry (MFC) or molecular techniques (e.g., qPCR, NGS). Among existing methods, MFC remains the most widely used for MRD monitoring due to its rapid turnaround time, relatively easy standardization and affordability (Buldini et al., 2019; van Dongen et al., 2015). International working groups have introduced multicolor panels and gating strategies that allow the detection of leukemic cells with an aberrant phenotype at levels of 0.01% or even less (Abou Dalle et al., 2020; Jaso et al., 2014). However, interpretation of MFC results can be quite challenging due to the immunophenotypic shifts observed in normal and leukemic cells in highly perturbed post-HSCT hematopoiesis. Interpretation of these shifts is particularly challenging when targeted treatment (e.g., CD19- or CD22-directed moAbs or CAR-T cells) is used before or after HSCT (Illarionova et al., 2017; Kiss et al., 2008; Mejstríková et al., 2017; Xu et al., 2019). Since such changes may form the basis for misinterpretation of MRD, a simple approach for distinguishing between the donor and recipient origin of cells with aberrant phenotypes is critically important for validation of results obtained by MFC–MRD monitoring. For this reason, isolation of cell populations under question using fluorescence-activated flow sorting with subsequent chimerism testing could be applicable. The present work aimed to determine the ability of flow cell sorting with further chimerism evaluation to validate the MFC-based results of MRD detection in patients after HSCT.
2 PATIENTS AND METHODS
2.1 Sample selection
To define a lower limit of sorted cells needed for chimerism evaluation, we used normal lymphocytes from peripheral blood samples of three healthy donors (10 aliquots from each donor, with 100–1000 cells per aliquot and a step value of 100 cells).
A test cohort was formed out of seven bone marrow (BM) samples from post-HSCT patients (age 3–13 years; median age 8 years) with B cell precursor ALL (BCP-ALL, n = 5) and acute myeloid leukemia (AML, n = 2). In these samples, the level of residual disease was greater than 1.0% of nucleated cells. The test cohort was used to explore whether chimerism testing could confirm the recipient origin of cells reported as residual leukemia. We performed purity tests after each sorting procedure in this cohort, and median purity achieved 95.1% (n = 7, range 93.0%–99.1%).
A validation cohort was formed out of 52 BM samples. Among them, 32 samples showed 0.002%–0.771% MRD-suspected cells, 19 samples had normal cells with slight immunophenotypic differences from normal BM differentiation patterns, and one sample contained 22.98% cells with immunophenotype indicating potential relapse. Post-sorting purity tests were performed only in the case of potential relapse because of the high yield of sorted cells (>1 × 106 cells of each sorted cell population). Samples of validation cohort were obtained during routine MRD detection in 37 post-HSCT patients (age 3–21 years; median age 10 years) with T-lineage ALL (n = 14), BCP-ALL (n = 16), and AML (n = 7). Eleven out of 52 BM samples (21.15%) were obtained before day 100 post HSCT. Time points of MRD monitoring are specified in Table S1. Follow-up MRD assays were performed by conventional multicolor flow cytometry (10–12 colors) (Popov et al., 2016; Schuurhuis et al., 2018). The investigated samples were stained with appropriate antibody panels according to a standard procedure (Abou Dalle et al., 2020; Popov et al., 2016).
Information about patients and samples of both cohorts is represented in Table S1.
2.2 Chemotherapy regimens and post-transplant care
A total of 37 patients of the validation cohort received allo-HSCT between September 2015 and March 2019. Among 37 donors, 33 (89%) were haploidentical related donors (parents), 4 (11%) were fully matched related donors. Disease status at the time of HSCT was as follows: complete remission in 26 patients, active disease in 11 patients. TBI-based regimen was used in 20 (54%) patients (ALL, n = 18; AML, n = 2), 16 patients (43%) received treosulfan-based myeloablative preparative regimen (ALL, n = 9; AML, n = 7), one patient (3%) received busulfan, TCR αβ+/CD19+ depletion of HSCT with CliniMACS technology (MiltenyiBiotec, UK) was implemented in 31 cases (peripheral blood stem cell transplant), post-transplant cyclophosphamide was used in two patients (BM transplant) and four patients received HLA-identical BM.
Pharmacological prophylaxis of graft-versus-host disease included tocilizumab on day −1 (8 mg/kg i.v.), abatacept on days −1, +7, +14 and +28 after HSCT (10 mg/kg i.v.), bortezomib on days −5, −2, +2 and +5 (1.3 mg/m2) and rituximab (100 mg/m2) on day −1 in 31 patients, CsA and MMF or rapamycin in four patients, post-transplant cyclophosphamide and CsA in two patients.
2.3 Flow cell sorting and chimerism testing
The cells of interest were separated using a flow cell sorter (FACS Aria III, Becton Dickinson, US). Unfixed antibody-stained cells were sorted in “Purity” sort mode into Eppendorf tubes with phosphate-buffered saline (Cell Wash, Becton Dickinson, US). The minimal number of isolated cells in both the test and validation cohorts was 526. After sorting, the obtained cell suspensions were centrifuged at 700×g for 10 min, and the cell pellets were stored at −80°C. Then, cell pellets were thawed, and DNA was isolated with a DNA-sorb-B kit (ILS, RU). The study of chimerism was performed by real-time quantitative polymerase chain reaction (RTQ-PCR) with insertion/deletion (InDel) polymorphisms as genetic markers (Gendzekhadze et al., 2013). InDel RTQ-PCR was performed with a PCR complex kit and individual primers and probes for each patient (Synthol, RU) and was based on the pre-established InDel profiles of patients and related donors. The PCRs were run in duplicate.
When establishing the lower limit of sorted cells needed for successful chimerism testing, we assessed the threshold cycle (Ct) values of PCRs of normal lymphocytes. The Ct value is defined as the cycle number when the fluorescent signal generated within a PCR crosses the threshold (background fluorescence). This assay-specific value indirectly reflects the amount of the target sequence presented at the start of the reaction. A high Ct number indicates the minimal initial quantities of the target template and enhances the chances of yielding false-positive results. We used a cut-off value of 22–24 cycles (depending on the investigated genetic marker). Above this level, the amplification results obtained in the PCR were indicated as nonvalid.
The aliquots of normal lymphocytes from healthy donors were also studied for InDel markers by PCR in duplicate, and the Ct values were determined for the PCRs of each aliquot. Then, the median number of successfully analyzed cells was calculated.
3 RESULTS AND DISCUSSION
Among the aliquots of normal lymphocytes that demonstrated Ct values lower than the established cut-off level (22–24 cycles), the median number of cells was defined as 500 sorted cells. This number of cells appeared to be a lower limit for a successful study of chimerism. This promising result demonstrates the potential use of the described approach for analysis of very rare events.
The leukemic cells of all seven samples of the test cohort were analyzed for chimerism and were all of recipient origin. Therefore, the reliability of this approach was confirmed, which provided a basis for subsequent studies.
The chimerism study of the validation cohort was successfully performed in 50 out of 52 cases (96.15%). In the remaining two samples , interpretation of the results was technically limited, although it was not dependent on the number of tested cells. As mentioned above, the smallest potentially leukemic population detected in the BM sample during routine MRD assessment amounted to 0.002% of nucleated cells (1 × 106 nucleated cells acquired). We managed to isolate 955 cells from the whole sample for further study. Interpreted initially as MRD, cell populations of 31 samples demonstrated up to 99% recipient origin (T cell neoplasms, n = 11; BCP-ALL, n = 13; AML, n = 7), whereas cells interpreted as normal demonstrated complete donor origin in 18 cases (T cell neoplasms, n = 6; BCP-ALL, n = 11; AML, n = 1). Of particular note, samples of four patients with BCP-ALL exhibited either questionable precursors or potential leukemic cells at different time points during the follow-up.
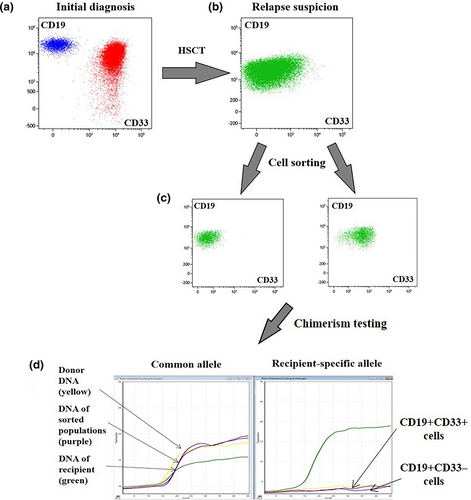
In one patient with suspected relapse, the cells showed a pro-B immunophenotype of leukemic blasts with coexpression of CD33 at initial diagnosis. At follow-up, MFC studies revealed an ambiguous cell population (22.98%) with CD19 positivity and heterogeneous dim expression of CD33, rising suspicion about relapse. This population was divided into two subpopulations according to CD33 expression (CD19 + CD33− and CD19 + CD33+) and sorted for chimerism testing. Both populations proved to be normal very immature BCPs of donor origin, and this finding helped to rule out impending relapse (Figure 1).
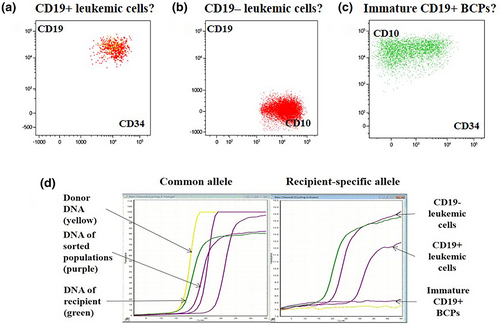
In another intriguing BCP-ALL case, the patient received HSCT in remission obtained after blinatumomab. At follow-up, a leukemic CD19+ population was found first in the cerebrospinal fluid and then in BM at the level of 0.013%. In addition, in the BM, two suspicious populations of cells were found: a presumed leukemic CD19- population (1.193%) and other early CD19+ BCPs (0.554%) with slight immunophenotype peculiarities (Figure 2). All three BM cell populations were sorted and tested for chimerism. The analysis showed that a questionable CD19+ population of early BCPs had donor origin, while both suspicious leukemic populations (CD19+ and CD19−) were of recipient origin. The detection of a significant population of cells lacking the target antigen indicated the futility of further CD19-directed therapy.
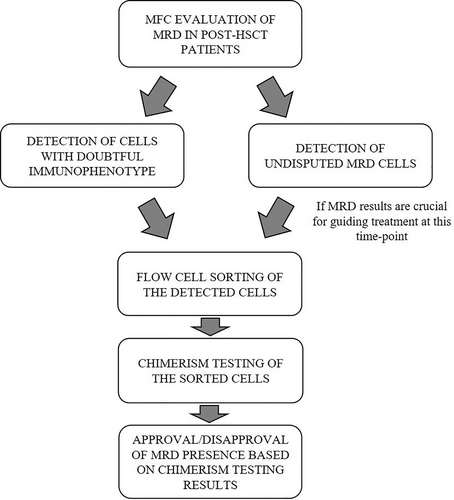
Combining MFC data with flow sorting and chimerism analysis is a reliable way to increase the specificity of MRD monitoring. Here, we present a flowchart of the confirmatory testing of the MRD investigation results (Figure 3).
4 CONCLUSIONS
In the HSCT setting, MFC-based MRD assessment can be complicated due to shifts in the normal patterns of antigen expression, especially if treatment directed to specific surface antigens is applied before or after HSCT. In such cases, questionable cell populations can be isolated by flow cell sorting and then analyzed for chimerism. We demonstrated that even low levels of ambiguous cells presumed leukemic or normal could be tested successfully. Employing this confirmatory procedure may eventually help adjust post-HSCT treatment. The combined approach described in the present study proved to be a beneficial tool for verifying MRD assessment results in virtually all complicated cases after HSCT.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
Informed consent was obtained from all patients for being included in the study.