CD4+ T lymphocytes enumeration by an easy-to-use single platform image cytometer for HIV monitoring in resource-constrained settings
Abstract
Background:
HIV monitoring in resource-constrained settings demands affordable and reliable CD4+ T lymphocytes enumeration methods. We developed a simple single platform image cytometer (SP ICM), which is a dedicated volumetric CD4+ T lymphocytes enumeration system that uses immunomagnetic and immunofluorescent technologies. The instrument was designed to be a low-cost, yet reliable and robust one. In this article we test the instrument and the immunochemical procedures used on blood from HIV negative and HIV positive patients.
Methods:
After CD4 immunomagnetic labeling in whole blood, CD4+ T lymphocytes, CD4+dim monocytes and some nonspecifically labeled cells are magnetically attracted to an analysis surface. Combining with CD3-Phycoerythrin (PE) labeling, only CD3+CD4+ T lymphocytes are fluorescently labeled and visible in a fluorescent image of the analysis surface. The number of CD4+ T lymphocytes is obtained by image analysis. Alternatively, CD3 immunomagnetic selection in combination with CD4 immunofluorescent labeling can also be applied for CD4+ T lymphocytes enumeration.
Results:
The SP ICM system was compared with two single platform flow cytometer (SP FCM) methods: tetraCXP and TruCount methods. The SP ICM system has excellent precision, accuracy and linearity for CD4+ T lymphocytes enumeration. Good correlations were obtained between the SP ICM and the SP FCM methods for blood specimens of 44 HIV− patients, and of 63 HIV+ patients. Bland–Altman plots showed interchangeability between the SP ICM and the SP FCM methods.
Conclusions:
The immunolabeling methods and the instrumentation are simple and easy-to-handle for less-trained operators. The SP ICM system is a good candidate for CD4+ T lymphocytes enumeration in point-of-care settings of resource-constrained countries. © 2007 Clinical Cytometry Society.
INTRODUCTION
AIDS is an extraordinary crisis that forms an immediate emergency and a long-term development issue. It is estimated that in 2005 40.3 million human beings were living with HIV, of whom approximately 95% were in developing countries (1).
In recent years, the price of antiretroviral treatment per person per year has dramatically fallen from US$ 10,000 to US$ 300. By the end of 2005, more than one million persons in middle- and low-income countries had received this treatment (2). For adults, the CD4+ T lymphocytes count is essential to decide when to start the antiretroviral treatment, to monitor the effect of this treatment, and to decide when to stop opportunistic infection prophylaxis (3-5).
While the cost of the antiretroviral treatment has dropped dramatically, CD4+ T lymphocytes enumeration remains too expensive for many resource-constrained countries. Currently, flow cytometry (FCM) is the most widely accepted method for CD4+ T lymphocytes enumeration. However, FCM instruments are expensive and the price of an FCM assay is relatively high. Although dedicated CD4 flow cytometry systems are less expensive, the costs of the instruments and the assays are yet not affordable for resource-constrained countries. Furthermore, the operation and maintenance of an FCM requires well-trained technicians and stable electricity. Alternative methods (e.g. microbead separation methods) are cheap, but they are labor-intensive and may have a high operator-to-operator variations. Developing affordable, simple, easy-to-use, and reliable CD4+ T lymphocytes enumeration systems is therefore urgently demanded (6-8).
Recently, Rodriguez et al. reported a microchip-based CD4 counting method for HIV monitoring in resource-poor settings (8). In this method, blood cells are stained with CD3 and CD4 fluorescent antibodies, captured on a membrane in a miniaturized flow cell, and then imaged through microscope optics.
We previously developed a simple single platform image cytometer (SP ICM) for absolute enumeration of white blood cells and their sub-populations in whole blood. The instrument is based on antibody-specific immunomagnetic separation of target cells from whole blood samples, in which all the white blood cells have been labeled with a DNA fluorescent stain acridine orange (9-11). Immunomagnetically selected cells are magnetically attracted to an analysis surface and imaged by a CCD camera. Using this method, cell types that are well defined by single type of cell surface antigen, i.e. white blood cells (CD45), T lymphocytes (CD3), and B lymphocytes (CD19), can be accurately enumerated. However, as both CD4+ T lymphocytes and CD4+dim monocytes can be selected by CD4 immunomagnetic labeling, this procedure is not ready for the enumeration of CD4+ T lymphocytes for HIV monitoring.
To overcome this limitation, we apply immunofluorescent labeling in addition to the immunomagnetic labeling. Correspondingly, a dedicated SP ICM for CD4+ T lymphocytes enumeration has been developed. This system identifies CD4+ T lymphocytes in the fluorescent images of the analysis surface as the CD3+CD4+ population. The instrumentation of this system will be described in great detail elsewhere. In this article, the immunochemical methodology is described and the overall method is tested. Test results on HIV negative (HIV−) and HIV positive (HIV+) patients are presented and compared with those obtained from single platform FCM (SP FCM) methods.
MATERIALS AND METHODS
Blood Collection
Blood samples from randomly selected adult HIV− patients and adult HIV+ patients were supplied by MST hospital, Enschede, The Netherlands. Blood samples from healthy donors were obtained from volunteers. All blood samples were collected in sterile K3EDTA blood collection tubes (BD Biosciences) by venipuncture and processed within 8 hrs after draw.
Immuno Labeling for Single Platform Image Cytometry (SP ICM)
-
Scheme a: CD4 immunomagnetic labeling and CD3 immunofluorescent labeling.
-
Scheme b: CD3 immunomagnetic labeling and CD4 immunofluorescent labeling.
The appropriate amounts of immunomagnetic labels, incubation time, and magnetic separation time to be applied in SP ICM were determined by titration using blood samples from a healthy donor. CD4+ T lymphocytes counts of the same blood samples enumerated with SP FCM (Beckman Coulter tetraCXP 4-color method) (tetraCXP method) were used as reference. In separate titration experiments, 0.5 μl, 1 μl, 2 μl, 3 μl, 5 μl, 8 μl, 10 μl, and 20 μl of immunofluorescent labels were applied. The appropriate amounts of immunofluorescent labels were determined according to the Signal-to-Noise Ratios (SNRs) of the fluorescent images and the accuracy of CD4+ T lymphocytes counts obtained by SP ICM. (SNR = (〈Icell〉 − 〈Ibackg〉) / 〈σnoise〉, where 〈Icell〉 and 〈Ibackg〉 are respectively the average fluorescence intensity of the CD4+ T lymphocytes and that of the background in a same image, and 〈σnoise〉 is the standard deviation of the total noise present in the same image (12)). Blood specimens of three HIV+ patients, whose CD4+ T lymphocytes counts being ca. 200/μl, 400/μl, and 600/μl (determined by SP ICM method), were used. From these titration experiments the following immunolabeling protocols evolved:
Scheme a
To 100 μl of EDTA anticoagulated whole blood, 10 μl of reagent cocktail, which contains 2 μl of 0.88 mg/ml CD4-FerroFluid (CD4FF, clone: EDU-2, isotype: mouse IgG2a-κ, Immunicon, Inc.), 3 μl of 25 μg/ml CD3 conjugated with Phycoerythrin (CD3PE, clone: UCHT1, isotype: Mouse IgG1-κ, BD Pharmingen, USA) and 5 μl of system buffer (Immunicon), was added and mixed. After 15 min incubation, the sample was diluted with 290 μl of system buffer to a final volume of 400 μl. Approximately 340 μl of the sample solution was transferred into the analysis chamber. The chamber was plugged and placed into a magnet assembly (Magnest®; Immunicon) inside the SP ICM instrument. After 20 min magnetic separation, the sample was ready to be analyzed.
Scheme b
The 10 μl of reagent cocktail contains: 3 μl of 0.655 mg/ml CD3-FerroFluid (CD3FF, clone: CRIS-7, isotype: mouse IgG2a-κ, Immunicon), 5 μl of 12.5 μg/ml CD4 conjugated with PE (CD4PE, clone: RPA-T4, isotype: Mouse IgG1-κ, BD Pharmingen) and 2 μl of system buffer. Other details are the same as those described in scheme a.
CD4+ T Lymphocytes Enumeration by SP ICM
During the magnetic separation period, the immunomagnetically labeled cells were subjected to a homogenous magnetic force pointing to the positive Z-direction of the magnetic chamber (9-11), thus moved to the upper glass surface of the chamber (analysis surface). For excitation of Phycoerythrin (PE), 4 × 5 W Light Emitting Diodes (LEDs) (470 nm, Lumileds, Luxeon) are mounted symmetrically above the magnet. In front of each LED, an exciter filter (475AF50, Omega optical) is placed and used to suppress the light emitted by the LED specifically in the region overlapping with the emission fluorescent signal of PE. The emission fluorescent signal is collected by a 10× objective (NA 0.2, Lomo Optics), filtered by an emission filter (59.5AF60, Omega optical) that selects the PE fluorescence, and then focused onto an ST-402ME CCD camera (SBIG). The images recorded are transferred to a single board computer (EES-3610, Evalue Technology, Taiwan) with a touch-screen monitor (B084SN03 V2, AU Optronics, Taiwan), and analyzed using a dedicated freeware image analysis algorithm that determines the number of CD4+ T lymphocytes per μl. For each test, three images from separate positions, each corresponding to 0.80 μl of whole blood in volume, are recorded and the counts are summed. The total volume (2.40 μl) was chosen to obtain a theoretical statistical Poisson variation of approximately 6% at 100 cells/μl and approximately 9% at 50 cells/μl (see below).
The dimensions of our SP ICM instrument are 25 cm × 25 cm × 20 cm. The system operates on 125–240 V, or on one 12V rechargeable battery.
CD4+ T Lymphocytes Enumeration by Single Platform Flow Cytometry (SP FCM)
TetraCXP method (Beckman Coulter, USA)
Samples were prepared according to the manufacturer's recommendation (13). To 100 μl of EDTA anticoagulated whole blood, 5 μl of Cyto-stat tetraCHROME (CD45 (clone: B3821F4A, isotype: mouse IgG2b)-FITC/CD4 (clone: SFCI12T4D11, isotype: mouse IgG1)-RD1/CD8 (clone: SFCI21Thy2D3, isotype: mouse IgG1)-ECD/CD3 (clone: UCHT1, isotype: mouse IgG1)-PC5) was added and mixed. (FITC, fluorescein isothiocyanate; RD1, R-phycoerythrin; ECD, R-phycoerythrin/Texas Red tandem dye; PC5, R-phycoerythrin/cyanine5 tandem dye.) After 10 to 12 min incubation in dark at room temperature, the sample was lysed and fixed in a Multi-Q-Prep workstation. 100 μl of Flow-Count Fluorospheres was added and mixed before analysis. The samples were analyzed on a Cytomics FC500 using tetraCXP software, and absolute CD3, CD4, and CD8 counts were obtained. These tests were performed in MST hospital, Enschede, The Netherlands.
TruCount method (BD Bioscience, USA)
TruCount™ absolute count tubes were used and blood samples were processed using a lyse-no-wash method according to the manufacturer's recommendation (Immunocytometry System, BD Biosciences) (14). Reagents used were CD3 (clone: SK7, isotype: mouse IgG1-κ) FITC/CD4 (clone: SK3, isotype: mouse IgG1-κ) PE/CD45 (clone: 2D1, isotype: mouse IgG1-κ) PerCP (TriTEST, BD Biosciences). The samples were analyzed on the FACSCalibur using CELLQUEST™ software.
Evaluation of SP ICM on CD4+ T Lymphocytes Enumeration
Precision

Accuracy and linearity
To evaluate the accuracy and linearity of our system within the clinically important range of CD4+ T lymphocytes counts (0–500/μl) (3, 15, 16, 17), a series of blood samples were prepared by graded dilutions of CD4+ T lymphocytes through adding CD4+ T lymphocytes depleted blood into whole blood according to the following protocol:
From a whole blood specimen of a healthy donor, a CD4 depleted blood sample was prepared using CD4FF immunomagnetic separation method. Then the original whole blood specimen was mixed with the CD4 depleted blood sample in different ratios, resulting in a series of five specimens with known numbers of CD4+ T lymphocytes. The CD4+ T lymphocytes counts of the whole blood specimen, of the CD4 depleted blood sample, and of these mixed samples were enumerated by our system (scheme a). The accuracy and linearity were evaluated. As a reference, CD4+ T lymphocytes counts of the above mentioned samples were also measured by TruCount method.
Comparison of SP ICM and SP FCM on HIV − patients
TetraCXP method and TruCount method are frequently applied SP FCM methods for CD4+ T lymphocytes enumeration. The performance of these two methods was evaluated using blood specimens of 44 HIV− patients, and the results obtained using these two methods are compared with each other.
From the same blood specimens, the CD4+ T lymphocytes counts were also enumerated by the SP ICM methods, respectively following labeling scheme a and scheme b. The results obtained using these two SP ICM methods are compared with each other, and also compared with those of the two SP FCM methods.
Comparison of SP ICM and SP FCM on HIV+ Patients
The blood specimens of 63 HIV+ patients were tested using our SP ICM method (scheme a) and SP FCM (tetraCXP method), and the obtained CD4+ T lymphocytes counts were compared.
CD4+ T lymphocytes enumeration of ImmunoTrol cells by SP ICM
To evaluate the suitability of our SP ICM for the enumeration of CD4+ T lymphocytes in the stabilized cell products used in the External Quality Assurance (EQA) schemes, ImmunoTrol Cells (Beckman Coulter) were separately enumerated for 10 times. The obtained CD4+ T lymphocytes counts and the mean value were compared with the expected results (596 ± 180 CD4+ T lymphocytes /μl) supplied by the manufacturer.
Statistical Analysis
Based on the CD4+ T lymphocytes counts obtained from different methods, linear regression lines were drawn and correlation coefficients (R) were calculated. Bland–Altman plots (18-20) were used to evaluate the interchange-ability between methods. In Bland–Altman plots, the average of the CD4 counts obtained from two methods is plotted on the horizontal axis, and the difference/average% is plotted on the vertical axis. The solid line in the plot represents the bias (the average difference between the two methods), and the dashed lines in the plot illustrate the upper and lower limits of agreement (±1.96 SD).
Percentage similarity scatter-plot and percentage histogram (21) were also used to evaluate the accuracy and precision of these methods, and the interchange-ability between methods. In percentage similarity scatter-plots, SP FCM is used as a gold standard, and the data obtained from the gold standard (vertical axis) are plotted against the percentage similarity values (({SP ICM + gold standard}/2)/gold standard × 100) (horizontal axis). From the plot, a mean percentage similarity, a standard deviation and a CV (standard deviation/mean percentage similarity) can be calculated. 100% similarity means no difference between two methods. In percentage histogram, the percentage similarity data from the scatter-plot are grouped into 15 intervals, and the counts in each interval (vertical axis) were plotted against the percentage similarity (horizontal axis). A normal curve is plotted over the histogram. The spreading of the normal curve with respect to the 100% similarity reference line indicates both accuracy and precision of our method as compared to the gold standard.
RESULTS
Titration Experiments
The appropriate amounts of immunomagnetic reagents and magnetic separation time for selecting CD4+ T lymphocytes were determined by the titration experiments. Figure 1A illustrates the number of CD4+ T lymphocytes/μl determined using scheme a (CD4FF/CD3PE) as a function of magnetic separation time and that as a function of the amount of CD4FF applied. When 1 μl to 4 μl of CD4FF is used, the cell count reaches a plateau at 10 min of magnetic separation time. The plateau value is independent of the amount of CD4FF, and approximately equal to the CD4+ T lymphocytes obtained by SP FCM (tetraCXP method). Accordingly, we chose 2 μl of 0.88 mg/ml CD4FF to be applied in CD4+ T lymphocyte enumeration and 20 min as magnetic incubation time. These conditions ensure that all the CD4+ T lymphocytes could be attracted to the upper surface of the chamber.
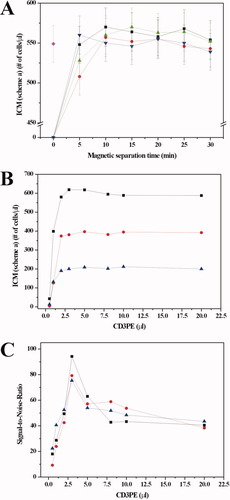
A: CD4FF titration. Number of CD4+ T lymphocytes/μl enumerated by ICM (scheme a) as a function of magnetic incubation time and that as a function of the amount of CD4FF applied. CD4+ T lymphocyte count obtained by SP FCM (tetraCXP method) was applied as a reference. Error bar is the square root of cell count. Black squares: 1μl of CD4FF, red spheres: 2μl of CD4FF, green up-triangles: 3μl of CD4FF, blue down-triangles: 4μl of CD4FF, magenta diamonds: by tetraCXP method. CD3PE titration. B: Number of CD4+ T lymphocytes obtained by SP ICM using scheme a, and C: Signal-to-Noise Ratios as a function of the amount of CD3PE. Black squares: the blood samples of the patient with approximately 600/μl CD4+ T lymphocytes, red spheres: approximately 400/μl CD4+ T lymphocytes, blue triangles: approximately 200/μl CD4+ T lymphocytes. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To find the appropriate amount of CD3PE used in scheme a, separate titration experiments were performed. Figure 1B and C illustrate the number of CD4+ T lymphocytes obtained by SP ICM (scheme a) and Signal-to-Noise Ratios as a function of the amount of CD3PE, respectively. It was found that the accurate CD4+ T lymphocytes counts could only be obtained when the amount of CD3PE is 2 μl and higher. The best SNR was obtained when using 3 μl of CD3PE. Taking the test cost into account, we chose 3 μl of CD3PE (25 μg/ml) in scheme a.
Alternatively, for scheme b (CD3FF/CD4PE), the titration experiments were performed in the same way. Three μl of CD3FF (0.655 mg/ml) and 5 μl of CD4PE (12.5 μg/ml) were chosen.
Precision of SP ICM
To assess the precision of the SP ICM on CD4+ T lymphocytes enumeration, five replicate blood samples from 20 HIV− patients (in total 100 samples) were enumerated by the SP ICM using labeling scheme a (CD4FF/CD3PE). For replicate blood samples of each patient, the calculated CV values and Poisson variations were compared.
Figure 2 shows the CV values and the expected Poisson variations from the five replicates of each of the 20 blood specimens, plotting against their mean CD4+ T lymphocytes counts. The mean CD4+ T lymphocytes counts range from 90/μl to 1495/μl; correspondingly, the Poisson variations range from 6.8% to 1.7%.

CV (diamonds) and Poisson variation values (squares) of CD4+ T lymphocytes counts from five replicates of each of the blood specimens of 20 HIV− patients plotted against their mean CD4+ T lymphocytes counts.
The measured CV values range from 7.8% to 1.8%, this range is comparable to that of the commercially available CD4+ T lymphocytes enumeration systems (flow cytometry and hematology analyzer) (22). Moreover, the measured CV values are close to the expected Poisson variations; this shows that the precision of the SP ICM system is mainly determined by Poisson statistics.
Accuracy and Linearity of SP ICM
To evaluate the accuracy and linearity of the system, a whole blood sample and a series of diluted samples containing graded, known numbers of CD4+ T lymphocytes were prepared. Both the original undiluted sample and the diluted preparations were checked in parallel with the “gold standard” BD TruCount method (13) and the SP ICM (scheme a). In Fig. 3A, the CD4+ T lymphocytes counts measured by SP ICM were plotted as a function of the percentage of the whole blood in these samples (0% for the CD4 depleted sample, and 100% for the original whole blood sample). A linear relation (R = 0.998) is found, demonstrating the linearity of our system.

Linearity and accuracy of the SP ICM system for CD4+ T lymphocytes enumeration. A: Observed SP ICM CD4+ T lymphocytes counts of whole blood sample of a healthy donor, its CD4 depleted blood sample, and a series of their mixtures vs. the percentage of the whole blood in these samples (for the original whole blood sample, 100%; for the CD4 depleted blood sample, 0%). B: Corrected SP ICM CD4+ T lymphocytes counts vs. CD4+ T lymphocytes counts obtained using SP FCM (TruCount method).
In Fig. 3A, an offset of 29 cells/μl was found for CD4 depleted samples. These cells are probably the CD4+ T lymphocytes that are left over in the CD4 depleted blood; they carry magnetic labels as a result of the depletion procedure, but have not been removed and therefore could be detected by the SP ICM. In SP FCM analysis, such an offset is not present. This result is logic: since the CD4 receptors on these remaining CD4+ T lymphocytes in the CD4 depleted blood have been covered with the immunomagnetic labels, these cells can not be immunofluorescently labeled and recognized in SP FCM analysis.
Considering the presence of these remaining CD4+ T lymphocytes, the cell counts of each sample obtained by SP ICM can be “corrected” by taking out the contribution of these remaining cells. Such “corrected” SP ICM counts are compared with the cell counts obtained from SP FCM, as shown in Fig. 3B. From the fact that the slope of the curve is close to 1.0 (0.98) and R is 0.997, we conclude that the SP ICM method and the SP FCM method give comparable and accurate results.
Comparing SP ICM with SP FCM on HIV− Patients
First, the two “gold standard” SP FCM methods, Tetra CXP method and TruCount, were compared with each other by enumerating CD4+ T lymphocytes for 44 HIV− patients (CD4+ T lymphocytes counts: 128–1958/μl, obtained by tetraCXP method). Figure 4 shows the linear regression plot (Fig. 4A) and the Bland–Altman plot (Fig. 4B) of this comparison. From Fig. 4A, a correlation coefficient (R) of 0.99 and a slope of 0.91 were determined, with the tetraCXP data being about 9% lower than the TruCount data. Figure 4B shows a bias of −8.0% and limits of agreement (mean value ± 1.96 Standard Deviation) of −19.1% to 3.0% (tetraCXP data vs. TruCount). These data show that the two gold standards yield comparable, but slightly different values.

Linear regression plot (A) and Bland–Altman plot (B) comparing CD4+ T lymphocytes enumerations using tetraCXP and TruCount methods. Blood specimens of 44 HIV− patients were tested.
Then we compared the SP ICM results obtained respectively using the labeling scheme a and scheme b for the same 44 HIV− patients. The results are shown in Fig. 5. From Fig. 5A, a correlation coefficient of 0.99 and a slope of 0.95 were calculated; the Bland–Altman plot in Fig. 5B shows a bias of −4.6% and limits of agreement of −19.9% to 10.8% (scheme b vs. scheme a). These results illustrate that the two labeling methods agree well with each other, with scheme b giving results about 5% lower than those from scheme a. This difference is smaller than that we found between the two “gold standards.”
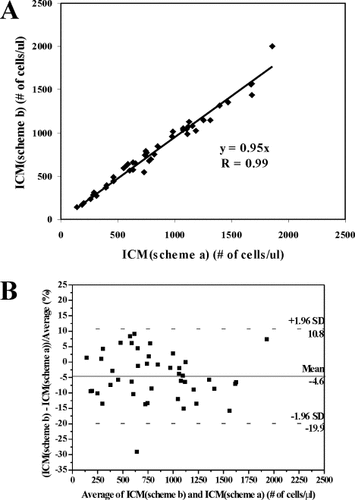
Linear regression plot (A) and Bland–Altman plot (B) comparing CD4+ T lymphocytes enumerations by SP ICM using labeling scheme b and scheme a. Blood specimens of 44 HIV− patients were tested.
The SP ICM results obtained from labeling scheme a and scheme b were respectively compared with the results obtained from the two “gold standard” SP FCM methods. The results are shown in Fig. 6 (comparing with TruCount) and Fig. 7 (comparing with tetraCXP).

Linear regression plots and Bland–Altman plots comparing CD4+ T lymphocytes enumerations by SP ICM using respectively scheme a (A and B) and scheme b (C and D) vs. the enumeration by TruCount SP FCM. Blood specimens of 44 HIV− patients were tested.

Linear regression plots and Bland–Altman plots comparing CD4+ T lymphocytes enumerations by SP ICM using respectively scheme a (A and B) and scheme b (C and D) vs. the enumeration by tetraCXP SP FCM. Blood specimens of 44 HIV− patients were tested.
The CD4+ T lymphocytes counts obtained by both SP ICM methods are comparable to and slightly less than those obtained by TruCount method. In case that labeling scheme a was applied, the correlation coefficient R is 0.98, the slope is 0.95, and the bias is −0.8%, with limits of agreement of −15.2% to 13.7 % (Fig. 6A and B). In case that labeling scheme b was applied, R is 0.97, the slope is 0.90, and the bias is −5.3%, with limits of agreement of −27.3% to 16.6% (Fig. 6C and D).
Figure 7 shows the comparison of the two SP ICM methods with the tetraCXP method. The CD4+ T lymphocytes counts obtained by both the SP ICM methods are comparable to and slightly more than those obtained by the tetraCXP method. In case of labeling scheme a, R is 0.99, the slope is 1.04, and the bias is 7.2% with limits of agreement of −6.9% to 21.3%, as illustrated in Fig. 7A and B. In case of labeling scheme b, R is 0.98, the slope is 0.99, and the bias is 2.7% with limits of agreement −19.4% to 24.8% (Fig. 7C and D).
These results indicate that the SP ICM methods following both immunolabeling schemes agree well with the two SP FCM methods. The bias values are largest between the two gold standards; and the SP ICM data are in between of those obtained from tetraCXP method and from TruCount method. The SP ICM methods are interchange-able with SP FCM methods for HIV− patients.
When comparing the data obtained from scheme a and from scheme b in SP ICM methods (using the ‘gold standards’ as reference), it appears that the range of limits of agreement between the SP ICM and the SP FCM is smaller when scheme a (see Fig. 6B and 7B) is used, as compared to the case using scheme b (see Fig. 6D and 7D).
From Fig. 4B, 6B, and 6D, it is noticed that for blood samples with CD4+ T lymphocytes counts above ∼1400/μl, the cell counts from both tetraCXP method and our two SP ICM methods are less than those from TruCount.
Comparing SP ICM with SP FCM on HIV+ Patients
In view of the abnormalities of the immune system in HIV infected patients (23), we evaluated the SP ICM method (scheme a) separately by enumerating the CD4+ T lymphocytes in blood samples of 63 adult HIV+ patients. SP FCM tetraCXP method was also used for the same blood samples (from tetraCXP method, the CD4+ T lymphocytes counts of these patients range between 8 and 1134/μl). The accuracy of the SP ICM method and the interchange-ability of the SP ICM method with the SP FCM were assessed (see Fig. 8). From Fig. 8A it is clear that a good correlation between the two methods was obtained, the correlation coefficient R is 0.97, and the slope of the regression line is 0.98. The Bland–Altman plot (Fig. 8B) shows a small bias of only 1.4% with the limits of agreement (±1.96 SD) ranging from −29.4% to 32.2% (SP ICM vs. TetraCXP). These results demonstrate the interchange-ability between the SP ICM and the tetraCXP method for testing blood samples from HIV+ patients.
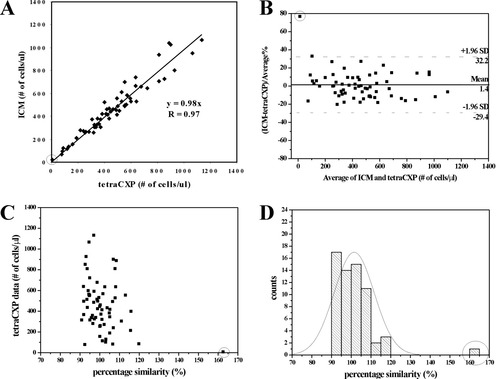
Linear regression plot (A), Bland–Altman plot (B), percentage similarity scatter-plot (C), and percentage similarity histogram (D) comparing CD4+ T lymphocytes enumeration by SP ICM using labeling scheme a with that by tetraCXP SP FCM (the gold standard). Blood specimens of 63 HIV+ patients were testes. An outlier is indicated with a circle in each figure. In Fig. 8D, a normal curve is plotted.
The percentage similarity scatter-plot (Fig. 8C) and the histogram (Fig. 8D) show an excellent agreement as well. In Fig. 8C, tetraCXP data are used as the gold standard, and the data obtained from the gold standard are plotted against percentage similarity values (({SP ICM + gold standard}/2)/gold standard × 100). A mean percentage similarity of 101.47% is calculated, with a standard deviation of 10.15% and a CV of 10.00%. If one outlier with extremely low CD4+ T lymphocytes count (tetraCXP: 8/μl, SP ICM: 18/μl, see Fig. 8 A–C) is excluded, the results are 100.49% for mean percentage similarity, 6.53% for standard deviation, and 6.50% for CV. Similarly, the normal curve in Fig. 8D spreads around the 100% similarity reference line, peaking at approximately 101.5%. These results confirm the accuracy and precision of the SP ICM method for CD4+ T lymphocytes enumeration of HIV+ patients.
CD4+ T Lymphocytes Enumeration of ImmunoTrol Cells by SP ICM
It is required by WHO that the new CD4 counting devices should work with specimens used in the External Quality Assurance (EQA) schemes. ImmunoTrol Cells are stabilized blood cells product which is used for QASI (Quality Assurance Systems International, an international program for quality assessment and standardization for T lymphocyte subset enumeration) (24). Therefore, our SP ICM system was used to count CD4+ T lymphocytes in ImmunoTrol Cells product. The mean CD4+ T lymphocytes count of 10 measurements was 585/μl, which is very close to the expected count (596 ± 180 CD4+ T lymphocytes/μl) as supplied by the manufacturer. Furthermore, the obtained CD4+ T lymphocytes counts ranged from 507 to 680/μl, this range is much smaller than that supplied by the manufacturer (from 416 to 776 CD4+ T lymphocytes/μl).
DISCUSSION
The results demonstrate that the SP ICM method for enumerating CD4+ T lymphocytes works well on blood samples of healthy donors, HIV− patients, HIV+ patients, and the stabilized blood cells product (ImmunoTrol cells).
Our method is based on the combination of immunomagnetic separation and immunofluorescent labeling of target cells. The counted cells are CD3+CD4+ T lymphocytes, while CD4+ monocytes are excluded in this way. The fact that the selected cells are positive for two antibodies brings in the options of two different labeling schemes, namely CD4FF/CD3PE (scheme a) and CD3FF/CD4PE (scheme b). Both schemes give exchangeable results with each other, and more importantly, with the two SP FCM methods that are frequently used as gold standard. The precision, the accuracy and the linearity of the SP ICM are excellent in the clinically important range of CD4+ T lymphocytes counts (0–500/μl) for monitoring HIV induced immunodeficiency.
Our system directly outputs the values of interest: A small computer controls the measurement cycle and supports image analysis program. Additionally, the sample measurement is an automatically controlled process, and the CD4+ T lymphocytes count is automatically displayed within 1 min after insertion of the analysis chamber into the instrument. The CD4+ T lymphocytes counts of the patients are available in about 40 min after blood draw. Accordingly, the clinician can make a decision about treatment or non-treatment of the patients during their first visit. This eliminates delays, costs and other problems that may be generated by shipping the blood samples to central hospital labs. One well-trained operator can carry out about 50 tests with one instrument and several Magnests® in 8 hrs.
Our SP ICM instrument applies well-known principles of fluorescent microscopy and cheap components where possible. For illumination, cheap and long lifetime LEDs are used. The SP ICM is simple and easy-to-handle, even for less trained operators. No data interpretation is needed, which prevents subjective errors. Acquiring pipetting skill for the three pipetting steps in sample preparation is the most essential part in training the operators. Moreover, the system is portable, robust, battery-operated, and suitable for point-of-care applications. It can be operated in extremely remote areas, can be operated immediately after arrival at site, and can even be operated on a car battery. Due to the simplicity and the fixed position of the optical components, no alignment calibration is required. All these aspects are especially advantageous for usage in developing countries.
Although the system operates well, in some aspects further improvements would be ideal. In the first place this concerns a further reduction of the price. The single-unit component price of the instrument is about US$ 3,000, much cheaper than the cost of a flow cytometer. A considerable percentage of the assay price (ca. US$ 3) comes from the disposable chamber (ca. US$ 1.8), suggesting the necessity to replace this chamber with a cheaper one. A different concern is the stabilization and packaging of the reagents for applications under tropical conditions. Pre-packaging of the reagents in a cheaper and smaller disposable test chamber would further decrease the manual steps and the amount of the employed reagents.
It is found that for some HIV+ patients when their blood specimens were aged over 24 hrs, our CD4+ T lymphocytes enumeration system (scheme a) gives over-counts, whereas for blood specimens of healthy donors, the CD4+ T lymphocytes could reliably be enumerated at up to 72 hrs after blood draw. This is likely caused by the fact that for aged blood samples of some HIV+ patients, a part of the CD3+CD8+ T lymphocytes are non-specifically (magnetically) selected, and counted as CD4+ T lymphocytes since they are labeled with CD3PE. Such non-specific selection probably arises due to the abnormalities of the immune system in HIV infected patients (23).
It is important to carefully select the source of immunomagnetic nanoparticles because unexpected effects may occur. In our study, EasySep nanoparticles (StemCell Technologies, Canada) and FerroFluid (FF) nanoparticles (Immunicon Inc., USA) have been tested. Both nanoparticles are iron-oxide particles with an average size of 170 nm. The EasySep particles give better image (less clustering of particles). However, using EasySep CD4 immunomagnetic nanoparticles in combination with CD3PE labels, we found two outliers amongst 76 HIV+ patients (results not shown). The CD4+ T lymphocytes counts of these two patients obtained from SP ICM method were 3–5 times higher than those obtained from SP FCM method. These over-counts were related to a particular batch of the EasySep immunomagnetic nanoparticles and to particular patients. These artefacts were not encountered when the same blood samples of these two patients were tested using FF nanoparticles. Consequently, we selected FF particles for use in our system for HIV+ patients. We could not find the reason for this phenomenon. Whether it is related to the over-count occurring upon aging blood of some HIV+ patients as we described above is unknown.
Determination of CD4:CD8 ratios is important for pediatric HIV diagnosis and monitoring (25, 26). Currently, a system that simultaneously counts CD4+ and CD8+ T lymphocytes applying the same methodology is under development. The SP ICM system described in this article and the new CD4+ and CD8+ enumeration system will be tested in resource-constrained countries.
In conclusion, the SP ICM system is a good candidate for CD4+ T lymphocytes enumeration in point-of-care settings of resource-constrained countries.
Acknowledgements
This study is financially supported by STW, the Dutch Technology Foundation (project TGT. 6146). We thank I. Vermes M.D. Ph.D and C.H.H. ten Napel M.D. Ph.D. from MST hospital in Enschede for kindly supplying fresh blood samples and FCM data. We acknowledge all patients and healthy donors whose blood was tested in this study.




