A pilot study of assessing whole genome sequencing in newborn screening in unselected children in China
Min Jian, Xiaohong Wang, Yuanyuan Sui, Mingyan Fang, Lennart Hammarström, Xiaojing Jiang, Junnian Liu, Ya Gao contributed equally to this work.
We investigated whether screening by whole genome sequencing (WGS) in unselected newborns provides more information of potentially curable or treatable medical conditions than routine newborn screening (NBS). We demonstrated that compared with routine NBS, WGS produced fewer false positive results and identified more actionable pathogenic or likely pathogenic variants in the selective 246 genes.
Previously, WGS has been used to identify mutated genes in newborn children with a suspected disease.1 However, sequencing of apparently healthy newborns has remained controversial due to technical concerns and ethical issues.2 In this study, 321 non-pre-selected newborns from a cohort of pregnant women in Qingdao, China were recruited (Table 1). DNA from 303 umbilical cord blood samples and 18 umbilical cords was extracted for 40X WGS. For data interpretation, we selected 251 genes associated with 59 Mendelian disorders, 164 primary immunodeficiency diseases (PIDs) and five pharmacogenetic (PGx) genes, following the guidelines by the Recommended Uniform Screening Panel (RUSP), the International Union of Immunologic Societies (IUIS) Expert Committee for Primary Immunodeficiency, the Dutch Pharmacogenetics Working Group (DPWG), and the Clinical Pharmacogenetics Implementation Consortium (CPIC).3-5 Sequencing protocol, data analysis pipeline, and criteria for sequence variants interpretation following the ACMG/AMP guidelines are described in the Supporting Information. The WGS results were compared with NBS results, including the mandatory checks of hearing impairment and four metabolic diseases, the metabolic testing of 48 inherited metabolic diseases (IMDs), and the genetic screening for 20 hearing loss loci incorporated into the local NBS program in China.6, 7
| Type | Number | Percentage (%) | |
|---|---|---|---|
| Pregnancy | Natural pregnancy | 306 | 95.4 |
| Assisted reproduction technology | 11 | 3.4 | |
| Unspecified | 4 | 1.20 | |
| Gestational weeks | Pre-term birth | 7 | 2.2 |
| Term birth | 314 | 97.80 | |
| Average delivery gestation | 39 weeks plus 5 days | – | |
| Gender of newborn | Male | 151 | 47.04 |
| Female | 170 | 52.96 | |
| Parental age at delivery | Father's age (ave. year) | 33 | – |
| Mother's age (ave. year) | 32 | – | |
| SD of father's age (year) | 5 | – | |
| SD of mother's age (year) | 4 | – | |
| Mandatory NBS screening of 4 metabolic diseases (PKU, CAH, CH and G6PD) by TRFIA | Phe + | 1 | 0.31 |
| Negative | 320 | 99.69 | |
| Mandatory hearing impairment screening by OAEs or AABR | Not passed | 0 | 0.00 |
| Passed | 321 | 100.00 | |
| 48 IMDs screening by tandem MS/MS | C5-OH + | 1 | 0.31 |
| Phe + | 1 | 0.31 | |
| Negative | 310 | 96.57 | |
| Unspecified | 9 | 2.80 | |
| Genetic hearing loss screening by MALDI-TOF | Carrier | 18 | 5.61 |
| Negative | 294 | 91.59 | |
| Unspecified | 9 | 2.80 |
- AABR, automated auditory brainstem response; CAH, congenital adrenal hyperplasia; CH, congenital hypothyroidism; CH G6PD, glucose-6-phosphate dehydrogenase; C5-OH+, isovalerylcarnitine positive; MS/MS, mass spectrometry; MALDI-TOF, matrix-assisted laser desorption/ionization time of flight; OAEs, otoacoustic emissions; PKU, phenylketonuria; Phe+, phenylalanine positive; SD, standard deviation; TRFIA, time resolved fluoroimmunoassay.
Among the analysed DNA samples of 321 newborns, the average sequencing depth was 47.42X (28.84X–82.90X) and the average coverage was 99.48% (99.01%–99.89%) (Figure 1A). For the 59 Mendelian disorders, a total of 131 pathogenic or likely pathogenic (P/LP) mutations and 5 pathogenic copy number variations were detected in 107 of the 321 newborns (33.33%), corresponding to 106 carriers of 28 diseases and 1 patient with phenylketonuria (PKU) (Figure 1B and Table 2). The 25.23% of newborns (n = 81) carried one P/LP mutations, and 7.17% and 0.93% of newborns (n = 23 and n = 3) carried two or three P/LP mutations, respectively. Hearing loss, methylmalonic acidemia (MMA), primary congenital hypothyroidism (CH), and PKU were diseases with the most carriers, while GJB2 (28/321, 8.72%), MMACHC (11/321, 3.43%), DUOX2 (10/321, 3.12%), PAH (8/321, 2.49%) and SLC26A4 (8/321, 2.49%) were the top five genes with the highest carrier frequencies of P/LP mutations (Table 2).
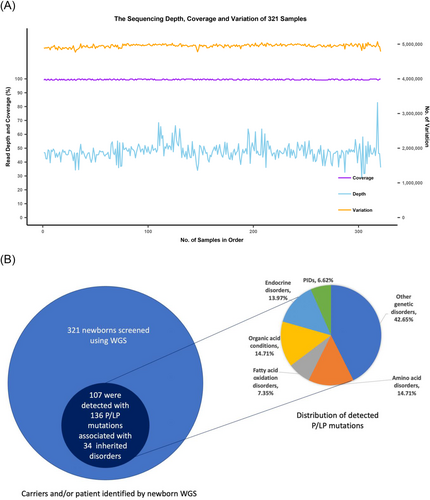

| Condition | Inheritance | Gene | Variation | Protein | Classification | Num of newborns | Het/Hom |
|---|---|---|---|---|---|---|---|
| Propionic acidemia | AR | PCCB | c.1364A > G | p.Y455C | P | 1 | Het |
| c.793G > A | p.G265S | LP | 1 | Het | |||
| Methylmalonic acidemia (methylmalonic-CoA mutase) | AR | MUT | c.2179C > T | p.R727* | P | 1 | Het |
| Methylmalonic acidemia (cobalamin disorders) | AR | MMAA | c.658G > A | p.V220M | LP | 1 | Het |
| AR | MMACHC | c.315C > G | p.Y105* | P | 1 | Het | |
| c.445_446del | p.C149Hfs32 | P | 1 | Het | |||
| c.482G > A | p.R161Q | P | 2 | Het | |||
| c.609G > A | p.W203* | P | 3 | Het | |||
| c.658_660del | p.*220del | P | 3 | Het | |||
| c.80A > G | p.Q27R | P | 1 | Het | |||
| Methylmalonic acidemia (cobalamin disorders)/methylmalonic acidemia with homocystinuria | AR | MMADHC | c.748C > T | p.R250* | P | 1 | Het |
| 3-Methylcrotonyl-CoA carboxylase deficiency | AR | MCCC1 | c.639+2T > A | / | P | 1 | Het |
| Holocarboxylase synthase deficiency | AR | HLCS | c.782del | p.G261Vfs20 | P | 2 | Het |
| Glutaric acidemia type I | AR | GCDH | c.1213A > G | p.M405V | P | 1 | Het |
| Carnitine uptake defect/carnitine transport defect | AR | SLC22A5 | c.1472C > G | p.S491C | P | 4 | Het |
| c.468G > A | p.W156* | P | 1 | Het | |||
| Medium-chain acyl-CoA Dehydrogenase deficiency | AR | ACADM | c.548_551del | p.T183Rfs4 | P | 2 | Het |
| Trifunctional protein deficiency | AR | HADHB | c.1175C > T | p.A392V | LP | 1 | Het |
| Citrullinemia, type I | AR | ASS1 | c.919C > T | p.R307C | P | 1 | Het |
| c.352G > A | p.A118T | LP | 1 | Het | |||
| Classic phenylketonuria | AR | PAH | c.1301C > A | p.A434D | LP | 1 | 7Het; 1 individual with compound Het |
| c.611A > G | p.Y204C | P | 2 | ||||
| c.728G > A | p.R243Q | P | 4 | ||||
| c.740G > T | p.G247V | P | 1 | ||||
| c.842+2T > A | / | P | 1 | ||||
| Primary congenital hypothyroidism | AR,AD | TSHR | c.1349G > A | p.R450H | P | 4 | Het |
| AR | DUOX2 | c.1588A > T | p.K530* | P | 6 | Het | |
| c.1883del | p.K628Rfs11 | P | 1 | Het | |||
| c.1946C > A | p.A649E | LP | 1 | Het | |||
| c.605_621del | p.Q202Rfs93 | P | 1 | Het | |||
| c.3329G > A | p.R1110Q | P | 1 | Het | |||
| AR | TPO | c.2422del | p.C808Afs24 | P | 1 | Het | |
| Congenital adrenal hyperplasia | AR | CYP21A2 | c.518T > A | p.I173N | P | 2 | Het |
| c.92C > T | p.P31L | P | 2 | Het | |||
| S,S disease (sickle cell anemia)/S, beta-thalassemia/S,C disease/other haemoglobinopathies | AR | HBB | c.126_129del | p.F42Lfs19 | P | 1 | Het |
| Cystic fibrosis | AR | CFTR | c.2052_2053insA | p.Q685Tfs4 | P | 1 | Het |
| Classic galactosemia | AR | GALT | c.821-7A > G | / | P | 1 | Het |
| AR | GALT | c.844C > G | p.L282V | LP | 1 | Het | |
| Glycogen storage disease type II (Pompe) | AR | GAA | c.2237G > C | p.W746S | P | 1 | Het |
| c.2662G > T | p.E888* | P | 1 | Het | |||
| c.2647-7G > A | / | LP | 1 | Het | |||
| Hearing loss | AD, AR, DD (digenic dominant) | GJB2 | c.109G > A | p.V37I | P | 14 | 26Het; 2 individuals with compound Het |
| c.235del | p.L79Cfs3 | P | 10 | ||||
| c.299_300del | p.H100Rfs14 | P | 4 | ||||
| c.605_606insAGAAGACTGTCTTCACAGTGTTCATGATTGCAGTGTCTGGAATTTG | p.C202* | P | 2 | ||||
| AR | SLC26A4 | c.1174A > T | p.N392Y | P | 1 | Het | |
| AR | c.1229C > T | p.T410M | P | 1 | Het | ||
| AR | c.1262A > C | p.Q421P | LP | 1 | Het | ||
| AR | c.2027T > A | p.L676Q | LP | 2 | Het | ||
| AR | c.2168A > G | p.H723R | P | 1 | Het | ||
| AR | c.919-2A > G | / | P | 2 | Het | ||
| AR | USH2A | c.2802T > G | p.C934W | P | 1 | Het | |
| c.100C > T | p.R34* | P | 1 | Het | |||
| c.8559-2A > G | / | P | 1 | Het | |||
| Maternal | MT-RNR1 | m.1095T > C | / | P | 4 | Hom | |
| Spinal muscular atrophy | AR | SMN1 | c.(723+1_724-1)_(834+1_835-1)del | p.I242_M278del | P | 4 | Het |
| c.(723+1_724-1)_(885+1_886-1)del | p.I242_L294del | P | 1 | Het | |||
| Short-chain acyl-CoA dehydrogenase deficiency | AR | ACADS | c.1031A > G | p.E344G | P | 1 | Het |
| Glutaric acidemia type II | AR | ETFDH | c.1211T > C | p.M404T | LP | 1 | Het |
| Citrullinemia, type II | AR | SLC25A13 | c.1180+1G > A | / | P | 1 | Het |
| c.852_855del | p.M285Pfs2 | P | 2 | Het | |||
| Hypermethioninemia | AR | GNMT | c.149T > C | p.L50P | LP | 1 | Het |
| Biopterin defect in cofactor biosynthesis/biopterin defect in cofactor regeneration | AR | PTS | c.166G > A | p.V56M | P | 1 | Het |
| c.259C > T | p.P87S | P | 1 | Het | |||
| c.84-291A > G | / | P | 3 | Het | |||
| Galactoepimerase deficiency | AR | GALE | c.505C > T | p.R169W | P | 1 | Het |
| Adenosine deaminase (ADA) deficiency | AR | ADA | c.424C > T | p.R142* | P | 1 | Het |
| c.872C > T | p.S291L | P | 1 | Het | |||
| Ataxia-telangiectasia | AR | ATM | c.67C > T | p.R23* | P | 1 | Het |
| Immunoskeletal dysplasia with neurodevelopmental abnormalities (EXTL3 deficiency) | AR | EXTL3 | c.1970A > G | p.N657S | P | 1 | Het |
| JAK3 deficiency | AR | JAK3 | c.1744C > T | p.R582W | LP | 1 | Het |
| c.307C > T | p.R103C | LP | 1 | Het | |||
| DNA ligase IV deficiency | AR | LIG4 | c.1271_1275del | p.K424Rfs20 | P | 1 | Het |
| TACI deficiency (immunodeficiency, common variable) | AD/AR | TNFRSF13B | c.542C > A | p.A181E | LP | 2 | Het |
- Het, heterozygous; Hom, homozgyous. P, pathogenic; LP, likely pathogenic.
For the 164 PIDs, 9 heterozygous P/LP variants in 6 genes were identified in 9 newborns (2.80%), all in a heterozygous state (Table 2). Four newborns were shown to carry heterozygous variant of unknown significance (VUS) in the gene SLC25A13 (c.2T>C, p.M1T), which was predicted as start loss and likely affecting the initiator methionine of the SLC25A13 mRNA. Two newborns carried a VUS in ASS1(c.-4C>T, p.?). Although these VUSs were not included in the final report to the participants, follow-up of the children with VUSs will be conducted till 3 years of age.
Sanger sequencing confirmed 143 out of 145 mutations identified by WGS, resulting in an accuracy of 98.62%. Carriers of SMN1 mutations were validated by multiplex ligation-dependent probe amplification and real-time quantitative PCR, showing that five out of the six predicted carriers were true.
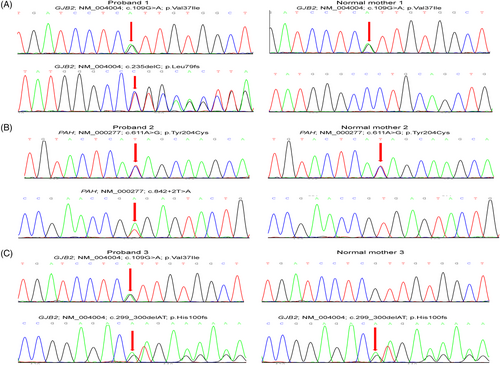
Of the 321 newborns, 312 (97.20%) had the results of 48 IMDs screening and genetic hearing loss screening on 20 loci, which identified one newborn with PKU and one infant with increased blood level of isovalerylcarnitine (Table 1). In addition, 18 carriers harbouring 20 pathogenic mutations causing hearing impairment were detected by genetic hearting loss screening, albeit all 321 children passed the physical hearing screening at hospital (Supporting Information). The newborn WGS also identified the PKU case and 18 hearing loss carriers (Figure 2A). However, the child with increased level of isovalerylcarnitine was confirmed to be a carrier of 3-methylcrotonyl-CoA carboxylase deficiency by WGS. In addition, WGS identified two infants carrying compound heterozygous P/LP variants in GJB2 (Figure 3) and four children carrying pathogenic mutations in MT-RNR1 (c1095T > C), suggesting an increased risk of late-onset deafness or drug-induced hearing loss, respectively. Although currently non-symptomatic, the two newborns with GJB2 variants were scheduled to undergo hearing tests every 6 months, and the four newborns with the m.1095T mutation in MT-RNR1 were advised to avoid using aminoglycosides.
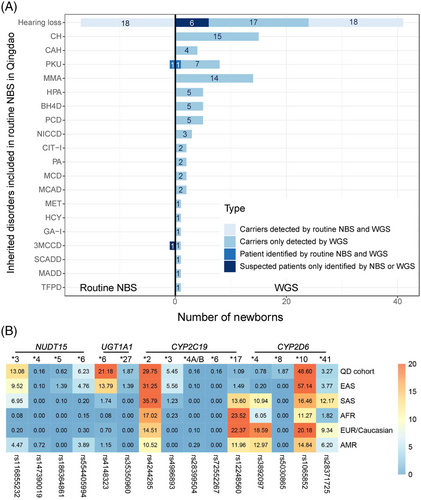
Interestingly, we observed that 313 newborns (97.51%) carried at least one actionable PGx variant (Figure 1C). This result is in line with a European 44 000 biobank participants study, where 99.8% of the participants had a genotype associated with increased risks to at least one medication.8 Furthermore, we found three common PGx variants in the Qingdao cohort, CYP2D6*10 (48.60%), NUDT15*3 (13.08%) and UGT1A1*6 (21.18%) (Figure 2B and Table 3), that showed significant frequency differences as compared to East Asian populations (p < 0.05). An important aspect when screening for disorders in a given population is the use of a matched control database as variants can be highly specific for a given ethnic group.9 Most databases published to date are based on individuals of European descent and many populations have limited or poor representation.
| Gene–drug pairs | Number of carriers in the Qingdao cohort | MAF$ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Gene | Star allele | dbSNP RS ID | Het | Hom | Variant No. | Carrier No. | Qingdao cohort (%) | EAS (%) | SAS (%) | AFR (%) | EUR/Caucasian (%) | AMR (%) |
| Azathioprine, mercaptopurine, tioguanine | NUDT15 | *3 | rs116855232 | 74 | 5 | 79 | 122 | 13.08 | 9.52 | 6.95 | 0.08 | 0.20 | 4.47 |
| *4 | rs147390019 | 1 | 0 | 1 | 0.16 | 0.10 | 0.00 | 0.00 | 0.00 | 0.72 | |||
| *5 | rs186364861 | 4 | 0 | 4 | 0.62 | 1.39 | 0.10 | 0.00 | 0.00 | 0.00 | |||
| *6 | rs554405994 | 36 | 2 | 38 | 6.23 | 4.76 | 0.20 | 0.15 | 0.30 | 3.89 | |||
| Irinotecan | UGT1A1 | *6 | rs4148323 | 106 | 15 | 121 | 133 | 21.18 | 13.79 | 1.74 | 0.08 | 0.70 | 1.15 |
| *27 | rs35350960 | 12 | 0 | 12 | 1.87 | 1.39 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Clopidogrel | CYP2C19 | *2 | rs4244285 | 141 | 25 | 166 | 209 | 29.75 | 31.25 | 35.79 | 17.02 | 14.51 | 10.52 |
| *3 | rs4986893 | 33 | 1 | 34 | 5.45 | 5.56 | 1.23 | 0.23 | 0.00 | 0.00 | |||
| *4A/B | rs28399504 | 1 | 0 | 1 | 0.16 | 0.10 | 0.00 | 0.00 | 0.10 | 0.29 | |||
| *6 | rs72552267 | 1 | 0 | 1 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| *17 | rs12248560 | 7 | 0 | 7 | 1.09 | 1.49 | 13.60 | 23.52 | 22.37 | 11.96 | |||
| Codeine | CYP2D6 | *4 | rs3892097 | 5 | 0 | 5 | 266 | 0.78 | 0.20 | 10.94 | 6.05 | 18.59 | 12.97 |
| *8 | rs5030865 | 10 | 1 | 11 | 1.87 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| *10 | rs1065852 | 150 | 81 | 231 | 48.60 | 57.14 | 16.46 | 11.27 | 20.18 | 14.84 | |||
| *41 | rs28371725 | 17 | 2 | 19 | 3.27 | 3.77 | 12.17 | 1.82 | 9.34 | 6.20 | |||
- * 1 denotes the default reference (wild type or fully functional) allele or haplotype, while other designations (e.g. *2 or *3) define haplotypes carrying one or more variants.
- AFR, africans; AMR, Americans; EAS, East Asians; EUR, Europeans; Het, heterozygous; Hom, homozygous; MAF, minor allelic frequency; SAS, South Asians; $, The MAF of EAS, SAS, AFR, EUR, and AMR refers to the 1000 Genome phase 3 dataset.
Limitations of the current study are the small sample size and restricted metabolic tests. A large-scale NBS effort is needed to validate our findings and fully investigate the treatable or curable medical conditions in newborns. The technical challenge of newborn WGS is to screen genes with high homology due to the misalignment of short-read sequencing. Therefore, the customized pipeline is needed to improve the accuracy and sensitivity of SNVs at genes with high-level homology. Albeit the present cost and turnaround time of WGS is several times more than the present NBS methods, in the forseeable future the pitfalls of WGS cost and turnaround time will likely facilitate the application of newborn WGS in NBS programs.
In our study, selective identification of genomic data, where therapeutic options are available, did not violate the Wilson–Jungner criteria10. Our work provides a basis for future research on expanding screening genes and diseases in newborn screening program. Given adequate cost-effectiveness, WGS should be considered in future newborn screening programs. Further discussion of the interpretation accuracy and ethical use of genomic information needs to take place on a global scale.
ACKNOWLEDGEMENTS
We appreciate the participation of the volunteers and their families. Without their support, this work would not have been possible. This work was also supported by the China National GeneBank (CNGB). This study was funded by the National Natural Science Foundation of China (No.31800765), the Shenzhen Municipal Government of China (JCY20170817145047361) and the Guangdong Provincial Key Laboratory of Genome Read and Write (No. 2017B030301011).
CONFLICT OF INTEREST
The authors declare no conflict of interest.




