Itaconate suppresses neonatal intestinal inflammation via metabolic reprogramming of M1 macrophage
Shuchen Huangfu, Chaoting Lan and Sitao Li are considered as joint first authors.
Abstract
Background
Necrotizing enterocolitis (NEC) is a rapidly progressive and severe gastrointestinal disorder in neonates that is marked by an inflammatory cascade initiated by mechanisms that remain incompletely understood, resulting in intestinal necrosis and systemic infections. This study demonstrated that itaconate (ITA) exerts a protective effect in NEC by regulating macrophage reprogramming.
Methods
Changes in ITA expression were investigated using immunofluorescence staining and liquid chromatography-mass spectrometry, and their effect on immune cell differentiation was verified through single-cell sequencing. In vivo experiments were performed using ACOD1−/- and ACOD1fl/flLysMcre NEC mouse models.
Results
We detected changes in ITA expression in clinical NEC samples and confirmed the effect of these changes on immune cell differentiation. In vivo experiments confirmed the therapeutic role of ITA in regulating macrophage differentiation in NEC, and we further investigated the mechanism by which ITA regulates macrophage metabolic reprogramming. The depletion of ITA in NEC correlates with an increased frequency of pro-inflammatory macrophage polarization, thereby exacerbating intestinal inflammatory injury. Importantly, our in vivo experiments revealed that treatment with 4-octyl itaconate (4OI) significantly mitigated intestinal symptoms associated with NEC in murine models. Mechanistic investigations showed that 4OI effectively suppressed M1 macrophage polarization by rescuing mitochondrial function and upregulating oxidative phosphorylation in macrophages.
Conclusions
Our results highlight ITA as a metabolic checkpoint of macrophage differentiation in NEC and suggest the therapeutic efficacy of 4OI in NEC.
Key points
-
Itaconate alleviates NEC by reprogramming M1 macrophage metabolism
-
ACOD1 deficiency exacerbates NEC severity
-
4OI maintains intestinal barrier integrity.
-
4OI rescues NEC by regulating macrophage mitochondrial activity.
1 INTRODUCTION
Necrotizing enterocolitis (NEC) is a prevalent and severe gastrointestinal emergency in neonates that effect premature newborns with low birth weight.1 Its defining feature is the rapid emergence of intestinal inflammation, leading to necrosis and systemic infection, which accounts for its high morbidity and mortality rates.2 Despite significant advances in neonatal treatment, the pathogenesis of NEC remains unclear and therapeutic options are limited, with surgery being the last resort.3 According to the data of a multicentre retrospective study in China, the incidence of NEC with a BELL stage ≥ stage II is 3.3% to 7.6% and increases as the gestational age and birth weight decreases. Despite aggressive medical and surgical interventions, the in-hospital mortality rate of NEC in children is still 9.4% to 17.6%.4 The lack of effective medical interventions highlights the urgent need to elucidate the mechanisms underlying NEC pathophysiology.
Recent data have shown that the immune system plays a central role in the development of NEC, with dysregulated inflammatory responses being critical for the initiation and progression of the disease.5 Macrophages are key players in immune response that have great plasticity and are implicated in the inflammatory cascade of NEC.6, 7 Emerging evidence suggests that metabolic reprogramming of macrophages is a key mechanism that affects their function.8, 9 This metabolic reprogramming not only affects macrophage energy production and the biosynthesis of metabolic intermediates but also affects their immune regulatory functions.10 Depending on their reliance on aerobic glycolysis and oxidative phosphorylation (OXPHOS) for energy production, macrophages can alter between a pro-inflammatory M1 phenotype, which aggravates tissue damage, and an anti-inflammatory M2 phenotype, which facilitates tissue repair and resolution of inflammation.11 The metabolic flexibility of macrophages represents a potential therapeutic target for controlling inflammation in NEC.
ITA has been widely studied as a metabolite with well-documented anti-inflammatory and immunomodulatory properties. It is produced by fumarate decarboxylase 1 (ACOD1, also known as immune-responsive gene 1, Irg1), which catalyses the decarboxylation of cis-aconitine.12 ITA is a crucial regulator of immune responses, particularly in macrophages, where it exerts significant anti-inflammatory effects.13, 14 Its mechanism of action is multifaceted. It can competitively inhibit succinate dehydrogenase (SDH), thus preventing the release of interleukin-1β (IL-1β) and reactive oxygen species (ROS) caused by the accumulation of succinate. It can also activate the Nrf2 signalling pathway, thereby inhibiting NF-κB signalling transduction. Moreover, ITA can enhance the antioxidant capacity of cells by modifying the KEAP1 protein.15-17 Furthermore, ITA significantly influences other immune cells. Tomlinson et al. showed that ITA from neutrophils protects the lung from excessive inflammation by inhibiting glycolytic and oxidative bursts of neutrophils during Staphylococcus. aureus infection.18 A previous study has shown that ITA can regulate the metabolic reprogramming of macrophages by inhibiting SDH and other means, influencing their polarization and function, thereby playing a significant role in many inflammatory diseases.19 The interrelated mechanism between metabolism and immunity makes ITA an promising target for the treatment of inflammatory disorders.
The function of ITA in regulating inflammation has been studied in various diseases. Although recent studies have shown that ITA protects the integrity of the intestinal epithelial barrier by enhancing autophagic flow and lysosomal function of intestinal epithelioid cells in NEC,20 its role in the intestinal immune microenvironment of NEC remains unclear. Recent studies have regarded metabolic reprogramming of targeted immune cells as a therapeutic strategy for alleviating inflammatory damage.21 Given the central role of macrophages in NEC and the established anti-inflammatory properties of ITA, we postulated that ITA confers protection against NEC by modulating macrophage polarization and metabolic activity. In addition, oxidative stress and redox imbalances are known to contribute to the pathogenesis of NEC; however, extent to which ITA regulates redox homeostasis and mitochondrial function in macrophages during NEC is unclear. Understanding the role of ITA in metabolism and function of macrophages will help advance our knowledge of NEC pathogenesis and lay the groundwork for novel therapeutic approaches.22
This study aimed to investigate whether ITA induces changes in the metabolic phenotype of macrophages by regulating their mitochondrial function, thereby exerting therapeutic effects against NEC.
2 RESULTS
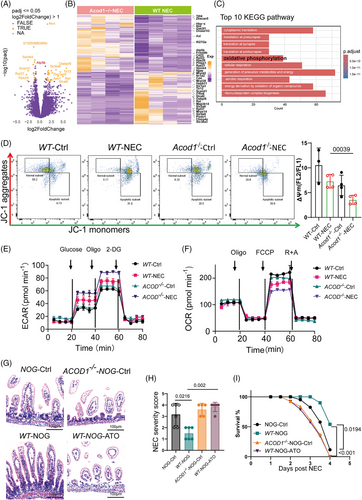
2.1 ITA deficiency occurs in the development of NEC
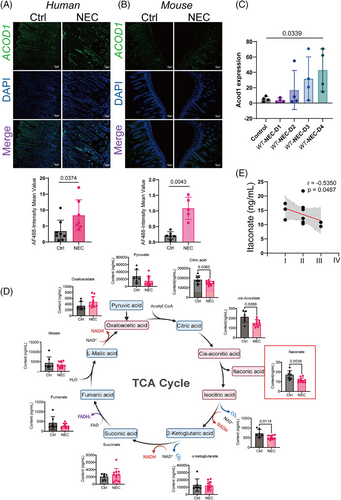
To investigate the changes in itaconate biosynthesis during NEC, we analysed ACOD1 expression in the intestines of humans and animals with and without NEC. This study included patients who were clinically and pathologically diagnosed with NEC according to the BELL staging system. ACOD1 expression levels were elevated in the intestinal tissues of patients with NEC and mice (Figure 1A,B) progressively increasing from day one to four after NEC induction (Figure 1C), which indicates its upregulation in response to inflammation. Metabolite analysis revealed a significant decrease in ITA levels in the peripheral blood of patients with NEC relative to healthy controls with citrate, cis-aconitate, and isocitrate levels also being affected (Figure 1D). A strong negative association was observed between ITA levels and NEC severity as classified the Bell classification (Figure 1E). Although the expression of genes that regulate ITA synthesis increased, the level of ITA and the substrates for its synthesis decreased with disease progression, suggesting that ITA plays a role in attenuating NEC-associated inflammation.
2.2 ACOD1 deficiency aggravates inflammatory injury in NEC
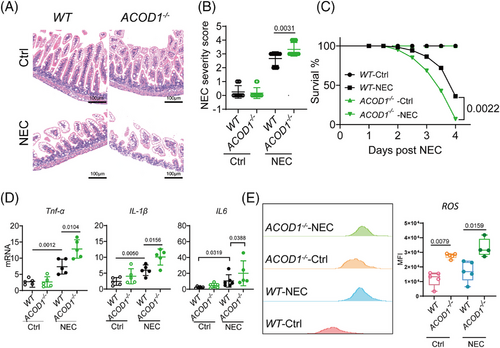
We examined the protective function of ITA in NEC using ACOD1 knockout (ACOD1−/−) mice randomly assigned to a control or NEC model group. Haematoxylin and eosin (HE) staining of intestinal epithelial tissue from ACOD1−/- mice revealed significantly greater epithelial damage during NEC progression (Figure 2A). Histological analysis indicated markedly increased tissue damage scores in necrotic lesions of ACOD1−/− mice compared with controls (Figure 2B). Survival analysis further demonstrated a significantly lower survival rate in ACOD1−/− mice compared with wild-type mice following NEC induction, consistent with the increased disease severity scores (Figure 2C). Additionally, ACOD1−/− mice exhibited heightened inflammatory responses compared with wide-type mice, having significantly elevated levels of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α (Figure 2D). Flow cytometry analysis revealed significantly elevated ROS levels in ACOD1−/− mice under both physiological and experimental conditions (Figure 2E). Our findings suggest that the lack of ITA leads to higher ROS levels and exacerbates NEC-induced inflammation.
2.3 ACOD1 deficiency promotes the proinflammatory polarization of macrophages during NEC
We evaluated the effect of ACOD1 deficiency on different immune cell populations during NEC using intestinal tissues from both WT and ACOD1−/− mice with and without NEC. The UMAP dimensionality reduction analysis was performed using multiparameter flow cytometry data from intestinal immune cells. The gating strategy used in the analysis is shown in Figure S1A. UMAP dimensionality reduction analysis was performed based on the results of multicolour flow cytometry analysis of specific immune-cell population marker features (Figure S1B). The results revealed that the presence or absence of ACOD1 significantly influenced macrophage infiltration in both control and NEC groups (Figure 3A). Statistical analysis revealed that ACOD1 loss significantly enhanced macrophage infiltration in intestinal tissues compared with that in the WT-NEC group (Figure S1C). The absence of ACOD1 in the intestinal tissue led to a marked reduction in the population of myeloid-derived suppressor cells (MDSCs) (Figure S1B), which maintain intestinal immune tolerance. These results indicate that ACOD1 deficiency induces an amplified inflammatory response in NEC mice.
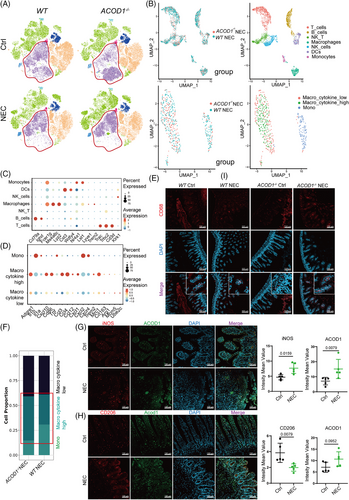
We investigated the effect of ITA on immune cell infiltration in NEC mice by performing single-cell omics analysis of intestinal tissues from WT and ACOD1−/- NEC mice. We identified multiple immune cell categories (Figure S2A,B). We performed single-cell sequencing to identify markers following labelling with a specific probe. Genes exhibiting elevated expression levels in macrophages included Fcer1g, Ms4a6c, and Lyz2 (Figure 3B,C, Figure S2C). Two macrophage subpopulations distinguished by their cytokine expression levels were identified: one with high expression, typically associated with M1 macrophage activation, and the other with low expression linked to immunosuppressive M2 macrophages (Figure 3B,D). Immunofluorescence analysis showed increased expression of CD68, a macrophage marker, in the intestinal epithelium of ACOD1−/− NEC mice compared with WT mice (Figure 3E, Figure S2E). According to the results of single-cell analysis, the proportion of macrophages exhibiting elevated cytokine expression was markedly higher in ACOD1−/− NEC mice than in WT mice (Figure 3F). Flow cytometry further confirmed a significant increase in the number of pro-inflammatory M1 macrophages in ACOD1−/− NEC mice (Figure S2D). Immunofluorescence analysis of small intestine revealed an increase in iNOS and Acod1 (Figure 3G) and decrease in the CD206 level in the NEC group (Figure 3H). The results of the in vivo macrophage differentiation induction experiments showed that 4-octyl itaconate (4OI) inhibited the M1 phenotype differentiation of THP-1 cells (Figure S2F).
2.4 ACOD1 in macrophages protects mice from NEC
Immunofluorescence results further showed the co-localization of ACOD1 and CD68, suggesting a close association between ACOD1 expression and macrophages. (Figure 4A). Therefore, we further investigated the role of macrophage-derived ITA in limiting NEC-induced inflammatory damage. We transplanted macrophages isolated from WT and ACOD1−/− mice into NOD/SCID/IL2Rγnull mice (NOG), followed by NEC induction (Figure 4B). H&E staining revealed that NOG mice that received ACOD1−/− macrophages exhibited intestinal epithelial damage comparable to that in untreated NOG mice (Figure 4C). In contrast, NOG mice that received WT macrophages exhibited a marked decrease in epithelial damage and lower mortality rates (Figure 4D,E).
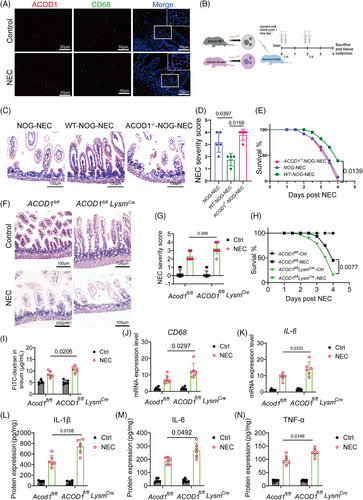
We established conditional ACOD1-knockout mice (ACOD1fl/flLysMcre) using myeloid-specific lysosomal-M (LysM)-Cre mice to investigate the role of ACOD1 in macrophages during NEC development. NEC were induced in ACOD1fl/flLysMcre mice and their ACOD11fl/fl (control) littermates. The results showed that ACOD11fl/flLysMcre mice had more severe intestinal damage, higher pathological scores (Figure 4F,G), and a significantly lower survival rate during NEC than ACOD1fl/fl mice (Figure 4H, p = 0.0077). FITC-dextran tests revealed increased intestinal permeability in ACOD1fl/fl LysMcre NEC mice, indicating that insufficient ITA synthesis by macrophages exacerbated epithelial barrier damage in NEC (Figure 4I). The ACOD1fl/flLysMcre-NEC group exhibited significantly elevated expression levels of CD68 and IL-6 increased macrophage migration and infiltration (Figure 4J,K). The protein expression of inflammation-related genes was considerably elevated in the ACOD1fl/flLysMcre-NEC group compared with that in the controls (Figure 4L–N). Collectively, our finding indicated that macrophage-derived ACOD1 inhibits excessive inflammatory responses macrophage chemotaxis and NEC infiltration.
2.5 ACOD1 deficiency accelerates the switch of macrophages towards glycolysis in NEC
We performed Smart-RNA-sequencing of macrophages from NEC WT and ACOD1−/− mice to create a differential gene expression matrix in order to examine how ACOD1 deficiency enhances proinflammatory polarization in NEC (Figure 5A,B). We identified a significant enrichment of differentially expressed genes in the OXPHOS pathway, as revealed by gene ontology (GO) enrichment (Figure S3A,B and E). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis further revealed that these genes in WT and ACOD1−/− macrophages are primarily involved in mitochondrial functions, including OXPHOS, generation of precursor metabolism and energy and energy derivation by organic compounds (Figure 5C, Figure S3A,B and E). These findings indicate that the differences in macrophage differentiation resulting from ACOD1 deficiency may be mediated by mitochondrial function, especially the modulation of cellular OXPHOS. As master regulators of metabolism, mitochondria play crucial roles in the function of macrophages. Mitochondrial membrane potential (MMP) depolarization is a crucial step in mitochondrial malfunction. Staining of JC-1 which exists in its monomeric form and emits green fluorescence when MMP drops, was performed to assess the effect of ITA on MMP in macrophages. As shown in Figure 5D, the MMP of macrophages in ACOD1−/− NEC group mice decreased compared with that of WT NEC group mice (Figure 5D). To assess the effect of ACOD1 deletion on macrophage metabolism in NEC, we performed mitochondrial stress assays. ACOD1 knockout increased glycolytic activity in NEC macrophages (Figure 5E), while reducing mitochondrial respiration and oxygen consumption (Figure 5F). Quantitative analysis confirmed that ACOD1−/− macrophages exhibited a higher glycolytic capacity than wild-type controls (62.4 ± 0.5 vs 67.6 ± 1.3 mpH/min, p < 0.05). ACOD1−/− macrophages had a greater increase in glycolytic capacity in the NEC group (67.6 ± 1.3 vs. 88.8 ± 0.9 mpH/min, p < 0.05) (Table S2). To verify whether the effect of ITA depletion in promoting intestinal inflammatory injury in NEC occurred through mitochondrial function, we injected mitochondrial inhibitor atovaquone (ATO) into the peritoneal cavity of NOG mice after macrophage transplantation to block mitochondrial function. Intestinal pathological damage during NEC was significantly reduced in NOG mice that received WT macrophage transplants (Figure 5G). In contrast, ATO-treated mice showed no significant reduction in intestinal damage, with disease pathology scores being markedly higher than those of the WT-NOG group (Figure 5H). Furthermore, survival rates in the ATO intervention group were markedly worse to those in the WT-NOG cohort (Figure 5I). These results suggest that ACOD1 deficiency promotes NEC by enhancing mitochondrial function in macrophages.
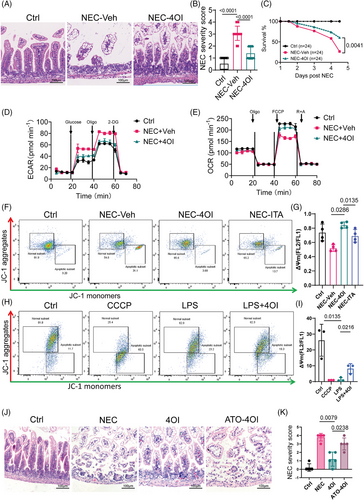
2.6 4OI plays a therapeutic role in NEC by promoting mitochondrial activity
We evaluated ITA's role in NEC by intraperitoneally administering 4OI, a membrane-permeable ITA mimic, for 4d alongside NEC induction. H&E staining of the intestinal epithelial tissue showed that 4OI supplementation effectively reduced typical intestinal damage (Figure 6A) and significantly lowered the pathological damage score during NEC (Figure 6B). Additionally, 4OI treatment significantly improved the survival rate of NEC mice (Figure 6C). Furthermore, 4OI treatment reduced macrophage infiltration and the expression of IL-6 and CD68 (Figure S4D,E). Subsequently, we aimed to confirm the possible control of macrophage metabolism by 4OI in NEC. Consistent with the previous findings, the reduced extracellular acidification rate (ECAR) indicated that 4OI supplementation reduced the reliance of macrophages on glycolysis (Figure 6D). Furthermore, compared with vehicle-treated NEC animals, 4OI-treated mice showed a substantial improvement in mitochondrial respiratory capacity of macrophages (Figure 6E). Quantitative analysis confirmed that glycolytic capacity was suppressed in NEC mice treated with 4OI compared with the controls (64.8 ± 0.4 vs. 80.5 ± 0.2 mpH/min, p < 0.001) (Table S3). As shown in Figure 6F, the MMP of macrophages from the 4OI treated NEC mice was higher than that of the positive control group (NEC-vehicle group). Furthermore, the specific marker CD64 in M1 macrophages in the intestine tissues of the 4OI treatment cohort mice was significantly downregulated (Figure S4G). However, no significant change in free ROS (Figure S4F). We conducted in vitro using THP-1 cells treated with CCCP as a positive control group. The results showed that LPS also induced a decline in mitochondrial function in cells (Figure 6H). Meanwhile, 4OI treatment effectively inhibited the LPS-induced reduction in MMP (Figure 6H). The increase in superoxide level in the mitochondria is an important indicator of mitochondrial dysfunction. The fluorescence of MitoSOX Red, a fluorescent probe for imaging superoxide radicals in the mitochondria of living cells, weakened upon 4OI treatment in THP1 cells (Figure S4I). To further validate the therapeutic role of ITA in NEC by promoting mitochondrial activity, we used macrophages from ACOD1−/− mice after ATO intervention and transplanted them into NOG mice, which were intraperitoneally injected with 4OI. The experimental results showed that in the NOG mouse model of ACOD1−/− mice, 4OI treatment effectively alleviated NEC-induced intestinal mucosal injury. However, 4OI failed to significantly protect against NEC-induced intestinal mucosal injury in a mouse model where ATO inhibited mitochondrial activity. (Figure 6J,K). Since 4OI is not a natural product, to evaluate its in vivo toxicity, we administered it via intraperitoneal injection at a dose of 40 mg/kg/d for 3 consecutive days. On the 4th day, the mice were euthanized, and the liver and kidneys were harvested. H&E staining revealed no apparent liver or kidney lesions. (Figure S5). In addition, we administered ITA to NEC mice via intraperitoneal injection and H&E staining indicated that pathological damage to the intestinal epithelial tissue in NEC mice was significantly mitigated after ITA intervention, accompanied by a reduction in the pathological severity score (Figure S4A,B). Furthermore, in contrast to that of the solvent injected group, the survival curve of the ITA-treated group showed an upward trend (Figure S4C). In conclusion, 4OI supplementation improves NEC by reversing the macrophage redox balance.
3 DISCUSSION
ACOD1 is the key enzyme responsible for synthesizing ITA and plays broader roles in immune regulation, such as the modulation of mitochondrial function, redox homeostasis, and inflammasome activity. Current evidence strongly indicates that the immune regulatory function of ACOD1 is primarily driven by ITA synthesis. Our results suggest that ITA is depleted during NEC progression due to a decrease in its synthetic precursor. This finding aligns with those of previous studies indicating that Acod1 expression is elevated under inflammatory conditions.23 Because ACOD1 expression is the sole regulator of ITA synthesis, we used an ACOD1 knockout mouse model to mimic the in vivo environment of ITA depletion. Using ACOD1-deficient mice, we showed that the lack of ACOD1 exacerbates intestinal damage and inflammation in NEC, characterized by increased pro-inflammatory cytokine production and damage to the intestinal lamina propria.
An imbalance between pro-inflammatory and anti-inflammatory signals is a significant contributor to the cascade of inflammation during intestinal inflammatory damage. Elevated levels of pro-inflammatory mediators, including TLR4, NF-κB, TNF, PAF, IL-18, interferon-γ, IL-6, IL-8 and IL-1β, lead to sustained intestinal damage. Simultaneously, defects in anti-inflammatory regulatory mechanisms, such as those involving IL-1Ra, TLR9, PAF lyase, TGF-β1 and 2, IL-10, and regulatory T cells reinforce the pro-inflammatory environment.24 This imbalance triggers an amplified immune response, which not only disrupts local intestinal homeostasis but also exacerbates the condition by inducing systemic metabolic alterations.25
In NEC, the inflammatory cascade resulting from a reduction in immunosuppressive cells and an increase in pro-inflammatory signals is a significant contributor to intestinal barrier impairment. Our previous research demonstrated that both the quantity and functionality of MDSCs within the immunosuppressive cell population were markedly diminished in preterm infants with NEC compared with their term counterparts.26 As shown in this research, ITA deficiency amplified the inflammatory cascade in NEC models. But where do these increased levels of inflammatory cytokines come from?
The results of this study indicate that ITA deletion not only promotes the transformation of macrophages to the pro-inflammatory M1 phenotype but also leads to a significant shift in the metabolic phenotype of macrophages towards glycolysis. Macrophages exhibit metabolic plasticity.27 Among them, pro-inflammatory M1 macrophages mainly rely on glycolysis to provide energy, and their metabolic characteristics are characterized by TCA cycle disruption and mitochondrial dysfunction.10 M1 macrophages express low levels of mitochondrial genes (e.g., PPARγ), high levels of glycolytic enzymes (e.g., HK2 and LDHA), and pro-inflammatory factors (e.g., HIF-1α and NF-κB). Their metabolism and immune functions are regulated by TCA cycle enzymes.11 Previous studies have shown that ITA can effectively inhibit LPS-induced elevation of ROS levels and ECAR by activating the anti-inflammatory program through Nrf2 activation and SDH inhibition.28 Upon inflammatory stimuli, ITA modifies the Cys 22 residue of GAPDH with a similar dipropylene glycol moiety, inhibiting glycolysis.29 In the present study, gene expression analyses revealed downregulation of genes involved in OXPHOS and mitochondrial function in ACOD1-deficient macrophages. In contrast, M2 macrophages depend on the TCA cycle and OXPHOS. Thus, macrophage differentiation is thus regulated by metabolic reprogramming balance.11 Our results indicate that 4OI, a derivative of ITA, influences the metabolic phenotype of macrophages and effectively alleviates intestinal inflammatory damage in NEC. This suggests that 4OI can be used to treat NEC by regulating the metabolism of macrophages, and that targeting the ACOD1-ITA axis offers a novel therapeutic strategy for NEC.
Macrophages are central players in the innate immune response and are highly plastic cells that play dual roles in regulation of immune responses and immune tolerance in NEC.6, 26 Skewing towards the M1 phenotype contributes to the maintenance of inflammation and tissue damage in NEC.
Metabolic reprogramming is a critical aspect of macrophage function and polarization.30 OXPHOS is the main biochemical pathway through which cells generate adenosine triphosphate (ATP) via located on the inner mitochondrial membrane and the electrochemical gradient. Damage to the mitochondrial electron transport chain leads to increased electron leakage, resulting in excessive ROS production and decrease in MMP levels.31 This damage reduces the efficiency of OXPHOS and forces cells to switch to the glycolytic pathway to rapidly generate ATP. Macrophage differentiation is metabolism dependent, and M1 macrophages rely on glycolysis for energy production.27
Our research findings indicate that ITA-deficient mice, the MMP levels of macrophages exhibited a significant decrease, accompanied by enhanced glycolysis. Mitochondrial dysfunction induced by the absence of ITA promotes the transformation of macrophages to a pro-inflammatory phenotype and further aggravates the inflammatory cascade reaction in NEC. Additionally, the increased ROS production observed in these macrophages may result from mitochondrial dysfunction further exacerbating tissue damage.32
Prior studies have shown that ITA inhibits mitochondrial respiratory chain complex II activity and diminishes mitochondrial respiratory capacity by suppressing SDH.33 However, our study revealed that 4OI intervention significantly recovered the mitochondrial function of macrophages. This phenomenon may be explained by following reasons. First, low-dose ITA may enhance the cell's antioxidant capacity by activating the Nrf2-KEAP1 pathway, thereby alleviating ROS-induced mitochondrial damage.28 Second, it may activate the transcription factor TFEB to clear damaged mitochondria and enhance the functionality of the remaining mitochondria.20
The therapeutic potential of 4OI in NEC was a significant finding in the present study. Treatment with 4OI reduced intestinal damage, decreased pro-inflammatory cytokine levels, and improved survival rates in NEC mice. 4OI is a cell-permeable derivative of ITA that mimics anti-inflammatory effects.16 Previous studies have indirectly reported the distribution of 4OI in multiple organs in mice following intraperitoneal injection using pharmacodynamic experiments.34-38 Using proteomics, Liao et al. confirmed that 4OI targeted GAPDH protein, which is widely present in all organs after intraperitoneal injection, suggesting its multiorgan bioavailability.39 Our data suggest that 4OI treatment restores mitochondrial function and redox balance in macrophages, as evidenced by increased mitochondrial respiration and decreased glycolytic reliance. Furthermore, 4OI treatment reduced ROS production, which is crucial in preventing oxidative stress-induced cellular damage. Excessive ROS levels not only disrupt the electron transport chain but may also damage the mitochondrial DNA, resulting in a compromised cellular energy supply.40, 41
ATO is a mitochondrial function inhibitor. It blocks electron transfer by inhibiting complex III of the mitochondrial electron transport chain thereby inhibiting ATP synthesis. It is an important tool for studying mitochondrial dysfunction and energy metabolism disorders. After intervention with ATO, macrophages were unable to perform their functions in NEC mice, an outcome consistent with that of macrophages lacking ACOD1. The importance of mitochondrial function in macrophage-mediated inflammation is highlighted by our findings using the mitochondrial inhibitor ATO.42 Inhibition of mitochondrial activity abrogated the protective effects of macrophage transplantation and 4OI treatment in NEC models. This suggests that the beneficial effects of 4OI are partly mediated through the preservation of mitochondrial function and promotion of OXPHOS in macrophages. Mitochondrial integrity is essential for regulating macrophage polarization and controlling inflammatory responses. Therefore, interventions that support mitochondrial function may have therapeutic benefits in inflammatory diseases such as NEC.
Despite the findings, our study has several limitations that should be considered. First, although we demonstrated the protective role of 4-OI in mouse models of NEC, the translation of these findings to human patients should be further investigated. Although 4-OI mimics the anti-inflammatory effects of ITA, Swain et al. highlighted functional differences,16 therefore, future clinical studies should prioritize the use of unmodified ITA and optimize its poor membrane permeability. Second, the exact mechanisms by which ITA modulates mitochondrial function and macrophage metabolism remain unclear. Further research is necessary to elucidate the signalling pathways involved and to identify potential targets for therapeutic intervention. Lastly, NEC is a complex disease impacted by both hereditary and environmental factors. While our study focused on the role of macrophages and ITA, other cell types and pathways may also contribute to disease progression and should be explored in future studies.
4 CONCLUSION
Our findings underscore the critical role of Acod1-mediated ITA production in regulation of macrophage metabolism and function in NEC. ITA deficiency leads to enhanced pro-inflammatory macrophage polarization, increased glycolysis, mitochondrial dysfunction, and increased ROS production, all of which contribute to intestinal inflammation and damage. Supplementation with the ITA derivative 4OI mitigated these effects via rescuing macrophage mitochondrial respiratory function, highlighting its therapeutic potential. For clinical translation, unmodified ITA should be considered as the preferred candidate owing to its well-documented immuno-regulatory properties. Nonetheless, 4OI can serve as a valuable compound during the proof-of-concept phase, providing essential support for preliminary investigations.
5 MATERIALS AND METHODS
5.1 Human participants
The included participants were neonates diagnosed via imaging examinations and met the clinical diagnostic criteria for NEC. Specifically, they included neonates with abdominal radiographs showing intestinal wall gas accumulation or portal vein gas accumulation or those diagnosed by surgery.
The exclusion criteria were as follows: severe congenital diseases such as complex congenital heart disease or chromosomal abnormalities; other intestinal diseases such as congenital intestinal atresia or intestinal malrotation; receipt of immunomodulatory treatment immediately after birth; or sepsis or other severe systemic infections for other reasons. Clinical plasma samples were obtained from the Department of Hematology at Guangzhou Women and Children's Medical Center, and these included peripheral blood samples from 14 patients diagnosed with NEC and a control group consisting of 7 premature infants and neonatal dyspnoea patients (NC). Tissue samples were collected from the Guangdong Women and Children's Medical Center. All participants provided written informed consent before sample collection (K-2022-030-01). All NEC patients were diagnosed according to the standard diagnostic criteria for neonatal acute enterocolitis. According to the widely used BELL scale in clinical practice, the included cases were graded based on clinical manifestations, imaging examinations, and laboratory tests.43 The detailed clinical information is provided in Table S1.
5.2 Animals
C57BL/6 mice were obtained from Zhuhai BesTest Biotechnology Co., Ltd. (China), while ACOD1−/− mice were provided by the Yun Zhao's team at the Department of General Surgery, BenQ Medical Center, Affiliated BenQ Hospital of Nanjing Medical University. Myeloid-specific ACOD1-knockout (ACOD11fl/flLysMcre) mice were generated by crossing ACOD1fl/fl mice (S-CKO-03141) with LysM-Cre mice (004781) purchased from Cyagen Biosciences and Jackson Laboratory (ME, USA), respectively. Floxed littermates (ACOD1fl/fl) were used as controls for ACOD1fl/flLysMcre mice. All animal experiments were approved by the Institutional Animal Care and Use Committee (the approval number: 202312005).
5.3 Construction of a neonatal NEC mouse model
Seven-day-old C57BL/6 pups were divided into two groups: control group (Ctrl) and experimental (NEC). The NC group pups were naturally nurtured by their mothers, whereas the NEC pups were gavaged daily with 30 mg/kg of lipopolysaccharide (LPS, Sigma-Aldrich) using a clean peripherally inserted central catheter. They were then fed a formula consisting of Similac Advance infant formula (Abbott Nutrition) and Esbilac puppy milk replacer (PetAg) in a 2:1 ratio.44 Subsequently, the pups were exposed to 5% oxygen and 95% nitrogen for 10 min to induce asphyxia, followed by cold stress at 4°C for 10 min. This procedure was conducted twice daily for 3 consecutive days. For exogenous ITA administration experiments, mice were intraperitoneally injected with 40 mg/kg 4OI, Selleckchem, Cat No. S5929) or ITA (Cat No. SS3095; Selleckchem) from the day before NEC modelling until the third day of modelling.
5.4 Cell culture
THP-1 cells were obtained from the Cell Bank of Chinese Academy of Sciences. They were cultured in Roswell Park Memorial Institute 1640 Medium (Gibco/Thermo Fisher Scientific) with 10% fetal bovine serum (Gibco) at 37°C in a humidified incubator with 5% CO2.
5.5 Cell transplantation
Macrophages were isolated from the spleens of wild-type C57BL/6 or Acod1−/− mice, sorted, and intraperitoneally injected into NOG (NOD/Shi-scid/IL-2Rγnull) immunodeficient mice (106 cells per mouse) on days 1 and 3 of NEC induction. The mice were sacrificed 4d post-induction and samples were collected for subsequent analyses.45
5.6 Targeted metabolomics
Within 15 min of collecting peripheral blood in ethylenediaminetetraacetic acid anticoagulating tubes, the plasma was separated via centrifugation (1500 × g, 4°C, 10 min) for TCA cycle metabolites detection. Targeted metabolomic analysis was performed on a Thermo Scientific™ TSQ Altis™ triple quadrupole mass spectrometer coupled with a Thermo Scientific Vanquish™ Flex ultra-high-performance liquid chromatography (UHPLC) system to quantify 13 organic acids associated with the TCA cycle. Standards for the 13 organic acids were obtained from Shanghai Zhenzhun Biotechnology Co., Ltd. Methanol, acetonitrile, and formic acid (all LC-MS grade; Thermo Fisher Scientific), and ultrapure water (Mill-Q; Millipore), were used as reagents.
5.7 Flow cytometry
For intracellular cytokine staining, the cell suspension was stimulated using a Cell Stimulation Kit at 37°C in a 5% CO₂ for 4 h. To minimize nonspecific binding, cells were pre-treated with Fc Block CD16/CD32 antibodies (Cat No. 14-0161-81, Invitrogen). Specific surface molecule antibodies were carefully chosen and used to stain the cells at a 1:100 dilution for 30 min at 4°C. After staining for surface markers, the cells were fixed and permeabilized using a Foxp3 Cytofix/Cytoperm kit (Cat No. 554714, Tonbo Biosciences). Finally, intracellular and nuclear staining was performed at 4°C with the appropriate antibodies, following the manufacturer's instructions. Details of the antibodies used are provided in the key resource table (Supplementary Materials). Additionally, cells were incubated with Mito-Green (Cat No. C1048, Beyotime) at 37°C for 30 min to evaluate mitochondrial function. All flow cytometry data were collected on a BD LSRFortessa-X20 instrument (BD Biosciences) and subsequently analysed using FlowJo software (version 10; FlowJo LLC).
5.8 Fluorescence-activated cell sorting
After preparing the cell suspensions from the tissues, cells were pre-treated with Fc Block CD16/CD32 antibodies. The cells were then stained with specific surface molecule antibodies, carefully selected for their targets, at a 1:100 dilution for 30 min at 4°C. After staining, the cells were resuspended in flow cytometry tubes and analysed using a BD FACSAria™ III flow cytometer (BD Biosciences). Specific information regarding the antibodies used for flow cytometry is provided in key resources table (Supplementary Materials).
5.9 Single cell RNA sequencing
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive of the BIG Data Center, Chinese Academy of Sciences under the accession code CRA024721 and are publicly accessible at http://bigd.big.ac.cn/gsa. Single-cell RNA sequencing (scRNA-seq) data were obtained from resected intestinal tissues of ACOD1−/− and WT NEC mice. First, individual cells were encapsulated in gel beads containing barcodes and primers using 10x™ GemCode™ Technology to form Gel Bead-In-Emulsions (GEMs). mRNA from each cell is reverse transcribed into barcoded cDNA within the GEMs, followed by amplification and purification. Next, sequencing libraries were constructed and sequenced on an Illumina platform (Illumina) to generate sequence data using cell-specific barcodes. Finally, the data were processed using the Cell Ranger pipeline (v4.0.0) to align the reads and generate gene-cell count matrices. Cells with fewer than 200 expressed genes or genes detected in fewer than three cells were removed. After merging all samples in Seurat (v4.3.0, R v4.3.1), low-quality cells were filtered out based on the following criteria: < 1900 UMIs, < 1000 genes per cell, > 30% mitochondrial content, or > 10% erythrocyte marker expression. The Seurat object was normalized and scaled using the LogNormalize and ScaleData functions, respectively. Highly variable genes (n = 2,000) were identified using FindVariableGenes, followed by principal component analysis (PCA). Clustering was performed using the top 20 principal components via the graph-based FindClusters function with a resolution of 0.3. Uniform Manifold Approximation and Projection (UMAP) was used for dimensionality reduction with the RunUMAP function.
Cluster-specific differentially expressed genes were identified using the Wilcoxon rank-sum test via FindAllMarkers (parameters: min.pct = 0.25, only.pos = TRUE, logfc.threshold = 0.25, and p.adjust.method = “BH”). Clusters were annotated based on the expression of canonical marker genes.
5.10 Statistical analysis
All data are presented as the mean ± standard error of the mean or as individual data points. Statistical analysis between two groups was conducted using an unpaired t-test or Wilcoxon rank-sum test based on whether the data were normally distributed. Correlations were analysed using Pearson's correlation analysis. Survival time was analysed using a simple survival analysis (Kaplan–Meier) as implemented in GraphPad Prism (version 9).
More details please refer to Supplementary Materials and methods in Supplementary materials.
AUTHOR CONTRIBUTIONS
Shuchen Huangfu, Chaoting Lan and Sitao Li wrote the main manuscript text and drafted the work. Shuchen Huangfu and Huijuan Wang contributions to the in vivo experiment and the analysis of data. Chun Yan, Yuling Yang and Yijia Wang contributions to the interpretation of data; Bowen Tian, Yide Mu, Peizhi Zhao and Yan Tian contributions to the acquisition of data; Wei Zhong and Sitao Li revised it critically for important intellectual content; and Limei Zhong, Yongyan Shi and Yufeng Liu contributions to the conception or design of the work and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The authors would like to thank the team of Yun Zhao at the Department of General Surgery, BenQ Medical Center, The Affiliated BenQ Hospital of Nanjing Medical University for providing the experimental animals.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
For Yufeng Liu, National Natural Science Funds (grant no. 82171695); Science and Technology Program of Guangzhou (SL2024A03J01319; SL2024A04J00240). Chaoting Lan is supported by the grant National Natural Science Foundation of China (grant no. 82301955), the Research Foundation of Guangzhou Women and Children's Medical Center for Clinical Doctor (grant no. 2023BS015), the China Postdoctor Science Foundation (grant no. 2023M730791), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2024A1515013190) and the Science and Technology Project of Guangzhou (grant no. 2024A03J1238). Wei Zhong is supported by the grant National Natural Science Foundation of China (grant no. 82370526) and the Science and Technology Project of Guangzhou (grant no. 2024A03J1171). Yan Tian is supported by the Science and Technology Research Project of Education Department of Jiangxi Province (grant no. GJJ2203559) and the Jiangxi Provincial Children's Hospital 2024 First Batch “Qingmiao” Scientific Research Projects (grant no. 2024JXEYQM02). Yongyan Shi is supported by National Natural Science Foundation of China (grant no. 82171709) and the 345 Talent Project of Shengjing Hospital (grant no. M1392).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval of this study was granted by the Department of Hematology at Guangzhou Women and Children's Medical Center (approval number: 307B01) The study was conducted in accordance with the Declaration of Helsinki principles.
CONSENT FOR PUBLICATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




