Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities
Abstract
Background
Serotonin (5-hydroxytryptamine) is a multifunctional bioamine serving as a neurotransmitter, peripheral hormone and mitogen in the vertebrate system. It has pleiotropic activities in central nervous system and gastrointestinal function via an orchestrated action of serotonergic elements, particularly serotonin receptor-mediated signalling cascades. The mitogenic properties of serotonin have garnered recognition for years and have been exploited for repurposing serotonergic-targeted drugs in cancer therapy. However, emerging conflicting findings necessitate a more comprehensive elucidation of serotonin's role in cancer pathogenesis.
Main body and conclusion
Here, we provide an overview of the biosynthesis, metabolism and action modes of serotonin. We summarise our current knowledge regarding the effects of the peripheral serotonergic system on tumourigenesis, with a specific emphasis on its immunomodulatory activities in human cancers. We also discuss the dual roles of serotonin in tumour pathogenesis and elucidate the potential of serotonergic drugs, some of which display favourable safety profiles and impressive efficacy in clinical trials, as a promising avenue in cancer treatment.
Key points
-
Primary synthesis and metabolic routes of peripheral 5-hydroxytryptamine in the gastrointestinal tract.
-
Advanced research has established a strong association between the serotonergic components and carcinogenic mechanisms.
-
The interplay between serotonergic signalling and the immune system within the tumour microenvironment orchestrates antitumour immune responses.
-
Serotonergic-targeted drugs offer valuable clinical options for cancer therapy.
1 INTRODUCTION
Serotonin, also known as 5-hydroxytryptamine (5-HT), was initially named enteramine following its discovery in 1937 by Italian pharmacologist Vittorio Erspamer, who extracted it from enterochromaffin (EC) cells in the gastrointestinal (GI) tract.1 About a decade later (1948), Maurice Rapport and Irvine Page isolated a substance from bovine serum and called it serotonin, derived from the Latin term ‘serum’ (where it was discovered) and the Greek word ‘tonic’ (referring to its earliest known function).2-4 Enteramine was found to induce smooth muscle contraction and was considered as an exclusive signalling molecule in the GI tract until 1952, when Dr. Erspamer confirmed that enteramine and serotonin shared the same structure.5
The discovery of serotonin's noted function as a neurotransmitter can be traced back to 1953 in hard-shell clams, which led to the subsequent identification of serotonergic system in the vertebrate brain.6-8 Its most noteworthy clinical role in the central nervous system (CNS) revolves around its function in psychiatric disorders such as depression, schizophrenia and anxiety. Thus, numerous pharmaceutical drugs were developed to target the serotonergic system, including antidepressants and antipsychotics.9 While the name serotonin stuck, the historical importance of enteramine has been recognised as the multifaceted GI roles of 5-HT continue to be revealed. It acts as a peripheral hormone that plays a multitude of functions such as GI motility and emesis, vasoconstriction,4 angiogenesis,10 osteoporosis,11 wound healing and maintaining the glucose homeostasis and obesity.12 It also functions as a mitogen, modulating the cell cycle, inflammation and immunity. More recently, multiple studies have envisioned serotonin's carcinogenic properties, which sparked further investigation into its potential as a biomarker for carcinogenesis, the feasibility of serotonin pathways as potential therapeutic targets, and the repurposing of serotonergic-targeted drugs (such as 5-HT receptors [5-HTRs] antagonists, serotonin reuptake inhibitors, serotonin synthesis inhibitors and monoamine oxidase inhibitors [MAOIs]) for cancer therapy. However, the functions of serotonin in cancer pathogenesis remain scanty and contradictory, largely attributed to the diversity and tissue-specific distribution of 5-HTRs and the complexity of serotonergic signalling. Here, we provide a holistic view of the serotonergic system and the signalling events downstream of serotonin. We then systematically discuss the original and intriguing signalling mechanisms of peripheral serotonin on tumourigenesis and summarise the latest advances in serotonergic-targeted cancer therapies.
2 BIOSYNTHESIS AND METABOLISM OF 5-HT
2.1 Biosynthesis of 5-HT
5-HT is synthesised in the body through two steps involving the conversion of the dietary amino acid L-tryptophan. The initial step is the rate-limiting step and is catalysed by tryptophan hydroxylase (TPH). The second step involves decarboxylation of 5-hydroxytryptophan through the enzymatic action of aromatic amino acid decarboxylase, as depicted in Figure 1. TPH exhibits two different isoforms: TPH2 is restricted to the CNS and enteric neurons, whereas TPH1 is widely distributed in the periphery and pineal gland, indicating the presence of two separate serotonin reservoirs in the body.13, 14 5-HT is produced and stored in presynaptic neurons within the CNS, where it primarily acts as a neurotransmitter. However, roughly 95% of the body's 5-HT is believed to be synthesised by EC cells in the intestinal mucosa.15, 16 Interestingly, the production of 5-HT in EC cells could be modulated by intestinal microbiota, which in turn, could be altered by 5-HT.17, 18 Tumour-associated microbiota also promotes 5-HT production by augmenting Tph2 expression.19 The intricate interplay between the microbiome and the serotonergic system is considered to regulate systemic serotonin homeostasis.
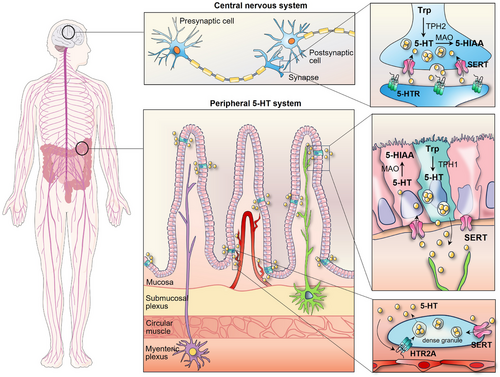
2.2 Storage and metabolism of 5-HT
The majority of 5-HT synthesised peripherally by EC cells is absorbed by platelets after being released into blood plasma via a serotonin reuptake transporter (SERT/SLC6A4), with less than 1% of serotonin circulating in the blood in an unbound state.20, 21 Platelets, as the main circulating reservoir of serotonin, have little ability to produce serotonin.22, 23 Generally, the serum serotonin level is typically maintained within the range of 1−5 ng/mL, but it can increase up to 1000-fold when released from platelet dense granules at sites of inflammation or injury.24, 25 Intracellular serotonin sequestration facilitated by the vesicular monoamine transporter shields it from monoamine oxidase (MAO)-mediated enzymatic degradation.26 MAO metabolises 5-HT into 5-hydroxyindoleacetic acid, that is, primarily eliminated through urine. The GI tract, brain and platelets are major sites where MAO activity exists. In addition, serotonin undergoes minor metabolic pathways such as glucuronidation and sulphation, which takes place in the liver, lung, kidney and brain.27 Within the CNS, MAO-mediated metabolism occurs in the cytosol of the neuron, whereas in the pineal gland, serotonin is converted into melatonin through an alternative pathway. Due to its inability to penetrate the blood‒brain barrier, the central and peripheral serotonin systems are anatomically and functionally separate.28
3 FUNCTIONAL MODES OF SEROTONIN
3.1 Serotonin receptor families
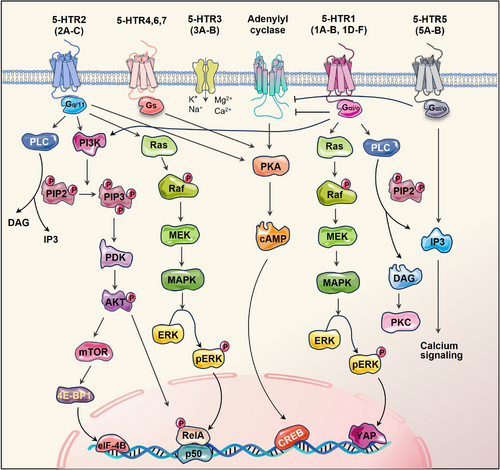
Our understanding of serotonin's function has significantly broadened over the last 20 years with the cloning of over 15 serotonin receptors, categorised into seven families based on the genetics and signalling mechanisms.29 Most receptors exhibited heterogeneity and were further subclassified. Of the families, 5-HTR3 is distinctive since it engages a ligand-gated Na+/K+ ion channel, while the rest six subtypes belong to the G-protein-coupled receptors.30 Generally, the 5-HTR1 (1A-1F) and 5-HTR5 (5A/5B) families couple via inhibitory Gαi/o proteins and suppress adenylyl cyclase, resulting in the reduction of cyclic adenosine monophosphate (cAMP) levels. HTR1A exhibits cell type-specific variations in its signalling repertoire, including extracellular signal-regulated kinase 1/2 (ERK1/2), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and phospholipase C/protein kinase C (PLC/PKC). Whereas 5-HTR4/6/7 activate adenylyl cyclase, increasing cAMP activity. In addition, 5-HTR2 stimulates intracellular calcium signalling by activating PLC.31, 32 The distribution and subcellular location of diverse 5-HTRs are crucial for determining the specific paracrine effects of 5-HT. The complexity of serotonergic signalling is manifested by the activity of various 5-HTRs that integrate multiple inputs into converging or diverging signals, ultimately resulting in a wide spectrum of physiological effects (Figure 2).
3.2 Serotonin transporter
The effects of 5-HT rely on its availability, which is determined in part by the SERT. SERT uses sodium to transport monoamine and is accountable for clearing free serotonin from the extracellular space, thereby terminating the subseuent impacts of 5-HTR activation. In the CNS, the depolarisation of neurons leads to the release of serotonin into the synaptic cleft, where it attaches to either postsynaptic 5-HTR or presynaptic SERT.33 The interaction with SERT functions as a negative feedback mechanism, restraining additional serotonin discharge into the synaptic cleft.
Thus, the selective serotonin reuptake inhibitors (SSRIs), which specifically target SERT, increase the availability of serotonin at the synaptic junction, influencing the duration and intensity of 5-HT signalling. These are the most widely used medications to treat obsessive compulsive disorder, depression and anxiety disorders.34 Treatment with SSRIs also leads to a depletion of platelet 5-HT storage.
3.3 Receptor-independent 5-HT signalling—serotonylation
Serotonylation is a chemical modification through which 5-HT is incorporated into acceptor proteins through the formation of glutamyl-amide bonds in a transglutaminase (TGM)-dependent way.23
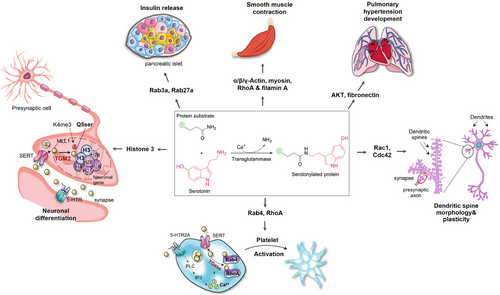
Seven TGMs have been identified, primarily localising intracellularly, with TGM2 being the most ubiquitous and abundant one. Blood coagulation factor XIII, activated by thrombin during coagulation to form factor XIIIa, also exhibits extracellular TGM activity.35 Serotonylation occurs in both extracellular and intracellular compartments during thrombus formation, exemplifying this process in the body. Extracellularly, serotonylated procoagulant proteins bind to fibrinogen and thrombospondin, increasing the stability of essential protein complexes.36 Concurrently, 5-HT attaches to platelet HTR2A, triggering the phosphatidylinositol pathway through a Gαq-protein-dependent mechanism. This activation leads to a rise in cytoplasmic Ca2+, which is necessary for TGM activity.23 Once 5-HT is transported into the cytoplasm, TGM crosslinks it to small G-proteins (such as RhoA and Rab4), which constantly trigger α-granule exocytosis.23 Due to the hydrophilic properties of 5-HT, serotonylation is thought to exclusively occur in SERT-expressing cells, including smooth muscle cells, pancreatic β cells, valve interstitial cells, neurons and glial cells.37 Besides platelet activation, serotonylation participates in various physiological functions, such as smooth muscle contraction,38 insulin release,39 dendritic spine plasticity40 and cardiac valve degeneration.41
One of the most striking findings on serotonylation is its remarkable function as an epigenetic marker regulating gene expression: adding serotonin molecule to the glutamine 5 residue on histone H3, known as H3Q5Ser, has been recognised as a permissive post-translational modification which exists in conjunction with neighboring lysine 4 trimethylation (H3Kme3).42 H3Q5Ser potentiates the function of H3K4me3 either via stabilising H3K4me3, preventing dynamic turnover, or by improving its recognition by downstream effectors43 (Figure 3). Despite a smattering of identified examples of serotonylation so far, it has yielded innovative mechanistic insights and opened therapeutic avenues for a myriad of physiological and pathophysiological processes.
4 SEROTONIN'S PRO-TUMOURIGENIC ROLES IN CANCER CELLS
4.1 Mitogenic activity through serotonin receptor signalling
Elevated 5-HT, as well as its receptors, has been demonstrated to be involved in oncogenic progression, as an potent trophic, mitogenic and anti-apoptotic factor.32 5-HTRs are present in numerous types of cancer, including colorectal cancer (CRC),19 hepatocellular carcinoma (HCC),44 gastric cancer (GC),45 breast cancer (BC),46, 47 melanoma,48, 49 pancreatic cancer,50 prostate cancer (PCa),51-53 lung adenocarcinoma,54 ovarian cancer (OC),55 bladder cancer56 and cholangiocarcinoma57 (Table 1). The intracellular reaction to 5-HT varies between normal colon cells and CRC cells, since 5-HT facilitated CRC cells growth without increasing the cell division rate of normal colonic crypt cells.58 The growth of normal colonic crypts is regulated by endocrine and autonomic neural mechanisms, while the division of CRC cells only requires endocrine signalling.59 5-HT stimulates the growth but inhibits apoptosis of CRC cells through a variety of 5-HTRs.60-64 HCC cells express different serotonin receptors, which were demonstrated to increase tumour cell proliferation and metastasis.44, 65 5-HT also inhibited autophagy through HTR2B in HCC, leading to continuous phosphorylation of p70s6k and 4E-BP1, two downstream targets of mammalian target of rapamycin (mTOR).66 In a cultured murine melanoma cell line, zebrafish embryos and human skin, the protein kinase A/cAMP-response element binding protein (PKA/CREB) signalling pathway has been revealed as a mediator of HTR2A, promoting melanogenesis.67
| Cancer type | 5-HTRs expression | Experimental models | Reference |
|---|---|---|---|
| Colorectal cancer | 1A, 1B, 1D, 1F, 3 |
Xenograft tumour model of human colorectal cancer cell lines (LoVo, HT29, SW480) and mice colorectal cancer cell (CT26, MC38) Orthotopic metastatic mouse model (HCT116) Tumour organoids isolated from human colon cancer tissues AOM/DSS-induced colorectal cancer mouse model |
19, 60-64, 118, 161, 162, 164 |
| Hepatocellular carcinoma | 1B, 2B | Human hepatocellular cancer cell lines (Huh7 and HepG2) | 44, 65, 66, 83, 84 |
| Gastric cancer | 2A, 2B, 3A, 7 |
Human stomach cancer tissues Human gastric cancer cell lines (AGS and HGC27) |
45, 86 |
| Breast cancer | 1A, 1B, 1D, 2A, 2B, 2C, 3, 4, 7 |
TNBC cell lines TNBC xenograft models established using LM2 and MCF10 Ca1a cell lines Tissue microarrays Hormone-responsive breast cancer cell lines (MCF-7, T47D) |
46, 47, 68-71, 82, 85 |
| Melanoma | 2A, 2B |
Murine melanoma cell line (B16F10) Human melanoma cell lines (SK-MEL-2) Human skin and zebrafish embryos Primary tumours of various patients diagnosed with uveal melanoma cell lines |
48, 49, 67 |
| Pancreatic cancer | 1B, 1D, 2B |
Human pancreatic cancer cell lines (AsPC-1, BxPC-3, Capan-2, CFPAC-1, HPAC, PANC-1 and SW1990) Tissue microarrays Patient-derived xenograft mode |
50, 72-74 |
| Prostate cancer | 1A, 1B, 1D, 2B, 4 |
PC cell lines (PC-3, DU145, LNCaP) Benign prostatic stromal cell line (human prostate cell preparation) Xenografts of PC-3 cells |
51-53, 76 |
| Lung cancer | 1A, 1B, 7 |
Chronic stress and depressive-like behaviour murine model Human lung adenocarcinoma patients tissue samples Lung small cell carcinoma cell line GLC-8 |
54, 77-80 |
| Ovarian cancer | 1A, 1B, 2B, 4 | Human ovarian cancer tissues | 55 |
| Bladder cancer | 1A, 1B |
Human bladder cancer cell lines (HT1376) Human bladder cancer tissue specimens |
56 |
- Abbreviation: AOM, azoxymethane; DSS, dextran sulfate sodium salt; TNBC, triple-negative breast cancer.
Mammary epithelial homeostatic mechanisms play a vital role in maintaining normal tissue function amidst the significant alterations linked to pregnancy, lactation and involution. As a crucial local controller of epithelial homeostasis in the breast, it has been observed that the biosynthetic capacity of 5-HT was increased, associated with multiple alterations in 5-HTRs expression in BC.46, 68, 69 These abnormal signals favour malignant progression of human BC cells.46, 69-71 5-HT was reported to amplify Warburg effect of pancreatic cancer cells through the PI3K/mTOR axis mediated by HTR2B-LYN-p85 complex.50 It also implicates in maintenance of cancer stem cells (CSCs) populations and gemcitabine resistance in pancreatic ductal adenocarcinoma (PDAC) through 5-HTR7-PI3K/AKT and 5-HTR7/JAK2/STAT3 pathways.72 The downregulation of diverse 5-HTRs in pancreatic cancer cells was revealed to suppress tumour proliferation and migration.73-75 In the case of PCa, 5-HT generated by neuroendocrine (NE) cells exerted a pro-proliferative effect on PCa cells via HTR1A-MAPK/ERK and HTR1A-PI3K/AKT signalling pathways and suppressed apoptosis via the HTR1B-PI3K/AKT axis.76 The mitogenic effects of 5-HT on small cell lung cancer (SCLC), involving HTR1A and HTR1D, were discovered in the 1990s.77, 78 Later, various 5-HTR subtypes, including HTR3A, HTR3C and HTR7, were reported to promote lung adenocarcinoma proliferation79, 80 and correlate with poorer survival outcomes in lung cancer patients.81
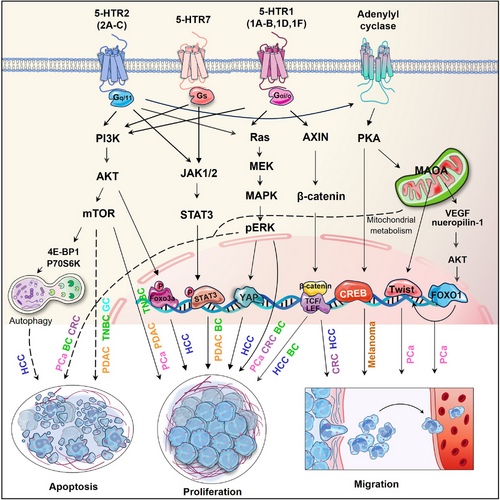
Besides the canonical signalling pathways initiating phosphorylation cascade in the four major protein kinase pathways, several non-canonical signalling mechanisms of 5-HTRs have also been implicated in the pro-carcinogenic effects of serotonin. For example, 5-HT triggered the association between HTR1(B/D/F) and AXIN1, activating the Wnt/β-catenin signalling. This activation occurs by preventing β-catenin degradation, leading to promoted self-renewal of colorectal CSCs and tumourigenesis.19 HTR1A interacts with TRIM21 and PSMD7 to prevent the degradation of TβRII mediated by ubiquitin‒proteasome. This action simultaneously suppressed the downstream Smad and MEK-ERK-Myc pathway, consequently hindering cytoskeletal rearrangement and epithelial‒mesenchymal transition (EMT) in BC.82 5-HT enhanced BC cell proliferation by PKM2-facilitated glycolysis, a process dependent on HTR2A/JAK1/STAT3 signalling.71 HTR1D stabilised PI3KR1, exerting a potent oncogenic effect on HCC, and activated the PI3K/AKT/FoxO6 pathway.83 5-HTR7 was reported to promote the growth and migration of HCC through activating Wnt/β-catenin signalling,84 and support triple negative BC cell proliferation through FOXM1, and cyclin D1 signalling.85 In GC, HTR2B was revealed to enhance the PI3K-AKT-mTOR pathway independent of its interaction with receptor tyrosine kinases, instead through crosstalk with Fyn. This activation increased the expression of HIF1α and ABCD1, concurrently reducing ferroptosis86 (Figure 4).
4.2 Pro-tumourigenesis through serotonylation
Dysregulated serotonylation has been implicated in tumourigenesis. The small G family proteins are the primary cellular targets of TGM2-mediated serotonylation. RhoA (Ras homologue gene family, member A) was activated by TGM2-mediated serotonylation in CRC, enhancing yes-associated protein (YAP) expression and promoting the carcinogenesis of CRC.87 Serotonylation of Rac1 in PDAC was revealed to promote its activation and be essential for the trans-differentiation process of acinar cells into acinar-to-ductal metaplasia (ADM), a critical determinant in PDAC development. This phenotype was leveraged to explore the administration of SSRIs as a potential intervention to prevent the development of ADM lesions in PDAC.88, 89 In addition to the small G family proteins, 5-HT has been reported to activate mTOR1 through serotonylation and promote CRC proliferation, independent of 5-HTRs.90
4.3 Other pathways
5-HT has been shown to facilitate the progression of PDAC from chronic pancreatitis (CP) by activating RhoA/Rho-associated, coiled-coil containing protein kinase (ROCK) signalling cascades. The 5-HT-RhoA/ROCK axis subsequently increased the nuclear translocation of nuclear factor-kappa B (NF-κB) and the expression of α-smooth muscle actin (α-SMA), enhancing inflammatory reactions and fibrosis in pancreatic tissues.91 Intriguingly, a recent study has demonstrated that 5-HT suppresses ferroptosis (a type of regulated cell death characterised by lipid reactive oxygen species accumulation and iron dependency) independent of 5-HTRs. Instead, it acts as a potent radical-trapping antioxidant, eliminating lipid peroxidation.92 An in vitro study revealed that treating non-small cell lung cancer (NSCLC) cells with 5-HT enhanced their proliferation and migration. This effect was accompanied by the inhibition of c-Myc ubiquitination and upregulation of SERT, thereby establishing a 5-HT-Myc-SERT-5-HT feedback loop.79 SERT was also reported to be responsible for transporting serotonin into clone cancer cells, activating the RhoA/ROCK/YAP signalling and promoting carcinogenesis.87 Furthermore, the suppression of 5-HT uptake by Azaphen dihydrochloride monohydrate reduced the tumourigenicity and inhibited the distant metastasis of NSCLC cells in vivo, underscoring the importance of SERT in tumourigenesis.
5 SEROTONIN'S ANGIOGENIC EFFECTS ON TUMOUR VASCULATURE
Serotonin also functions as an angiokine in tumour angiogenesis. Platelet activation results in a substantial release of 5-HT in the tumour microenvironment (TME), where it can directly contact neighboring endothelial cells and activate angiogenic pathways. 5-HT triggered a comparable array of signalling kinases as those stimulated by vascular endothelial growth factor from endothelial cells, such as PI3K-AKT-mTOR signalling, and the orphan nuclear receptor and transcription factor TR3.10, 93 Utilising a serotonin deficiency genetic mouse model (Tph1−/−), it has been reported that 5-HT regulates angiogenesis in subcutaneous CRC allografts. This regulation is achieved by modulating MMP-12 in tumour-infiltrating macrophages, which in turn affects the generation of circulating angiostatin.94 Allografts of SCLC and melanoma in Sert−/− mice also displayed tumour retardation, which may attribute to decreased endothelial nitric oxide synthase expression and insufficient blood supply.95 The effect of serotonin on tumour vasculature is intricate, relying on its engagement with a diverse array of receptors, and presenting an opportunity to target these receptors as potential strategies to hinder tumour progression.95-97
6 IMMUNOMODULATORY FUNCTION OF SEROTONIN IN TUMOUR IMMUNITY
6.1 Serotonin signalling in immune cells
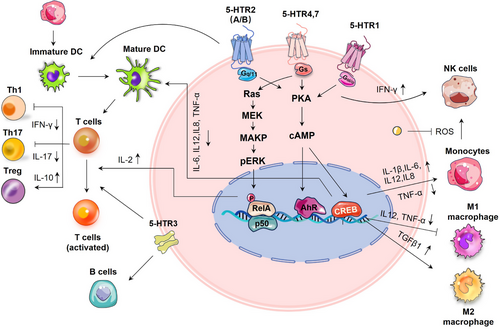
Immune cells express serotonergic components, including 5-HTRs, SERT, TPH and MAO, which govern their effector capabilities and regulatory mechanisms.98 For example, serotonin skewed human macrophages towards anti-inflammatory M2 phenotype via HTR2B/HTR7 and promoted a pro-fibrotic gene signature through HTR7-PKA axis.99-101 In dextran sulfate sodium salt (DSS)-induced colitis mice, 5-HT demonstrated anti-inflammatory effects on macrophages through the HTR2A/NF-κB pathway. Serotonin modulated cytokine production (such as interleukin [IL]1-β, IL-6, IL12 and tumour necrosis factor alpha [TNF-α]) in monocytes by activating 5-HTR3, 4 and 7 subtypes.102 It promoted the differentiation of immature CD1a+ human monocyte-derived dendritic cells (DCs) following TLR3 activation through HTR2B,103 and reduced the release of proinflammatory cytokines from mature DCs through 5-HTR4/HTR7-cAMP signalling.104 The serotonin-induced reduction of IL12 in DCs has been shown to reduce DC-induced interferon-gamma (IFN-γ+) Th1 polarisation and Th17 polarisation.103 SERT on DCs allow for the uptake of serotonin from activated T cells and its subsequent release through Ca2+-sensitive exocytosis, activating T cells.105 T cells possess a functional serotonergic system that enables them to produce, store, metabolise and respond to serotonin.106 Serotonin was reported to activate T cells through various 5-HTR signalling pathways.98 It inhibits T-cell polarisation to inflammatory Th1, or Th17 lymphocytes, whereas stimulating the proliferation and activation of anti-inflammatory Tregs in various inflammatory settings.107
Serotonin has been demonstrated to enhance mitogen-stimulated B-cell proliferation via 5-HTR1A and 5-HTR3A,108-110 but it induced apoptosis through SERT in Burkitt's lymphoma cells, independent of 5-HTRs.111 Long-term treatment with SSRIs has been associated with an elevation of B lymphocytes in patients, implicating an intricate relationship between serotonin signalling and the determination of cell fate across different biological contexts.112 With autologous monocytes, serotonin was reported to boost the cytotoxic capability of natural killer (NK) cells through HTR1A signalling.113 In the TME, monocytes restrict the cytotoxic effects of NK cells by releasing extracellular H2O2 and myeloperoxidase. Serotonin was revealed to protect NK cells against monocyte-induced apoptosis in vitro by scavenging peroxidase-derived reactive oxygen species (ROS).114 Conversely, inhibiting serotonin uptake with SSRIs has been shown to boost the cytosolic functions of NK cells in vitro.115 Platelet serotonin has also been reported to enhance the accumulation of innate immune cells, including monocytes and neutrophils at the inflammation sites.116 Therefore, numerous evidence suggests that serotonergic signalling affects immune cells in ways that facilitate tumour development by suppressing antitumour immunity (Figure 5).
6.2 Serotonin signalling in the tumour microenvironment
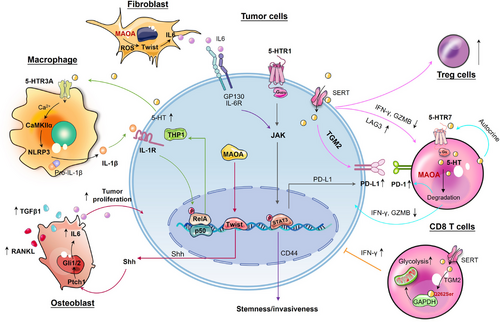
Besides its well-known mitogenic roles in tumourigenesis, serotonin also functions pivotally in immune modulation within the TME. A recent investigation has indicated that 5-HT not only influences tumour cells, but also aids in immune evasion for lung cancer patients with depression.54 These effects were mediated through the HTR1A/autophagy/p-STAT3/PD-L1 axis, which conferred resistance to cytotoxic T lymphocyte-mediated lysis in cancer cells. Further evidence showed that peripheral serotonin could orchestrate the TME: serotonin diminished the effector capabilities of CD8+ T cells and upregulated the expression of PD-L1 in subcutaneous syngeneic colorectal and pancreatic murine cancer models. Intriguingly, serotonin mediated PD-L1 upregulation in cancer cells via serotonylation could be effectively blocked by TGM2 inhibitors.117 These findings underscore the importance of TGM2-mediated serotonylation in defining the pro-tumour effect of serotonin. The pro-tumourigenic activities of serotonin also involve the interplay between tumour and immune cells residing within the TME: overproduced 5-HT by CRC cells paracrinally enhanced NLRP3 inflammasome activation through HTR3A on macrophages, leading to the production of IL1β. As a result, IL1β induced TPH1 transcription and 5-HT synthesis in CRC cells, thereby creating a reinforcing cycle between 5-HT and NLRP3 signalling in the TME. This loop assisted in sustaining chronic inflammation to facilitate CRC progression.118 Additionally, in a murine model with overexpressed human TIAM2S, ectopic TIAM2S expression provoked a pro-inflammatory environment that facilitated CRC tumourigenesis via 5-HT-triggered immunomodulatory effects.119 SSRIs, including fluoxetine and sertraline, restored antitumour immune responses in a chronic stress-induced mouse model of lymphoma, restricting tumour growth and cell dissemination.120 However, a recent study presented conflicting evidence that TGM2-mediated serotonylation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at glutamine 262 in CD8+ T cells induced a metabolic shift towards glycolysis, consequently promoting antitumour immune response121 (Figure 6).
6.3 Serotonin metabolism in tumour immunity
Serotonin metabolism disorders in TME can promote tumourigenesis. Maoa−/− mice displayed heightened T-cell antitumour immunity and inhibited growth of colorectal and melanoma tumours in mouse synergetic models. MAOA negatively regulated the antitumour immunity of CD8+ T cells (including IFN-γ, Granzyme B and PD1 expression) partly through the modulation of autocrine serotonin signalling in CD8+ T cells within the TME.122 Increased MAOA expression in PCa cells promoted bone and visceral metastasis by enhancing paracrine Shh-IL6-RANKL signalling in tumour‒stromal interactions. Within the bone microenvironment, MAOA stimulated osteoblast-derived IL6 secretion and triggered skeletal colonisation via inducing osteoclastogenesis through RANKL and IL6 produced by osteoblasts, forming a feedforward loop.123 Reciprocally, upregulated MAOA in stromal fibroblasts was revealed to offer growth advantages to tumour cells through paracrine IL6-STAT3 signalling, which transcriptionally activated the expression of CSCs marker CD44 in PCa cells.124 These findings indicate that MAOA originating from tumour or stromal cells dictates the interaction between these two cell types, favouring the reprogramming of naïve stroma towards a tumour-supportive phenotype (Figure 6).
7 SEROTONIN'S ANTITUMOUR FUNCTION
Physiological responses to serotonin, albeit to a lesser extent, also display tumour-suppressing activities, primarily mediated through a diverse array of 5-HTRs. These receptors, acting as tumour suppressors, often demonstrate decreased expression levels within tumours. The transcriptional silencing of HTR1B in NSLCL owing to abnormal hypermethylation of its gene promoter has been implicated in endowing with growth advantage to lung cancer cells.125 In OC patients, reduced expression of HTR1E in peritoneal disseminated OC cells correlated with unfavourable clinical outcomes. The researchers combined the mouse stress model with the OC orthotopic mouse model and observed that HTR1E suppressed downstream pathways activated by non-receptor tyrosine kinases Src family kinases (SRC). Consequently, the cell growth and migration that favour OC dissemination were restrained. Utilisation of HTR1E agonist or SRC inhibitor demonstrated potential in inhibiting chronic stress-promoted OC progression.126 In TNBC, HTR1A expression levels were notably reduced compared to adjacent normal tissues. HTR1A inhibited the ubiquitin‒proteasome-dependent degradation of TβRII by binding to TRIM21 and PSMD7, which downregulated the canonical Smad signalling and non-canonical MEK/ERK/c-Myc pathway. These suppressed signalling pathways inhibited cytoskeletal rearrangement and EMT in BC.82
Employing mouse models with defective 5-HT biosynthesis (Tph1KO and Tph1fl/flVillinCre), researchers revealed a protective role for 5-HT in enhancing DNA repair during the initial phases of colitis-dependent colorectal carcinogenesis.127 But in colitis-independent tumour mouse models, 5-HT was shown to promote CRC.94, 117 The dual role of 5-HT in CRC progression seems to rely on the stage of the disease and the underlying pathophysiological context: 5-HT shields colorectal stem cell niche from DNA damage, thus decreasing early carcinogenic events. However, at a late stage, it facilitates the growth of established colorectal tumours. In another colitis-associated colorectal cancer (CAC) study (azoxymethane [AOM]/DSS-induced model), the two side effects of 5-HT on both the onset and progression of CAC were clarified. 5-HT-HTR2B inhibited CAC initiation by modulating transforming growth factor-beta (TGF-β)-Smad signalling, protecting from epithelial damage and inflammation. On the contrary, 5-HT facilitated CAC progression via non-canonical TGF-β signal (including PI3K-AKT and angiogenesis) in a later stage of CAC.128 In a murine model of syngeneic CRC, the accumulation of 5-HT within CD8+ T cells was shown to enhance their glycolytic metabolism and antitumour activity, leading to the suppression of subcutaneous tumour growth.121 These various effects of 5-HT in CRC underscore the intricacy of serotonin signalling and its context-dependent nature in cancer development.
In addition, as a crucial NE stress mediator involved in various psychiatric disorders, the neural and plasma levels of serotonin are susceptible to psychological stress. For example, reduced serum serotonin levels have been detected in murine stress and rat depression models.126, 129, 130 The chronic unpredictable mild stress-induced reduction of serotonin resulted in increased OC dissemination by attenuating HTR1E-mediated tumour suppressive signalling.126 While elevated levels of gut-derived serotonin were observed in a chronic mild stress mouse model, contributing to the promotion of bone metastasis in BC.131 These findings suggest that stress-induced fluctuations in serotonin levels could significantly contribute to finely tuned carcinogenesis in a psychiatric context-dependent manner.
8 THERAPEUTIC POTENTIAL OF SEROTONERGIC PATHWAY IN CANCER
8.1 5-HT receptor-directed therapy
Agonists and antagonists that target 5-HTRs are commonly utilised to elucidate the roles of serotonin in tumourigenesis and the concept that serotonin receptor-directed pharmacotherapy has emerged. For example, 5-HTR3 antagonists are effective and safe antiemetic agents commonly used to treat nausea and vomiting following surgery and chemotherapy.132 Various clinical studies have been conducted or are being underway to evaluate their effectiveness in the treatment of chemotherapy-induced nausea and vomiting in patients with different types of cancer (Table 2). Numerous in vitro studies have demonstrated the antineoplastic effects of 5-HTR antagonists in varieties of cancer, including CRC,64, 133, 134 GC,135, 136 HCC,44 BC,85, 137 melanoma,48 lung cancer,77 pancreatic cancer,138 glioblastoma,139 OC96 and placental choriocarcinoma.140 Different inhibitors designed based on 5-HTR1A antagonist showed inhibitory and cytotoxic effects on PCa cell lines.141, 142 A non-selective 5-HTRs antagonist (methiothepin) could increase doxorubicin cytotoxicity in melanoma cells,143 and targeting HTR2A and HTR2C with selective serotonergic receptor ligands (SER) ameliorated tamoxifen effectiveness in ER+ BC cells.144 Vortioxetine, a potent inhibitor for 5-HTR3A, 5-HTR7 and SERT, induced apoptosis and autophagy in GC cells through the PI3K-AKT pathway.145 These antagonists are promising modulators of immune cells as well. For instance, HTR2B antagonist (SB204741) and 5-HTR7 antagonist (SB269970) decreased the differentiation of anti-inflammatory M2 macrophages99; 5-HTR7 antagonist (SB269970) treatment reduced the velocity of migratory-active of DCs in mouse colon,146 and inhibited ERK signalling in T cells.147
| Compounds | FDA approved | Target | Cancer type | Study stage in cancer | Effects in cancer |
|---|---|---|---|---|---|
| Pimavanserin (antagonist) | Yes | 5-HT2A | TNBC | Preclinical | Induced mitochondria-dependent intrinsic apoptosis and cause cytoprotective autophagy through the PI3K/Akt/mTOR pathway in TNBC cells in vitro137 |
| PDAC | Preclinical | Inhibited the growth of PDAC by inducing autophagy mediated apoptosis138 | |||
| GBM | Preclinical | Suppressed the proliferation of U87 glioblastoma cells in vitro and in vivo139 | |||
| Risperidone (antagonist) | Yes | 5-HT2A, 5-HT2D | GC | Preclinical | Inhibited the proliferation of KATO-III cells by inducing ROS and apoptosis in vitro and in vivo136 |
| Methiothepin (antagonist) | Yes | 5-HT2 | Placental choriocarcinoma | Preclinical | Attenuated mitochondrial function and induced ER stress, reducing oxidative phosphorylation, and causing metabolic shifting140 |
| Melanoma | Preclinical | Overcome the resistance of BRAFV600E melanoma cells by enhancing the cytotoxicity of vemurafenib and trametinib on these cells leading to melanoma cells death143 | |||
| OC | Preclinical | Suppressed OC growth by repressing mitochondrion-mediated metabolism and inhibited angiogenesis in vivo96 | |||
| Dolasetron (antagonist) | Yes | 5-HT3 | CRC | Preclinical | Induced apoptosis in colon cancer cells by inhibiting PUM1134 |
| Palonosetron (antagonist) | Yes | 5-HT3 | GC | Phase II clinical trial NCT04308837 | To assess this multi-modality approach in inducing pathological complete response; decreased rates of disease progression during neoadjuvant therapy; and increased overall, disease-free and peritoneal disease-free survival |
| BC | Phase IV clinical trial NCT05841849 | Efficacy and safety of intravenous versus oral 5-HT3 antagonists combined with NK-1 receptor antagonists for the prevention of CINV in BC | |||
| HNSCC | Clinical trial NCT06102447 | Efficacy and safety of Palonosetron hydrochloride capsules in preventing nausea and vomiting induced by radio chemotherapy in head and neck squamous cell carcinoma | |||
| Brain cancer | Phase II clinical trial NCT00636805 | Efficacy and tolerability of palonosetron and dexamethasone in preventing acute CINV in brain tumour patients | |||
| CRC | Phase II clinical trial NCT00381862 | To study how well giving aprepitant together with palonosetron and dexamethasone works in preventing nausea and vomiting caused by chemotherapy in patients receiving chemotherapy for metastatic CRC | |||
| Ramosetron (antagonist) | Yes | 5-HT3 | BC | Phase IV clinical trial NCT05326360 | To evaluate the effectiveness of additional ramosetron injection for controlling late PONV after breast surgery in high-risk PONV patients |
| OC | Phase II clinical trial NCT01012336 | To evaluate if new combination (aprepitant/ramosetron/dexamethasone) may improve actual CINV control in OC patients treated with taxane/carboplatin. | |||
| Lung cancer | Preclinical | Inhibited lung cancer cell growth and migration by inducing autophagic cell death through the ERK pathway148 | |||
| Tropisetron (antagonist) | Yes | 5-HT3 | Cervical cancer | Phase III clinical trial NCT05564286 | To evaluate the antiemetic effect of adding fosaprepitant to biplet regimen of tropisetron and dexamethasone for patients with cervical cancer |
| Lung cancer | Preclinical | Exerted antineoplastic effects in part through modulating inflammatory and proliferating markers149 | |||
| CRC | Preclinical | Alleviated the tumour progression in an AOM/DOS-induced CRC mouse model118 | |||
| Lung cancer | Preclinical | 148 | |||
| Tegaserod (angonist) | Yes | 5-HT4 | GC | Preclinical | Inhibited the proliferation of gastric cancer cell lines and PDX models by targeting MEK1/2151 |
| Melanoma | Preclinical | Reduced tumour growth, metastases, and induced apoptosis through PI3K/AKT/mTOR signalling150 | |||
| Vortioxetine | Yes | 5-HTR3A, 5-HTR7 | GC | Preclinical | Induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway145 |
| Pimavanserin tartrate (antagonist) | Yes | 5-HT2A | GBM | Preclinical | Suppressed the proliferation & migration of U87 glioblastoma cells, induced G1/S phase arrest and promoted apoptosis139 |
| NAN-190 (antagonist) | No | 5-HT1A | PCa | Preclinical | Antiproliferative on PCa cell lines51, 53 |
| 8-OHDPAT | No | 5-HT1A | TNBC | Preclinical | Inhibited the progression of TNBC via TGF-β canonical and non-canonical pathways82 |
| CP93129 (agonist) | No | 5-HT1B | CRC | Preclinical | Stimulated growth of HT29 cells60 |
| SB224289 (antagonist) | No | 5-HT1B | CRC | Preclinical | 60 |
| GR127935 (antagonist) | No | 5-HT1B, 5-HT1D | CRC | Preclinical | Inhibited CRC metastasis through targeting Axin164 |
| BRL54443 (agonist) | No | 5-HT1E, 5-HT1F | OC | Preclinical | Suppressed tumour progression in OC mouse model126 |
| SB204741 (antagonist) | No | 5-HT2B | HCC | Preclinical | Inhibited cell proliferation of Huh7 cells by reducing the expression of FOXO3a44, 75 |
| Sb-699551 (antagonist) | No | 5-HT5A | BC | Preclinical | Reduced the frequency of tumour sphere initiating cells PDXs70 |
| Metergoline (antagonist) | No | 5-HT7 | TNBC | Preclinical | Antiproliferative effects on TNBC cells85 |
| BJ-1113 (antagonist) | No | 5-HT7 | TNBC | Preclinical | Exhibited antiproliferative and anti-invasive activities against MDA-MB-231 cells69 |
| Y25130 hydrochloride (antagonist) | No | 5-HT3 | CRC | Preclinical | Antimitogenic and apoptotic effect on HT29 cells62 |
- Abbreviations: AKT, protein kinase B; AOM, azoxymethane; BC, breast cancer; CINV, chemotherapy-induced nausea and vomiting; CRC, colorectal cancer; GBM, glioblastoma; GC, gastric cancer; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; mTOR, mammalian target of rapamycin; OC, ovarian cancer; PCa, prostate cancer; PDAC, pancreatic ductal adenocarcinoma; PDX, patient-derived xenograft; PI3K, phosphoinositide 3-kinase; PONV, postoperative nausea and vomiting; ROS, reactive oxygen species; TGF-β, transforming growth factor-beta; TNBC, triple negative breast cancer.
In vivo, treatment with 5-HTR3 antagonist tropisetron alleviated tumour progression in an AOM/DSS-induced CRC mouse model118 and a lung cancer mouse model.148 Selective antagonists of HTR5A decreased the prevalence of tumour sphere initiating cells in BC patient-derived xenografts,70 and a non-selective 5-HTR2 antagonist methiothepin was shown to boost the anticancer efficacy of paclitaxel in OC model.96 A retrospective clinical study demonstrated that perioperative use of 5-HTR3 antagonist such as palonosetron or ramosetron displays potential anticancer effects with improved recurrence-free survival in patients following open thoracotomy for lung cancer.149 Subsequently, the antineoplastic activities of 5-HTR3 antagonists were confirmed and deciphered in both in vitro studies and mouse models of lung cancer.148, 149
However, an FDA-approved HTR4 agonist called Tegaserod, which is typically employed in treating irritable bowel syndrome, has been found to effectively induce apoptosis in both BRAFV600E and BRAFWT melanoma. Tegaserod inhibited PI3K-AKT-mTOR signalling and synergistically enhanced the effects of a standard treatment, Vemurafenib, in human melanoma cell lines.150 It also exerted antineoplastic effects in GC by targeting MEK1/2.151 In addition, corroborating the antitumour properties of HTR1E in OC and HTR1A in TNBC, selective agonists targeting these receptors have been shown to suppress tumour progression, respectively82, 126 (Table 2).
8.2 Targeting SERT with SSRIs
Targeting serotonin transporter to prevent the absorption of serotonin confers antitumour effects in a diverse range of cancers, and the utility of SSRIs as anticancer agents is being extensively evaluated. SSRIs are the most commonly prescribed medications for alleviating depression among the general populace and individuals with cancer experiencing depressive symptoms. An epidemiological study reported that the administration of SSRIs in patients with persistent clinical depression significantly decreased their likelihood of developing CRC.152 Population-based cohort studies also revealed that there is a correlation between the utilisation of SSRIs and a potentially lower chances of developing CRC in people with a family history of this disease.153 A recent evaluation and meta-analysis of published observational studies unveiled that taking SSRIs was associated with decreased likelihood of developing HCC, with a dose-dependent tendency.154 SSRIs have also shown an association with a decreased risk of HCC and may lower the chances of HCC in patients with HBV in a dosage-dependent fashion.155, 156 The capacity of SSRIs to penetrate the blood‒brain barrier makes them suitable for treating conditions such as glioblastoma or brain metastases. It has been documented that the highly brain-penetrant SSRI fluoxetine killed GBM by inhibiting sphingomyelinase activity. When fluoxetine was combined with temozolomide (a standard treatment for GBM), at doses equivalent to those within the FDA-approved range for patients, it resulted in tumour shrinkage and extended the survival of mice carrying GBMs from patients.157 Furthermore, there was a correlation between the utilisation of selective SSRIs and a reduced risk of OC or bladder cancer among patients.158, 159
Blocking SERT with citalopram reversed the pro-tumourigenic effects of serotonin and reduced the distant metastasis of CRC in preclinical studies.87, 160 Vilazodone, a selective SSRI exerted effective antimetastatic action for CRC by targeting TRIM21 (tripartite motif 21), which ubiquitinates MST2 to deactivate YAP signalling.161 Multiple lines of evidence have shown that the selective SSRI sertraline exhibited antitumour effects in CRC and BC cells.162-165 One well-studied mechanism involves sertraline enhancing protein levels of p53 by neutralising the action of translational controlled tumour protein on the MDM2-p53 axis, thereby facilitating P53-mediated apoptosis.166, 167 SSRIs sertraline and fluoxetine were reported to prevent tumourigenesis of melanoma, BC, NSCLC and HCC by targeting multiple protein kinase signalling pathways.168-170 The combination of these two SSRIs with sorafenib synergistically suppressed the proliferation of HCC in vitro and a mouse model of liver damage induced by diethyl nitrosamine/carbon tetrachloride (DEN/CCL4).171 SSRIs are recommended as a therapeutic option for BC patients to alleviate side effects such as hot flashes induced by anti-estrogen therapy.172 Treatment with SSRI paroxetine-induced apoptosis in BC cells through MAPK-dependent ROS generation.173 Sertraline (Zoloft) was revealed to restrain the viability of breast tumour-initiating cells or prostate CSCs, and synergised with chemotherapy (docetaxel), restraining the growth of BC xenograft in mice.174-176 Thus, SSRIs may be regarded as potential sensitisers in cancer treatment: sertraline enhanced the sensitivity of NSCLC to erlotinib by suppressing the AMPK/mTOR signalling pathway,177 and attenuated TRAIL resistance in lung cancer cells through inhibiting autophagic flux via upregulatoin of death receptor 5 (DR5).178 It also substantially reduced the tumour progression in a mouse model implanted with high-resistant human OC xenografts when combined with Doxil (pegylated liposomal doxorubicin), acting as a chemosensitiser.179 In addition, SSRI fluoxetine improved the effectiveness of chemotherapy agents such as doxorubicin, mitomycin C and paclitaxel by inhibiting multidrug resistance pumps in human xenograft mouse models180, 181 (Table 3). Of note, the human equivalent doses extrapolated from the antitumour doses of sertraline (2 mg/kg)179 and fluoxetine (.04 or 1 mg/kg)180, 181 employed in preclinical models were lower than the doses typically administered to human patients for antidepressant purpose. Similar to other antidepressants, SSRIs display a comparable side-effect profile, which includes GI disturbance, fatigue or insomnia, headache and transient increased anxiety following treatment initiation.182 Repurposing SSRIs as antineoplastic medications at higher doses may induce severe behavioural side effects. It is also crucial to ascertain whether the combination of SSRIs with conventional anticarcinogens results in synergistic or antagonistic effects. For example, certain SSRIs (such as fluoxetine, paroxetine), which exert potent inhibitory effects on cytochrome P450 (CYP450), should be avoided when used with anticancer agents (e.g., tamoxifen) metabolised via the CYP450 system.183
| Compounds | FDA approved | Cancer type | Study stage in cancer | Effects in cancer |
|---|---|---|---|---|
| Citalopram (Celexa) | Yes | CRC | Preclinical | Inhibited CRC tumourigenesis by targeting serotonin activated RhoA/ROCK/YAP signalling87 |
| CRC | Preclinical | Reduced tumour size and the number of circulating tumour cells and metastases in an orthotopic mouse model of CRC160 | ||
| Vilazodone | Yes | CRC | Preclinical | Reduced the metastasis of CRC cells via TRIM21-MST2-Hippo-YAP signalling161 |
| Sertraline (Zoloft) | Yes | BC | Clinical trial NCT00667121 | To study levels of tamoxifen in the blood of women with breast cancer and in women at high risk of BC who are receiving tamoxifen together with venlafaxine, citalopram, escitalopram, gabapentin or sertraline: no results posted |
| CRC | Preclinical | Exhibited proapoptotic activity with Bcl-2 inhibition in HT-29 cells162 | ||
| BC | Preclinical | Antiproliferative activity was aggravated in combination with mitochondrial inhibitors, and achieved through G1-S-cell cycle arrest165 | ||
| BC, CRC, melanoma | Preclinical | Increased the amount of p53 by neutralising TCTP's action on the MDM2-P53 axis, thereby facilitating P53-mediated apoptosis166, 167 | ||
| HCC | Preclinical | Induced apoptosis in HepG2 cells partially via activation of TNF-MAP4K4-JNK cascade signalling pathway169 | ||
| BC | Preclinical | Exerted antiproliferation activity by targeting the mTOR signalling pathway in a REDD1-dependent manner170 | ||
| HCC | Preclinical | Synergised with sorafenib to inhibit the viability of HCC cells in vitro and in vivo via targeting the AKT/mTOR pathway171 | ||
| BC | Preclinical | Reduced the frequency and sphere-forming ability of breast tumour-initiating cells174 | ||
| BC | Preclinical | Combination with docetaxel synergistically reduced tumour cell proliferation and induced cell death in mammary tumour allografts175 | ||
| PCa | Preclinical | Targeted prostate cancer stem cells through activation of apoptosis and autophagy signalling by deregulating redox balance176 | ||
| NSCLC | Preclinical | Enhanced NSCLC sensitivity to erlotinib by inhibiting AMPK/mTOR signalling pathway177 | ||
| Lung cancer | Preclinical | Inhibited autophagic flux through upregulation of DR5 on TRAIL-resistant lung cancer cells178 | ||
| OC | Preclinical | Reduces tumour growth and progression combination in combination with Doxil179 | ||
| Fluoxetine (Prozac) | Yes | NSCLC | Phase II clinical trial NCT00005850 | To test the efficacy of fluoxetine to improve patient's quality of life during chemotherapy: no results posted |
| CRC, pancreatic | Preclinical | Augmented the effects of PD-1 checkpoint blockade and triggered long-term tumour control in mice subcutaneously inoculated with syngeneic colorectal and pancreatic tumours117 | ||
| GBM | Preclinical | Promoted tumour regression and prolonged the survival of mice harbouring patient-derived orthotopic GBM in combination with temozolomide157 | ||
| BC, CRC | Preclinical | Synergistic growth inhibition of HT-29 and MCF-7 cells in combination with chemotherapy163, 164 | ||
| Lung cancer | Preclinical | Mediated ER stress and autophagy through the ATF4-AKT-mTOR signalling pathway168 | ||
| HCC | Preclinical | 171 | ||
| Paroxetine (Paxil) | Yes | CRC | Preclinical | 162 |
| Lung cancer | Preclinical | 168 | ||
| BC | Preclinical | Induced apoptosis in MCF7 cells through MAPK-dependent ROS generation173 | ||
| Vortioxetine | Yes | GC | Preclinical | Induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway145 |
- Abbreviations: AKT, protein kinase B; BC, breast cancer; CRC, colorectal cancer; GC, gastric cancer; GBM, glioblastoma; HCC, hepatocellular carcinoma; mTOR, mammalian target of rapamycin; NSCLC, non-small cell lung cancer; OC, ovarian cancer; PCa, prostate cancer; PD-1, programmed death 1; ROS, reactive oxygen species; TCTP, translational controlled tumour protein; YAP, yes-associated protein.
There are controversial data regarding their impact on cancer prognosis. In five retrospective cohort studies involving patients diagnosed with breast, prostate, lung, CRC and melanoma, the persistent use of SSRIs was correlated with reduced survival rates among cancer patients.184 A recent study revealed that duloxetine, a selective SSRI commonly used to treat major depression, can amplify the TGF-α-promoted activation of MAPK/AKT, JNK in HCC-derived cell line, leading to an increase in cell migration. Nevertheless, this phenotype needs further validation since the current study relies on a single cell line and lacks a thorough mechanistic exploration.185
8.3 Inhibition of serotonin biosynthesis with TPH1 inhibitors
Depletion of peripheral serotonin with Tph−/− mice displayed reduced growth of syngeneic murine pancreatic and CRCs. Additionally, treatment with a TPH1 inhibitor (telotristat ethyl [TE]) in this study reduced tumour progression and simultaneously enhanced the effectiveness of anti-PD1 therapy in mice.117 Administration of a TPH inhibitor also attenuated tumourigenesis in an AOM/DSS-induced CRC mouse model.118 Studies have reported that treatment with a TPH inhibitor LP-533401 suppressed the growth of BTIC and exhibited synergistic effects with chemotherapy (docetaxel), resulting in inhibition of BC xenograft growth in mice.174 5-HT produced by human cholangiocarcinoma cell lines was revealed to promote their growth in an autocrine manner. However, the proliferation of cholangiocarcinoma cells can be blocked by a TPH inhibitor p-chlorophenylalanine (CPA) both in vitro and in vivo.57
Over the past few years, several specific inhibitors targeting TPH1 have been developed, and TE has received FDA approval for the treatment of diarrhoea in patients with carcinoid syndrome.117 More recently, TE is undergoing evaluation in clinical trials as a potential therapy for metastatic NE tumours.186, 187 Another phase II clinical trial is ongoing to explore the effectiveness of TE in combination with the first-line chemotherapy for individuals diagnosed with advanced cholangiocarcinoma (ID: NCT03790111). Inhibition of serotonin biosynthesis thus represents a promising strategy for antitumour treatment (Table 4).
| Compounds | FDA approved | Target | Cancer type | Study stage in cancer | Effects in cancer |
|---|---|---|---|---|---|
| TE | Yes | TPH1 | NETs | A retrospective, single-arm, pre-post physician panel-based chart review of patients who received TE | Reduced peripheral serotonin and relieved carcinoid syndrome in NETs184, 187 |
| Cholangiocarcinoma | Phase II clinical trial NCT03790111 | A safety and efficacy study of XERMELO + first-line chemotherapy in patients with advanced biliary tract cancer (TELE-ABC) | |||
| CRC, pancreatic | Preclinical | Augmented the effects of PD-1 checkpoint blockade and triggered long-term tumour control in mouse syngeneic colorectal and pancreatic tumours117 | |||
| 4-Chloro-DL-phenylalanine (Fenclonine) | No | CRC | Preclinical | Alleviated tumour progression in an AOM/DSS-induced CRC mouse model118 | |
| LP-533401 | No | BC | Preclinical | Reduced BTIC frequency after transplanting drug-treated tumour cells into immune-compromised mice174 | |
| p-Chlorophenyl alanine | No | Cholangiocarcinoma | Preclinical | Blocked the proliferation of cholangiocarcinoma cells in vitro and in vivo57 | |
| Clorgyline | Yes | MAOA | PCa | Preclinical | Reduced PCa xenograft growth in mice189 |
| Preclinical | Inhibited tumour cell proliferation and reduced expression of CSC markers190 | ||||
| Preclinical | Reduces PNI in vitro and tumour-associated neurogenesis in vivo191 | ||||
| Preclinical | Decreased growth and proliferation of androgen-sensitive and castration-resistant prostate cancer cells193 | ||||
| Preclinical | Restored enzalutamide sensitivity to suppress EnzR cell growth194 | ||||
| Phenelzine | Yes | MAOA and MAOB | PCa | Phase II clinical trial NCT02217709 | Demonstrated efficacy in patients with biochemical recurrent castrate-sensitive PCa197 |
| PCa | Phase II clinical trial NCT01253642 | To study how well giving phenelzine with docetaxel works in treating patients with PCa that is growing, spreading, or getting worse after first-line therapy with docetaxel. | |||
| CRC, melanoma | Preclinical | Suppressed tumour growth in mouse syngeneic and human xenograft tumour models in a T-cell-dependent manner122 | |||
| PCa | Preclinical | 194 |
- Abbreviations: AOM, azoxymethane; BC, breast cancer; CRC, colorectal cancer; CSC, cancer stem cell; DSS, dextran sulfate sodium salt; NETs, neuroendocrine tumours; PCa, prostate cancer; PD-1, programmed death 1; TE, telotristat ethyl.
8.4 Targeting MAOA for PCa therapy
The heightened expression of MAOA has been observed to exhibit a strong correlation with the Gleason grade and preoperative serum prostate-specific antigen (PSA) levels of patients with PCa, making it a promising biomarker for PCa prognosis.188 MAOA-dependent HIF1α-VEGF-A-FOXO1-TWIST1 pathway promoted PCa growth and metastasis. Knockdown or pharmacological blocking MAOA suppressed tumour growth and metastasis in PCa xenograft mouse model.189 In a prostate conditional Maoa knockout mouse model, PCa development was significantly inhibited with slowed proliferation and reduced expression of CSC markers, such as CD44, α2β1 and CD133.190 Additionally, MAOA was found to promote perineural invasion (PNI) of PCa, characterised by the infiltration of tumour cells into surrounding nerves, serving as a prognostic indicator for poor outcomes and survival in this disease. At the mechanistic level, MAOA triggered the activation of SEMA3C through a Twist1-dependent transcriptional process, subsequently stimulating cMET to enhance PNI through interactions with co-activated NRP1 and PlexinA2191 (Figure 4). While MAOA expression is not universally upregulated in all cancer types. For example, in GC tissues, the expression of MAOA was found to be notably reduced, which correlated with an unfavourable patient prognosis.192
Substantial evidence indicates that targeting MAOA blocks PCa proliferation and metastasis,189-191 restores enzalutamide sensitivity,193, 194 and revokes immune suppression.122, 195, 196 Importantly, clinically available MAOIs, which are utilised in the treatment of depression and various neurological conditions, have demonstrated promising outcomes against PCa in both experimental models and clinical studies, presenting a promising chance for their repurposing as a treatment for PCa. A phase II clinical trial (ID: NCT02217709) reported that phenelzine, a non-selective MAOI, shows effectiveness since serum PSA levels decreased in individuals experiencing biochemically recurring, castration-sensitive PCa.197 In another phase II clinical trial (ID: NCT01253642), a treatment regimen involving both phenelzine and docetaxel was administered to patients with advanced PCa. Phenelzine hinders tumour progression and potentially improves the efficacy of docetaxel. Further studies are imperative to potentially broaden the application of MAOIs in cancer therapy, facilitating their transition into clinical practice (Table 4).
9 CONCLUSIONS AND PERSPECTIVES
Serotonin (5-HT) was first identified over 7 decades ago and initially characterised as a vasoconstrictor. It is a versatile neurotransmitter and peripheral hormone, with emerging mitogenic functions in carcinogenesis. However, conflicting evidence, coupled with the complex and multifaceted neoplastic effects of the serotonergic system, poses significant challenges for repurposing serotonergic-targeted pharmaceuticals in cancer therapy. A more comprehensive understanding of the context-dependent neuro-immuno-endocrine mechanisms through which the serotonergic system modulates carcinogenesis is essential for developing future evidence based and holistic therapeutic strategies.
The pro-tumour functions of serotonin involving in proliferation, anti-apoptosis, invasiveness and angiogenesis, were initially identified, and primarily studied in in vitro systems, largely due to its mitogenic properties. Although the carcinogenic effects of serotonin are gaining momentum in recent years, contradictory findings have emerged, especially with the development of 5-HTR-specific knockout and peripheral serotonin deficient (Tph−/−) mouse models, as well as 5-HTR subtype-selective drugs. The conflicting roles of serotonin in the pathogenesis of tumour may be attributed to the fact that (1) the tissue-specific expression patterns of 5-HTRs, for example, HTR1E has been observed to be downregulated in OC patients and functions as a cancer suppressor in the context of chronic stress-promoted OC progression; (2) the dose-dependent mitogenic effects of serotonin, for example, higher doses of 5-HT stimulate cell growth, while lower concentrations induce vasoconstriction in tumour vessels, resulting in repressed tumour progression; (3) the stage of carcinogenesis and the underlying pathophysiological context, for example, 5-HT facilitated DNA repair during the early phases of colitis-dependent CRC, but promoted CRC progression in the colitis-independent CRC through a plethora of mechanisms; (4) the complicated and obscure roles of serotonin in orchestrating the TME, for example, serotonin mediated immune evasion in lung cancer mouse model by upregulating PD-L1 expression via serotonylation, whereas circumstantial evidence indicates that serotonin contributes to enhance NK cells’ cytotoxic capabilities. Moreover, the intricate biological responses to serotonin are shaped by the combined effects of multiple 5-HTRs, and the dysregulated expression patterns of 5-HTRs are often observed in the progression of certain tumours. Therefore, gaining a more profound comprehension of the underlying mechanisms of serotonin/5-HTRs axis-mediated alterations in carcinogenesis, exploring serotonylation and other modes of serotonin signalling, and elucidating the intricate interplay between serotonin and the TME possess great promise for the development of serotonergic-targeted therapies against cancer.
5-HTRs, SERT and serotonin biosynthesis/metabolism pathways are potential molecular targets in cancer-directed pharmacotherapy. 5-HT binding agents, SSRIs, 5-HT synthesis inhibitors and MAOIs offer valuable clinical options for leveraging the translational potential of serotonin-mediated tumourigenesis, given the established arsenal of these drugs.198 However, more extensive in vivo studies that incorporate tissue-specific knock out strategies are urgently needed to thoroughly evaluate the therapeutic vulnerabilities, efficacy and safety profile of these medications in cancer treatment. The advancement of highly selective drugs that singularly target individual subtypes of 5-HTRs based on their structural characteristics is essential for minimising polypharmacology and reducing the risk of side effects. It is also crucial to determine the optimal treatment regimens for different types of cancer and patient populations. With the notion of serotonergic-targeted drugs for cancer, the therapeutic landscape is gradually unfolded. Elucidating the clinical benefit and improving tailored therapeutic approaches with these drugs will necessitate evidence based and scientifically guided clinical trial designs and comprehensive endeavour to discover predictive biomarkers.
AUTHOR CONTRIBUTIONS
Writing, figure preparation, conceptualisation and critical revision: Mei Song and Weiling He. Searching literature and initial draft preparation: Lulu Chen and Shuting Huang. Editing: Xiaoxue Wu. All authors have read and agreed to the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82303923 and 82022037), the National Key Research and Development Plan (2022YFC3401000), and the Guangdong Basic and Applied Basic Research Foundation (2021B1515230009, 2024A1515013156). We are grateful to the members of our laboratory and our collaborators for their attentive review of the text and insightful feedback.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.




