Delayed Neuropsychiatric Syndrome Due to Unrecognized Carbon Monoxide Toxicity: A Case Report
ABSTRACT
Introduction
Carbon monoxide (CO) is a leading cause of poison-related deaths in children and frequently results in nonspecific neurological symptoms and imaging findings. Rarely, pediatric patients develop a delayed neuropsychiatric syndrome (DNS) following a lucid interval. Although imaging findings of early bilateral globi pallidi injury and white matter demyelination in association with DNS are most common, these findings are not always present. It is important to consider CO toxicity in patients found unresponsive without a clear etiology.
Patient Presentation
This 16-year-old boy was found unresponsive at his workplace with initial labs notable for end-organ injury. After stabilization, he had deficits in language, attention, memory, and left-sided dysmetria on neurological assessment. Imaging demonstrated injury in the bilateral caudate, putamen, hippocampi, and cerebellum, concerning for anoxic injury. His symptoms initially improved, but he developed new agitation and dyskinetic movements 6 days after presentation. His imaging continued to evolve with late enhancement in areas of prior injury and the bilateral globi pallidi. Ultimately, it was discovered that he had CO toxicity from a leaky workplace water heater.
Discussion and Conclusions
We highlight a rare presentation of DNS in a pediatric patient with CO toxicity. Our patient demonstrates the spectrum of clinical and imaging findings associated with CO toxicity and DNS. The clinical and neuroimaging features of CO toxicity are variable, making diagnosis challenging without a known exposure. It is important to maintain CO toxicity on the differential for patients presenting with unexplained neurological symptoms.
1 Introduction
Carbon monoxide (CO) toxicity is a leading cause of poison-related deaths in children and is frequently associated with neurological complications [1-6]. Early identification is critical since treatment with hyperbaric oxygen may prevent neurological sequelae [3-6]. Neurological symptoms are often nonspecific, making diagnosis challenging without exposure history [3, 5, 6]. Additionally, although rare in pediatric patients, a delayed neuropsychiatric syndrome (DNS) can develop days to weeks after initial exposure following a lucid interval [2, 4]. Neuroimaging can assist with diagnosis and typically demonstrates early bilateral globi pallidi injury and white matter demyelination in association with DNS [1, 3-5, 7]. However, imaging is variable, and the lack of these features does not exclude CO toxicity [3, 5]. There is a paucity of literature describing DNS and the associated evolving neuroimaging patterns in pediatric patients. We describe a 16-year-old boy who was ultimately diagnosed with CO toxicity and DNS. Limited initial history and his atypical neuroimaging and clinical presentation led to diagnostic delays.
2 Patient Description
This 16-year-old boy was found unresponsive in a bathroom. He did not respond to naloxone, and he was intubated for airway protection. Initial labs were notable for end-organ injury with a lactic acid of 9.8 mmol/L (ref. range 0.5–1.9 mmol/L), creatinine kinase of 594 u/L (ref. range 50–388 u/L), alanine transaminase of 80 u/L (ref. range 9–24 u/L), aspartate aminotransferase of 92 u/L (ref. range 14–35 u/L), creatinine of 1.77 mg/dL (ref range < 1.34 mg/dL), troponin of 39 ng/L (ref range < 20 ng/L), and C-reactive protein of 118.7 mg/L (ref. range 0.1–1.7 mg/L). Urine toxicology was negative. Electrocardiogram demonstrated diffuse ST-segment elevations. It was later discovered that an individual in the same building had died 1 week prior, with subsequent investigation demonstrating high CO levels due to a leaky water heater. This information was not known at the time the patient was found, and serum CO levels were not tested.
The patient's neurological assessment 2 days later was notable for dysmetric along with impaired orientation, expressive language, attention, and memory. Electroencephalogram (EEG) was normal. Cerebrospinal fluid (CSF) analysis demonstrated a pleocytosis (13 E6/L WBC, 43% neutrophils) with normal glucose, protein, immunoglobulin G (IgG) index, oligoclonal bands, and infectious studies. Brain magnetic resonance imaging (MRI) showed T2 hyperintensities in the bilateral anterior putamen, caudate, hippocampi, and cerebellar folia along with reduced diffusion in the cerebellum, but no enhancement or susceptibility (Figure 1). Anoxic brain injury from an unwitnessed cardiac arrest was suspected, prompting further cardiac evaluation. Echocardiogram and cardiac MRI were normal, and telemetry did not demonstrate arrhythmias. His neurological symptoms improved over the next few days.
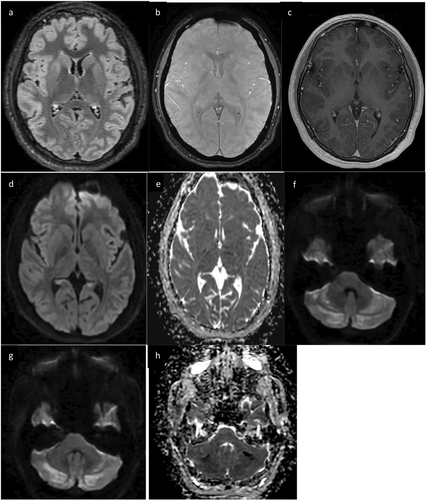
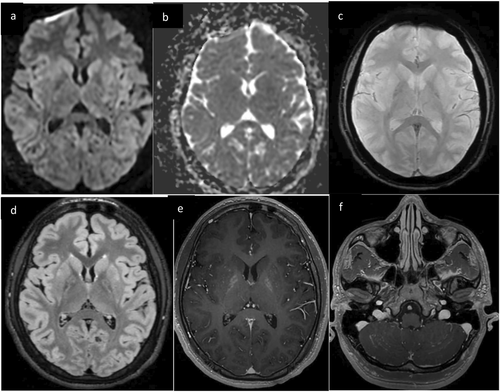
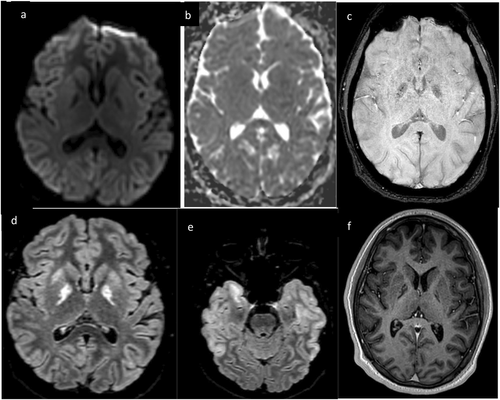
Six days after presentation, the patient developed new agitation, akathisia, chorea, and oral buccal dyskinesias, worsening perseveration, and language impairments. Repeat brain MRI demonstrated new enhancement in the cerebellar hemispheres, hippocampi, and bilateral basal ganglia including the globi pallidi (Figure 2). Repeat CSF analysis showed ongoing pleocytosis (7 E6/L WBC, 86% lymphocytes). Repeat EEG showed diffuse slowing. His new symptoms, contrast enhancement on MRI, and EEG findings raised concern for autoimmune or post-viral encephalitis due to a prodromal illness, and he was treated with pulse-dose solumedrol for 5 days. His hyperkinetic movements resolved after 3 days of solumedrol, but he had ongoing deficits in memory, attention, and language. CSF and serum autoimmune encephalitis panels were negative. MRI brain on day 13 demonstrated new supratentorial T2 hyperintensities in the bilateral temporal lobes and increased T2 signal in the globi pallidi, but resolution of prior enhancement (Figure 3). He was discharged home with plans for outpatient neurology follow-up.
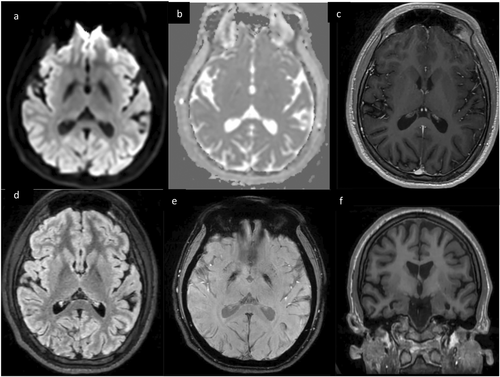
At the 4-month follow-up, his expressive language and attention had improved, but his memory impairment persisted. MRI brain showed global volume loss and worsening globi pallidi susceptibility (Figure 4). It was revealed at this time that the patient's grandfather had recently died in the same bathroom in which the patient was found. A first responder and the patient's father also developed symptoms after being in this bathroom, and the father had elevated serum CO levels. With this new information, in addition to his clinical presentation and imaging findings, CO toxicity was suspected as the etiology of the patient's symptoms.
3 Discussion
CO toxicity can present with nonspecific and evolving neurological symptoms and imaging findings. Acute toxicity manifests with headache, dizziness, syncope, and in severe cases, quick progression to coma and death [3, 5, 6]. Multiorgan involvement is common, particularly cardiac dysfunction [5, 6]. On imaging, early bilateral globi pallidi involvement is pathognomonic, related to watershed injury and direct CO binding [1, 3]. The globi pallidi may show T2 hyperintensities, diffusion restriction, or patchy enhancement within hours of exposure [1, 3, 7]. However, early neuroimaging features can be variable. Rarely, there may be early globi pallidi sparing with injury evolving over days to weeks, as demonstrated in our patient [3, 7]. Other patterns of gray matter involvement include T2 hyperintensities and diffusion restriction in the caudate, putamen, thalami, temporal lobes, hippocampi, and brainstem and cerebellum in more severe cases [1, 3, 5-7]. The areas of gray matter injury are similar to those seen in anoxic injury from other causes, making it difficult to diagnose CO toxicity based on neuroimaging without classic early globi pallidi injury.
Although 30%–50% of adults develop DNS, it occurs in only 3%–17% of pediatric patients [4, 8, 9]. DNS develops days to weeks after exposure, often following a lucid interval, and is characterized by cognitive impairments, decreased verbal fluency, memory and attention difficulties, emotional liability, psychosis, and movement disorders [2, 4, 8]. DNS is thought to be an immune-mediated process triggered by CO activation of the inflammatory cascade leading to demyelination [2, 5, 6, 8, 9]. Elevated inflammatory markers at presentation, as seen in our patient, as well as high CSF myelin basic protein are predictors for DNS development [2, 10, 11]. A similar phenomenon has been described after hypoxic injury from other causes and is also thought to be mediated by white matter demyelination from delayed excitoneurotoxicity and inflammation [12]. The inflammatory mechanism of DNS may explain the improvement in our patient's hyperkinetic movements after steroids, which was documented in a prior report [8].
White matter diffusion restriction and T2 hyperintensities are imaging features classically associated with DNS and likely relate to the demyelinating mechanism [1, 4, 5, 7]. However, white matter changes on MRI were not seen in our patient. When he developed DNS, his imaging instead demonstrated enhancement in areas of prior gray matter injury and new enhancement of the globi pallidi. Although globi pallidi enhancement in association with necrosis has been reported in CO toxicity, enhancement has not been documented in association with pediatric DNS [1]. This may relate to the proposed inflammatory mechanism and correspondingly resolved on interval imaging after pulse steroids in parallel with the patient's improved movement disorder. Additionally, the presence of imaging enhancement led us to focus our differential on infectious and inflammatory etiologies. Our patient illustrates that enhancement may be present in CO toxicity and should not preclude consideration of this diagnosis in patients with nonspecific neurological symptoms.
Unfortunately, long-term neurological deficits are common in CO toxicity and may persist more than 1 year in 20%–50% of patients [5, 6]. In patients with DNS, the prognosis is also variable, with some individuals recovering within a year and others having permanent deficits [2, 4, 8]. Late imaging findings include brain atrophy in regions corresponding to persistent neurological deficits [1, 5, 8]. Long-term sequelae from CO toxicity are frequently underrecognized and are important to address on follow-up [13].
In summary, the clinical and neuroimaging features of CO toxicity are variable, making diagnosis challenging without a known exposure. Early identification remains critical as initiating hyperbaric oxygen may improve outcomes. Initial neurological symptoms are nonspecific and can evolve in a delayed fashion. Our patient illustrates the development of DNS as a rare complication of CO toxicity in children. Despite the more common imaging presentation of early globi pallidi involvement and white matter injury associated with DNS, our patient illustrates the variability in CO toxicity neuroimaging features and their evolution. It is important to maintain CO toxicity in the differential for patients presenting with unexplained neurological symptoms and to consider interval imaging with symptom progression.
Author Contributions
Alexandria Valdrighi: conceptualization, investigation, writing – original draft, writing – review and editing, data curation. Greta Peng: conceptualization, investigation, writing – review and editing, data curation. Andreas Rauschecker: conceptualization, investigation, writing – review and editing, data curation. Mary Karalius: writing – review and editing, conceptualization, investigation, data curation, supervision.
Ethics Statement
Our institutional review board does not require committee review of case reports. Written informed consent for publication of this patient's clinical details and images was provided from the parents.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available for review at request subject to the confidentiality requirements.




