Advancing FIRES Treatment: The Potential of Intrathecal Dexamethasone
ABSTRACT
Background
Febrile infection-related epilepsy syndrome (FIRES) is a rare, devastating epilepsy syndrome characterized by refractory status epilepticus following a febrile illness.
Patient Description
We describe a 12-year-old girl with FIRES who exhibited a remarkable clinical and electrographic response to intrathecal dexamethasone (IT-DEX) after failing multiple antiseizure and immunomodulatory therapies. Despite persistent neuroinflammation, IT-DEX led to rapid seizure control and significant neurological recovery.
Conclusion
This report supports IT-DEX as a promising intervention in FIRES and underscores the need for further research into its mechanisms and long-term efficacy.
1 Introduction
Seizures that are unresponsive to medication and persist without resolution represent a particularly concerning group of conditions for both patients and healthcare providers. The severe and unpredictable nature of these cases has intensified research into new-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES). NORSE is a critical neurological disorder characterized by the sudden onset of refractory status epilepticus in patients without a history of epilepsy or significant neurological disorders, and without an identifiable structural, toxic, or metabolic cause. FIRES, a subset of NORSE, is defined by the occurrence of a febrile infection between 2 weeks and 24 h before seizure onset [1-3].
Historically, these conditions were associated with high mortality rates, with the current estimated mortality for NORSE/FIRES ranging between 12% and 20% [1]. Among survivors, approximately one-third maintain normal or near-normal cognitive function, one-third experience mild to moderate neurological impairment, and the remaining third experience severe disability or progress to a chronic vegetative state. Poor outcomes are often associated with prolonged medically induced comas during the acute phase, as well as the use of multiple anesthetics and high levels of inflammatory cytokines in both serum and cerebrospinal fluid (CSF) [4, 5]. In the chronic phase, drug-resistant multifocal epilepsy, intellectual disability, and cognitive impairment are frequently observed [5-7]. Kramer and colleagues, in their study of 77 FIRES patients, found that 12% died during the acute phase, 18% of survivors retained normal cognitive function, and 93% developed refractory epilepsy [8].
The predominant hypothesis regarding the pathogenesis of NORSE/FIRES suggests that an innate immune response triggers neuroinflammation [5]. Elevated levels of pro-inflammatory cytokines and chemokines, such as interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6), have been detected in the CSF of affected patients, and these molecules are known to have proconvulsant properties [9]. Howe and colleagues also reported on a patient with FIRES who had elevated serum levels of inflammatory markers—IL-6, chemokine (CXC motif) ligand 8 (CXCL8), high-mobility group box 1 (HMGB1), S100A8/A9, and C-reactive protein (CRP)—along with elevated CSF IL-6. Remarkably, these levels normalized after six doses of intrathecal dexamethasone (IT-DEX). They also found that this patient had a profound increase in inflammatory markers such as IL-6 and CXCL8 in response to bacterial stimulation but did not have much response to viral stimulation [10]. Hanin and colleagues also found elevated pro-inflammatory cytokines, including IL-6, TNF-α, CXCL8/IL-8, and MIP-1α, in 61 NORSE patients, including those with FIRES [4, 11].
Elevated neopterin levels in the CSF, an inflammatory marker indicative of T-helper cell type 1 and macrophage activation, further support the involvement of inflammatory-immune pathways in FIRES. This mechanism may explain the delay between the initial fever and the onset of seizures [10, 12].
Given this understanding of the pathogenesis, treatment strategies for FIRES have focused on targeting neuroinflammation. Published recommendations state that first-line immunotherapy with intravenous corticosteroids, PLEX (plasmapheresis), or IVIG (intravenous immunoglobulins) should be initiated within 72 h of seizure onset. If these treatments are not effective, then second-line immunotherapy is recommended to start within 1 week of seizure onset. These include anakinra and tocilizumab, which target IL-1 and IL-6 receptors, respectively [11]. One other second-line immunotherapy is IT-DEX. Recent studies have demonstrated promising outcomes with early administration of intrathecal steroids in up to 50% of pediatric FIRES patients [10, 13]. In Horino's study, all patients were successfully weaned off continuous antiseizure medications after a median of 5.5 days following the initiation of IT-DEX [9]. In a more recent report by Fisher and colleagues, two patients aged 5 and 7 received courses of IT-DEX beginning on day 42 and 25, respectively, with reported improvement in electroencephalogram (EEG) background/ictal-interictal continuum features and increased ability to wean off anesthetic agents [14].
We present a pediatric patient with FIRES at our institution, where early administration of IT-DEX resulted in marked clinical improvement.
2 Patient Description
This previously healthy 12-year-old girl developed new-onset seizures 5 to 7 days after a prodromal illness marked by intermittent fever, headache, and emesis.
She initially presented to an outside hospital with acute confusion and witnessed seizure-like activity. Her condition rapidly deteriorated as episodes of unresponsiveness, facial stiffening, oral automatisms, and oxygen desaturation clustered, requiring intubation for airway protection. Extensive diagnostic evaluations, including brain magnetic resonance imaging (MRI), magnetic resonance angiography, computed tomography of the chest, abdomen, and pelvis, and magnetic resonance venography were unremarkable. Serological tests (rheumatoid factor, thyroid peroxidase antibodies, and antinuclear antibodies) were normal. A thorough infectious evaluation (herpes simplex virus, Lyme disease, respiratory viral panel, and Epstein–Barr virus) was entirely unrevealing.
The initial CSF analysis revealed a white blood cell count (WBC) of 4 cells/µL, protein concentration of 58 mg/dL, and glucose of 72 mg/dL, with a negative meningitis/encephalitis panel. A repeat CSF analysis 2 days later showed a WBC of 19 cells/µL, protein of 69 mg/dL, glucose of 68 mg/dL, and lactate of 2.3 mmol/L. The meningitis/encephalitis panel remained negative. Both serum and CSF autoimmune panels were also negative.
Seizures persisted and her EEG showed diffuse background slowing as well as frequent invariant oscillations of electrographic seizures. On the quantitative EEG trends produced using Persyst, we are able to study the frequency of her seizures (Figure 1), chiefly, the only fast Fourier transform (FFT). In this spectrogram, increase in power in the higher frequency bands results in an oscillatory pattern, with the peaks appearing similar to flames of a fire, which we refer to as the “flame” sign (Figure 1). The patient's seizures were refractory to multiple antiseizure medications, including levetiracetam, lacosamide, phenobarbital, and a continuous midazolam infusion. On hospital day (HD) 2, she was started on intravenous methylprednisolone; anakinra was added on HD 3, and ketamine infusion was initiated on HD 4.
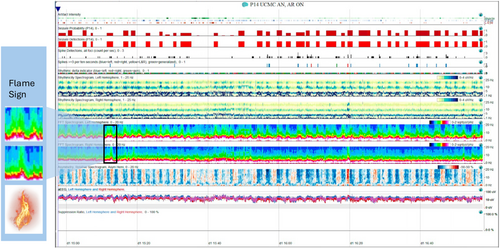
Despite escalating antiseizure treatment, her seizures persisted. Attempts to wean midazolam while continuing ketamine were unsuccessful. On HD 7, a ketogenic diet was initiated, followed by the addition of a continuous magnesium infusion on HD 8. Midazolam infusion was weaned off successfully by HD 9 as ketamine and lacosamide doses were increased.
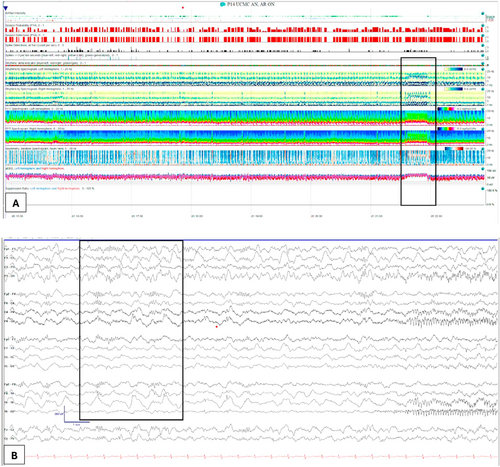
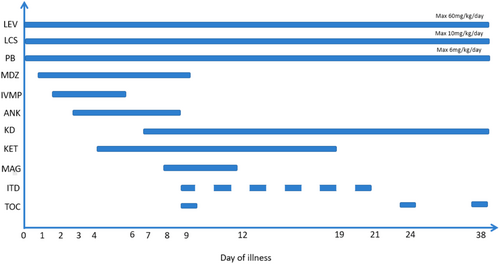
Nine days after seizure onset, anakinra was discontinued and replaced with tocilizumab. Due to ongoing status epilepticus, IT-DEX was initiated on the same day. The patient received six doses of IT-DEX between HD 9 and HD 21, at a dose of 0.25 mg/kg, using a preservative-free formulation. We administered six doses based on the methods included in the Howe et al. and Horino et al. studies [9, 10]. Serial EEGs during IT-DEX treatment showed gradual improvement starting from the first night after the first dose, including the emergence of sleep cycles and the resolution of ictal activity (Figure 2). The timeline of treatments is depicted in Figure 3. Our patient did not experience adverse effects from IT-DEX.
Initial assessments revealed elevated serum and CSF cytokine levels, as depicted in Figure 4. Of note, cytokine levels were obtained only twice during admission. The major cytokines that were elevated were IL-8, IL-10, and M1P1a.
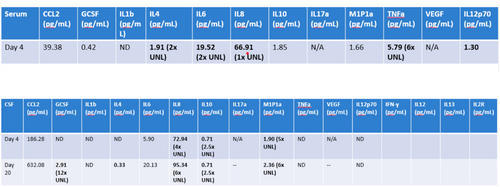
A subsequent CSF cytokine analysis collected 11 days after IT-DEX initiation demonstrated continued elevation of IL-8, IL-10, and M1P1α. This suggested ongoing inflammation even though the patient had significantly improved clinically.
Over the final 27 days of her hospital course, the patient's phenobarbital dosage was reduced due to sedative side effects, and two additional doses of tocilizumab were administered at 2-week intervals. Repeat MRI revealed cortical and deep hyperintensities with diffusion restriction. On HD 30, both a tracheostomy and a gastrostomy tube were placed. By HD 35, she showed notable clinical improvement, including increased alertness, purposeful movements, visual tracking, and verbalization of words.
The patient was discharged to acute rehabilitation on HD 48. At discharge, her medications included phenobarbital, levetiracetam, lacosamide, and cannabidiol, and she remained on a ketogenic diet. Tocilizumab was continued every 2 weeks post-discharge.
At a 3-month follow-up visit, she continued to show significant improvement. She was observed walking vigorously on a treadmill, waving to her mother, and responding to questions appropriately; however, she continued to have short-term and long-term memory problems. She had been seizure-free for about 5 months before developing breakthrough seizures while being weaned off phenobarbital. Theseizures included behavioral arrest, oral automatisms, and left gaze deviation, which were documented on EEG to have a left temporal onset. She continues to have brief once-weekly seizures, although provoked and unprovoked clustering did occur. She was maintained on levetiracetam, lacosamide, clobazam, and the ketogenic diet. She was able to have her tracheostomy decannulated 7 months after seizure onset.
3 Discussion
The remarkable improvement observed in our patient highlights the potential of IT-DEX in managing both FIRES and NORSE. Before IT-DEX treatment, she exhibited elevated serum levels of IL-4, IL-6, IL-8, TNF-α, and IL-12p70, indicating an inflammatory state with immune dysregulation. CSF analysis similarly revealed elevated IL-6, IL-8, M1P1α, and IL-2 receptor levels. This is a well-known common finding in patients with either FIRES or NORSE.
Despite the initial administration of anakinra, which targets IL-1 activity, our patient showed minimal improvement. Elevated IL-1 levels were also absent in both CSF and serum. However, the presence of elevated IL-8, a downstream product of IL-1β, suggested heightened IL-1β activity that may not have been captured at the time of analysis. The concurrent elevation of IL-8 and M1P1α indicated potential dysfunction of innate immunity. Therefore, it is unclear whether treatment with anakinra failed.
Following the administration of six doses of IT-DEX, our patient exhibited both clinical and electrographic improvement. The mechanism by which IT-DEX can be used to treat both FIRES and NORSE remains under investigation. Corticosteroids bind to glucocorticoid receptors, which dimerize and translocate to the nucleus, influencing gene transcription. They activate anti-inflammatory genes such as annexin A1 while suppressing those involved in cytokine and chemokine production [10]. Dexamethasone may also reduce monocyte and neutrophil binding to the endothelium through annexin A1 upregulation. It protects the blood-brain barrier, modulates neurotransmitter release, reduces brain edema, and inhibits corticotropin-releasing hormone production [10].
Posttreatment, the patient continued to exhibit elevated CSF cytokines and chemokines, including G-CSF, IL-4, IL-8, IL-10, and M1P1α, suggesting that IT-DEX did not fully resolve innate immune dysfunction, a finding that has also been documented recently by Fisher and colleagues [14]. Similarly, in Horino and colleagues' cohort of patients who received IT-DEX but not anakinra or tocilizumab, clinical improvement occurred despite ongoing elevations in a subset of cytokines, suggesting that IT-DEX may still be beneficial and possibly modify other immune-mediated pathways not captured on routine cytokine panels [9]. Kothur and colleagues studied inflammatory cytokines in children with FIRES, febrile status epilepticus, chronic epilepsy, and afebrile status epilepticus. Children with FIRES had abnormal cytokines that were associated with T-helper cell type 1, such as CXCL10 [15]. It is also recognized that not all patients with cryptogenic NORSE/FIRES display identical cytokine profiles (as mentioned by Hanin and colleagues [4]), suggesting variable underlying etiologies.
A potential confounding factor in our patient was the concurrent administration of tocilizumab with the first dose of IT-DEX. While EEG improvement was initially observed following both medications, the subsequent improvements seen on her EEG with each dose of IT-DEX suggested that the dexamethasone was the primary driver of clinical improvement. It is also possible that our patient had a positive outcome simply because that was the trajectory of her illness. FIRES and NORSE have a wide range of possible outcomes, and our patient might have had this outcome even without aggressive treatment. Further research needs to be done to learn more about treatment options and long-term outcomes for patients with FIRES/NORSE.
Limitations include the inability to measure posttreatment serum cytokine and chemokine levels, which would have provided further insight into the systemic effects of IT-DEX. Demonstrating reduced systemic inflammation following treatment could have further supported its impact on innate immune dysfunction.
Our patient continues to recover and make gains toward her pre-illness neurological baseline. Early intervention with IT-DEX may offer significant benefits in this challenging and often devastating condition, though long-term follow-up is essential to fully assess cognitive outcomes and overall recovery.
The NORSE/FIRES biorepository is supported by the NORSE/FIRES Research Fund at Yale and the NORSE Institute (for more information, see https://www.norseinstitute.org or email [email protected]).
Author Contributions
Carol Park: conceptualization, investigation, writing – original draft, data curation. Douglas R. Nordli III: writing – original draft, writing – review and editing. Mohamed Taha: investigation, writing – original draft. Henry David: investigation, writing – original draft. Shawn Kacker: investigation. Tareq Kass-Hout: investigation. Sonam Thind: investigation. Aurelie Hanin: investigation. Sana Said: investigation. Hiba A. Haider: supervision, resources, formal analysis, conceptualization, investigation, writing – original draft. Douglas R. Nordli Jr.: conceptualization, investigation, methodology, formal analysis, supervision.
Ethics Statement
This manuscript preparation and data collection was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accessibility Act compliant manner. This report did not require approval by our institutional review board for human research. The patient's family provided verbal and written consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, DN, upon reasonable request.




