Salvage Trans-Sylvian Peri-Insular Hemispherotomy After Embolic Hemispherectomy
ABSTRACT
Background
Hemispherectomy and hemispherotomy represent well-established treatments for drug-resistant hemispheric epilepsy. An alternative endovascular procedure has been explored for cases with challenging surgical anatomy, which seeks to achieve the clinical effect of hemispherectomy via embolization of the major cerebral arteries and subsequent hemispheric infarction. Neither the safety nor effectiveness of this procedure has been established.
Patient Description
A 4-month-old girl with a history of drug-resistant focal epilepsy due to left-sided hemimegalecephaly previously treated with endovascular hemispherectomy at another institution presented for surgical evaluation due to ongoing electroclinical seizures despite multiple antiseizure medications. Pre-operative magnetic resonance imaging (MRI) revealed viable tissue, including mesial temporal structures, and a salvage hemispherotomy was performed. The embolized cortex was surprisingly well-perfused intra-operatively. Postoperatively, she has had no further seizures at 1-year follow-up.
Conclusion
Embolization of the three large hemispheric arteries achieved neither complete hemispheric destruction nor complete disconnection in this case and did not resolve the patient's seizures, necessitating salvage hemispherotomy. While it is difficult to draw definitive conclusions from a single patient's course, our experience suggests that endovascular hemispheric destruction may not be an effective substitute for surgical hemispherectomy or hemispherotomy.
1 Introduction
Hemispherectomy and hemispherotomy represent well-established interventions for the treatment of patients with drug-resistant hemispheric epilepsy. Anatomic hemispherectomy achieves seizure control via removal of the parenchyma of the epileptogenic hemisphere, whereas hemispherotomy does so via disconnection of all seizure-causing structures (neocortex and archicortex) of the affected hemisphere, thereby isolating seizure activity from the deep structures, brainstem, body, and opposite hemisphere [1, 2]. Anatomic hemispherectomy has been associated with longer hospital stays and higher risk of shunt dependence, blood loss, and complications compared to hemispherotomy in retrospective studies [3].
Among hemispherotomy indications, congenital brain malformations, such as hemimegalencephaly, are thought to have some of the lowest seizure freedom rates [4], due to both the possibility of dysplasia in the contralateral hemisphere as well as more challenging surgical anatomy which may predispose to incomplete disconnection. Furthermore, some babies with hemimegalencephaly present with refractory seizures at a very young age, which increases the risk of hemodynamic collapse during surgery. Another challenge at that age can be the poor turgor of the pia, rendering subpial dissection more difficult.
Due to these obstacles, some have explored the possibility of achieving hemispheric destruction by endovascular techniques for patients with hemimegalencephaly. The cerebral hemispheres are supplied chiefly by three major vessels: the anterior, middle, and posterior cerebral arteries (ACA, MCA, PCA). The rationale for this procedure is that endovascular embolization of these three arteries, distal to the perforating branches supplying deep brain structures, might infarct the damaged hemisphere and thereby abrogate seizure activity. This strategy was initially pursued 30 years ago in a single case, in which it achieved a 1-year period of seizure freedom before a subsequent hemispherectomy was performed. Intra-operative electrocorticography done at that time demonstrated persistent, frequent sharp wave activity in the embolized tissue, which was then resected [5]. More recently, more favorable outcomes have been reported in a small case series, with total seizure freedom in three patients with limited follow-up duration [6]. Given the small number of patients reported in the literature, little is known about the safety and effectiveness of this procedure.
Here, we present the case of a 4-month-old girl with drug-resistant epilepsy due to hemimegalencephaly who had previously undergone an endovascular hemispherectomy, which had been unsuccessful in controlling her seizures.
2 Case Report
A 4-month-old girl with hemimegalencephaly and drug-resistant epilepsy status post endovascular hemispherectomy presented for surgical evaluation of ongoing seizures.
She initially presented to the emergency department of another institution at 30 days of age with seizures. At that time, her ictal semiology consisted of left greater than right eyelid jerking. Long-term electroencephalography (EEG) at that time revealed frequent focal medium-to-high voltage epileptiform sharp waves over the left hemisphere and two electroclinical seizures with left-sided spike-and-wave discharges with associated right leg jerking. A brain MRI obtained at that time revealed left-sided hemimegalencephaly. She was treated with phenobarbital, levetiracetam, and lacosamide and discharged on levetiracetam and lacosamide, which failed to control her seizures. She developed infantile spasms 1 week later.
Shortly thereafter, she underwent a series of five staged embolizations over the course of 2 months: (1) left PCA, (2) branches of the left MCA, (3) the remainder of the left MCA, (4) left ACA, and (5) a revision for part of the temporal lobe. After her last embolization, she exhibited some clinical seizures, and EEG revealed frequent subclinical seizures originating from the infarcted left hemisphere despite treatment with oxcarbazepine 44 mg/kg/day, levetiracetam 35 mg/kg/day, phenobarbital 5 mg/kg/day, and vigabatrin at 136 mg/kg/day.
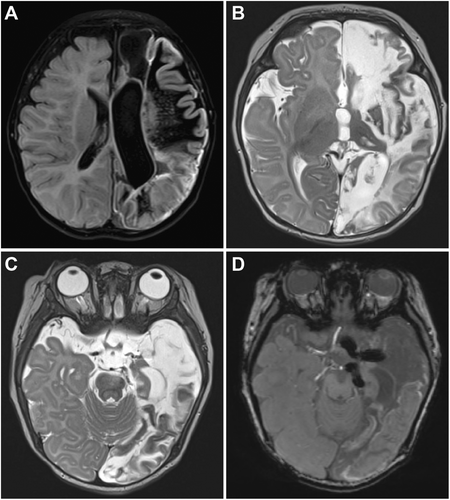
At the age of 4 months, she presented to Children's Hospital of Philadelphia for hemispherotomy. Neurological exam revealed right-sided weakness, hypertonia, and hyperreflexia consistent with expected hemispherectomy deficits. MRI performed at that time revealed extensive encephalomalacia, gliosis, and cortical laminar necrosis throughout most of the left hemisphere, as well as evidence of coils in the major cerebral arteries, consistent with her prior embolization procedures (Figure 1). The hemimegalencephalic cingulate and mesial temporal structures appeared completely unaffected, presumably due to collateral flow through the anterior communicating artery to the ipsilateral ACA and an unembolized anterior choroidal artery, arising proximal to the internal carotid artery (ICA) bifurcation. In a multidisciplinary epilepsy surgery conference, a consensus was reached to recommend a surgical hemispherotomy.
She was 4 months old and 7.2 kg on the day of surgery. Through a 6-cm linear incision, a 35-mm craniotomy was made over the Sylvian fissure. The tissue appeared surprisingly well-vascularized, more so than would be expected based on the prior embolization procedure and the pre-operative imaging (Figure 2A). The temporal lobe was much more well-vascularized than the frontoparietal operculum. The Sylvian fissure was split widely and the disconnection was performed using a trans-Sylvian peri-insular method [7]. During the procedure, the proximal ACA, MCA, and PCA were all encountered with easily identifiable intraarterial coils covered by a very thin arterial wall (Figure 2B). Great care was taken when handling these structures, as the thin arterial walls appeared delicate and liable to rupture with manipulation. Examination under the surgical microscope revealed the embolizations to be complete, as no blood was observed in the vessels in the segments occupied by coils. Of note, the proximal cerebral arteries were surrounded by a robust venous network, possibly reactive in nature due to the coiling. Bleeding from these was minimized with thrombin-soaked cottonoid patties. Much of the cortex of the embolized hemisphere was noted to be vascularized and bled, in particular, the lateral temporal neocortex (Figure 2C). The only region observed to be truly devoid of bleeding vessels was the frontoparietal white matter. The amygdala, hippocampus, and cingulate appeared particularly healthy, consistent with their appearance on MRI. Before surgery, the patient was anemic with a hemoglobin of 10.0. Early in the surgery, her hemoglobin had declined to 8.8 as a consequence of the dilutional effect of crystalloid. With the prospect of further blood loss during surgery, a 10 cc/kg blood transfusion was given, and her final hemoglobin was 10.5. The estimated blood loss was 20 cc.
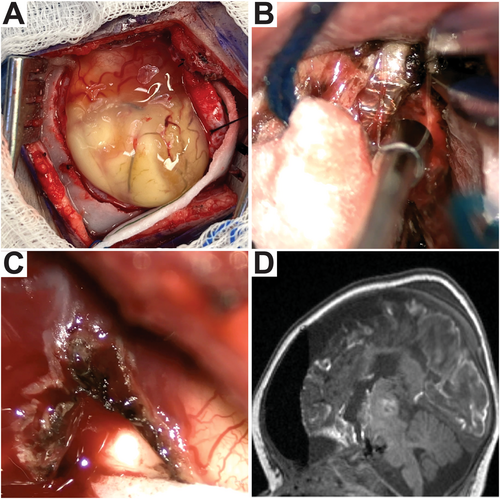
She readily woke up at her pre-operative neurologic baseline. Postoperative MRI revealed complete disconnection (Figure 2D), and she underwent CSF drainage for 3 days per institutional routine. She was discharged home on the sixth postoperative day, with outpatient physical and occupational therapy services already having been in place. At a 2-month follow-up visit, she had been seizure-free since surgery. She was moving her right leg nearly as well as her left and had begun making some purposeful movements with her right arm. This right-sided weakness is consistent with expected deficits following hemispherotomy. At 1-year follow-up, she was seizure-free.
3 Discussion
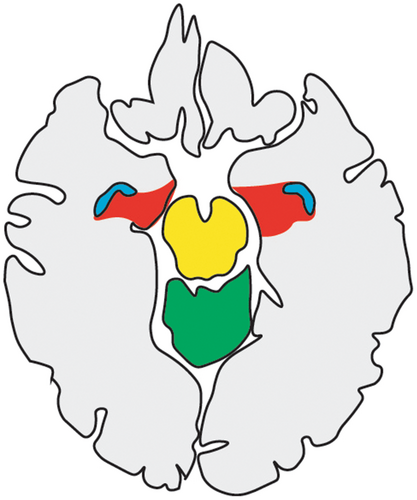
Despite this patient having undergone complete embolization of the three major cerebral arteries, as confirmed on MRI and under direct microscopic examination, much of the left hemimegalencephalic hemisphere appeared viable. Some of these findings were expected and some unexpected. The anterior choroidal artery is proximal to the ICA bifurcation and supplies mesial temporal structures, often including the uncus, hippocampus, dentate gyrus, and posteromedial part of the amygdala (Figure 3) [8]. It was therefore not unexpected that these structures appeared healthy radiographically and under the microscope. Similarly, a proximal ACA embolization may still allow arterial flow from the contralateral ACA through the anterior communicating artery into the ipsilateral ACA, so the healthy appearing ipsilateral cingulate was not unexpected either. This patient's post-embolization EEG revealed left-sided seizure activity despite maximal antiseizure medications, and the parenchyma beyond the cingulate and mesial temporal structures was noted to be well-vascularized intra-operatively. The extent of viability and vascularity appreciated intra-operatively was out of proportion relative to the encephalomalacia demonstrated on postembolization, presurgical MRI. One possible explanation is robust revascularization via collaterals from the contralateral circulation including the anterior cerebral artery, the ipsilateral anterior choroidal artery, or perforators originating at the circle of Willis. While the cerebral blood supply was compromised to the point of yielding significant tissue damage and disruption of normal function, it was not reduced to levels necessary for the complete death of all the epileptogenic dysplastic tissue. The damaged tissue likely lost its functional properties, as demonstrated by her post-embolization, pre-operative right hemiparesis and left gaze preference, but retained the ability to generate seizures, as demonstrated by post-embolization, pre-operative EEG studies and clinical seizures. This is consistent with the case report of the first endovascular hemispherectomy from 30 years ago, which confirmed epileptiform activity in the post-embolization encephalomalacic cortex with intra-operative electrocorticography [5].
While it has been reported that the endovascular hemispherectomy strategy may serve as a bridge to surgery in young patients [5, 9], the embolizations in this case did not address the patient's epilepsy, did not clearly increase the ease of surgery and may have presented intra-operative challenges. The proximal embolized vessels were surrounded by a robust and unusual venous network, which may have been reactive to the embolization. Manipulation of these vessels therefore resulted in an oozing-type bleeding, which necessitated increased care on the part of the surgeon to prevent serious blood loss. Methodical and liberal application of hemostatic agents was therefore necessary to maintain hemostasis in this small baby. Furthermore, the arterial walls around the intravascular coils were thin and translucent, appearing as fragile as a layer of arachnoid. There was concern for the integrity of these structures and therefore the possibility of catastrophic bleeding with manipulation. Greater than normal care was warranted in navigating these vessels intra-operatively.
Given the novelty of the endovascular hemispherectomy, it remains questionable whether this procedure is likely to achieve durable seizure freedom. Based on this and the initial case of follow-up surgery in a patient who had undergone an endovascular hemispherectomy, it appears unlikely that this strategy achieves complete destruction of epileptogenic structures of the hemisphere. Such an outcome may be considered analogous to an incomplete hemispherectomy. Consistent with this, a prior case series and a series of two patients treated with embolizations as a bridge to anatomic hemispherectomy revealed epileptiform activity in the embolized hemispheres before surgery [5, 9]. Furthermore, the vascular territories embolized would not be expected to destroy commissural fibers, such as the corpus callosum and anterior commissure, in their entireties. The surgical literature argues that an incomplete hemispherectomy and an incomplete hemispherotomy are unlikely to achieve durable seizure freedom [2, 10, 11]. Such findings can likely be extrapolated to the endovascular hemispherectomy.
The microhemorrhages present in the cerebellum on the postembolization, pre-operative MRI were almost certainly of no consequence to this patient but serve as a reminder that extensive embolic procedures in the very young can be technically challenging and are not without risk. Further, the finding of embolic material in the distal arterial tree (Figure 2) suggests that this material could cross the anterior communicating artery to the contralateral ACA territory. Unintended contralateral or brainstem stroke could be a catastrophic outcome, especially in a patient in whom one hemisphere is already being killed. These risks should be considered despite the minimally invasive nature of endovascular treatments.
4 Conclusion
Endovascular embolization with successfully completed occlusion of the ACA, MCA, and PCA did not achieve complete destruction of epileptogenic structures in the affected hemisphere in this patient. Blood flow sufficient to sustain pathologic epileptogenic activity was preserved, presumably due to collateral circulation and revascularization. Tissue that appears encephalomalacic on imaging may retain some degree of blood flow and remain epileptogenic. Although it is difficult to extrapolate from a single patient, our experience suggests that endovascular hemispheric destruction may not be an effective substitute for surgical hemispherectomy or hemispherotomy.
Author Contributions
Michael E. Baumgartner: conceptualization, writing – original draft, writing – review and editing. Sudha Kessler: conceptualization, writing – review and editing, methodology, investigation. Kathleen Galligan: writing – review and editing, investigation. James E. Baumgartner: writing – review and editing. Benjamin C. Kennedy: conceptualization, methodology, investigation, writing – original draft, writing – review and editing.
Conflicts of Interest
The authors declare no conflicts of interest.




