Complex epilepsy phenotype associated with chromosome 2q24.2-q24.3 deletion involving sodium channel gene cluster
Rima Madan and Fiorella S. Guido are co-first authors who contributed equally to this work.
Abstract
Objective
The 2q24.2-24.3 chromosome region encodes sodium channel genes important in severe childhood epilepsy, notably SCN1A linked to Dravet syndrome (DS). However, the roles of other genes, either within the SCN cluster or in the segments proximal to it, have not been clearly delineated. The combination of ketogenic diet and fenfluramine is known to provide substantial benefits to patients with DS, but there is a paucity of literature regarding its role in other developmental and epileptic encephalopathies (DEE). This report aims to further explore clinical findings and treatment outcomes in a patient with a complex epilepsy phenotype.
Methods
Our patient's extensive diagnostic evaluation revealed 14 deleted genes within the 2q24.2-24.3 chromosome region.
Results
The patient initially presented with clusters of focal motor seizures with apnea and cyanosis requiring intubation as well as prolonged hospitalizations for status epilepticus. He has baseline hypotonia, dysphagia, and developmental delay. He had a >50% reduction in his seizures following a combination of ketogenic diet and fenfluramine. His seizures are now responsive to rescue midazolam, and he no longer has status epilepticus.
Interpretation
Our patient's remarkable clinical improvement suggests that this dual therapy may be beneficial in patients with DEE exhibiting pathogenic variations in this region of chromosome 2, beyond just DS.
Introduction
The 2q24.2-q24.3 chromosome region encodes neuronal sodium channel (SCN) genes, which are important in severe childhood epilepsy phenotypes such as Dravet syndrome (DS) and epilepsy of infancy with migrating focal seizures (EIMFS).1 DS is an infantile-onset developmental and epileptic encephalopathy (DEE) characterized by neurodevelopmental delay and treatment-resistant seizures, often exacerbated by fever.2 The majority of DS patients have SCN1A loss-of-function variants. While many typical cases of DS do not involve SCN2A and SCN3A, complex epilepsy phenotypes such as EIMFS are associated with deletions of the whole SCN gene cluster.3 However, the role of other genes within the cluster (SCN7A and SCN9A) or in segments proximal to it have not clearly been delineated.3 Due to the large size of chromosome 2, partial monosomy of the long arm yields many specific phenotypes, including delayed growth, intellectual disability, early myoclonic seizures, and dysmorphic facial features.1
Treatment options for DEE are limited. These patients often have medically refractory epilepsy, even with multimodal therapy.1 The ketogenic diet is known to be useful in cases where pharmacological treatment or surgery was ineffective. The anticonvulsant mechanisms of the ketogenic diet are based on the concept that ketone bodies act as an alternative source of energy in the brain instead of glucose. Brain tissue exposed to ketosis is thought to be more resistant to metabolic stress, thus increasing the seizure threshold.4
Another promising therapy emerging for treatment-resistant seizures is the drug fenfluramine. In 2020, fenfluramine was approved for treatment of DS in the United States and Europe based on the results of three clinical trials that demonstrated reduction in the number of monthly convulsive seizures by 54%–65% compared with placebo.5 However, there is limited information about the potential efficacy of utilizing both ketogenic diet and fenfluramine in patients with complex epilepsy phenotypes involving larger deletions of chromosome 2.
In this report, we examine the extent that our patient's 2q24.2-q24.3 gene deletions contribute to his medically refractory epilepsy. We suggest that the combination of fenfluramine and ketogenic diet may be a valuable adjunctive treatment for patients with DEE involving the 2q24.2-q24.3 chromosome region.
Methods
Extensive genetic, metabolic, and electrodiagnostic studies were undertaken. An SNP microarray showed a 4259 kbp deletion of the long arm of chromosome 2 at band q24.2-24.3 in 98% of our patient's cells. The 14 deleted genes included SCN1A, SCN2A, SCN3A, SCN7A, SCN9A, IFIH1, GCA, KCNH7, FIGN, COBLL1, GRB14, SLC38A11, GALNT3, and TTC21B. Additionally, a 118 kbp deletion of the short arm of chromosome X at band p11.23 was also detected, including ZNF630 and SSX6.
Case Presentation
This 2-year-old boy was born at 39 weeks gestation from nonconsanguineous parents via in vitro fertilization and a surrogate pregnancy. The pregnancy was complicated by placenta previa that self-resolved. He was born via Caesarean delivery due to breech presentation. After birth, he had transient hypoxemia and was observed in the NICU for one night without complications. He has no evidence of prior central nervous system infection, head trauma, or relevant neurological family history.
His seizures began as febrile seizures at 6 months of age and became intractable. At his worst, he had clusters of focal motor seizures occurring up to 60 times a month. His seizures are characterized by vocalization accompanied by bilateral arm and leg stiffening and rhythmic shaking, followed by hypoxia and apnea for 30 s up to 2 min. These seizures were exacerbated by body temperature fluctuations. His seizures persisted through trials of levetiracetam, topiramate, phenobarbital, cannabidiol, and valproic acid. Titration of clobazam resulted in reduction of seizures to approximately 10 seizures per month. However, he developed excessive somnolence and tolerance to benzodiazepines, making rescue medications less effective. He had repeated status epilepticus characterized by clusters of seizures accompanied by apnea every three to four weeks requiring hospitalization and intubation.
At 20 months, he started the classic ketogenic diet at a 4:1 ratio and maintained adequate ketosis. At 21 months he was started on fenfluramine, and the dose was subsequently optimized. His seizures have reduced to clusters of three to four seizures every four to 6 weeks (>50% reduction) that are no longer accompanied by apnea and are responsive to rescue midazolam. He no longer requires hospitalizations for status epilepticus. This has led to a significant improvement in both his and his family's quality of life. He has profound global developmental delay. He is nonverbal and nonambulatory. He has significant hypotonia as well as dysphagia resulting in gastrostomy tube dependence.
Laboratory evaluation with plasma amino acids, creatinine kinase, and lipid panel was normal. Magnetic resonance imaging of the brain did not show a structural etiology for his seizures and was otherwise unremarkable.
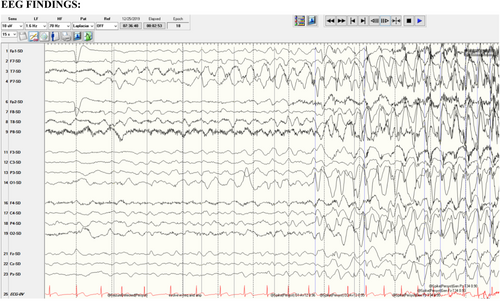
Our patient's EEG (Figure 1) documented focal seizure activity over the left parietal region when he was 11 months old. Clinically, the patient was awake and had sudden picking at the blanket with his right hand. The seizure lasted 38 seconds.
Discussion
Based on other individuals reported in the literature, the extent of the 2q24.2-q24.3 deletion likely plays a role in the severity of our patient's presentation. Patients who have different deletions within this region of chromosome 2 have demonstrated a variety of clinical presentations (Table 1), including generalized hypotonia occurring with or without seizures.7 Patients who exhibit larger deletions of the 2q24.3-q31.1 region have been reported to have atonic and hypomotor seizures.1 Most cases of DS arise from SCN1A loss-of-function variants. However, identifying other genes that may contribute to the complexity of the patient's epileptic phenotype may help guide additional therapeutic strategies.8 Precision medicine and targeted gene therapy can offer higher therapeutic value for our patients, allowing us to set a new standard for treatment of devastating DEE such as DS.5
| Patient 13 | Patient 23 | Patient 33 | Patient 43 | Patient 53 | Patient 66 | Patient 77 | Our patient | |
|---|---|---|---|---|---|---|---|---|
| Age of onset | 3 mo | 1 mo | 2 mo | 7 mo | 4 mo | 2 mo | 9 mo | 6 mo |
| Age at last follow-up | 5 yr 8 mo | 2 yr 5 mo | 1 yr 3 mo | 2 yr 9 mo | 9 yr 8 mo | 3 mo; died | 9 mo | 1 yr 9 mo |
| Hypotonia | − | − | − | − | − | + | + | + |
| Seizure type | MF, autonomic | MF, My, TC | MF, HC, autonomic | TC fever HC fever | HC, TC | EIMFS | None | FM |
| Multifocal EEG | + | + | + | − | + | + | + | + |
| Seizure outcome | Refractory | Refractory | Refractory | Response to LEV | Refractory | Refractory | N/A | Refractory |
| Brain MRI | Progressive diffuse atrophy | Mild diffuse atrophy | Mild diffuse atrophy | Normal | Mild diffuse atrophy | Mildly enlarged ventricles, age-delayed myelination | Normal | Normal |
| Chromosomal deletion | 2q24.3 | 2q24.3 | 2q24.3 | 2q24.3 | 2q24.3 | 2q22.1-q33.3 | 2q24.2-q24.3 | 2q24.2-q24.3 |
| Deletion size by CGH array | 8.4 Mb | 4.3 Mb | 1.5 Mb | 0.2 Mb | 0.2 Mb | 10.4 Mb | 5.2 Mb | 4.259 Mb |
| Deleted genes | SCN1A, SCN2A, SCN3A, SCN7A, SCN9A | SCN1A, SCN2A, SCN3A, SCN7A, SCN9A | SCN1A, SCN2A, SCN3A, SCN7A, SCN9A | SCN1A | SCN1A, SCN9A | SCN1A, SCN2A, SCN3A, SCN7A, SCN9A, GRB14, COBLL1, GALNT3, +44 other genes |
SCN2A, SCN3A, ITGB6; TBR1; SLC4A10; KCNH7 | SCN1A, SCN2A, SCN3A, SCN7A, SCN9A, IFIH1, GCA, KCNH7, FIGN, GRB14, COBLL1, SLC38A11, GALNT3, TTC21B |
- Abbreviations: CGH, comparative genomic hybridization; EIMFS, epilepsy of infancy with migrating focal seizures; FC, focal clonic seizure; FM, focal motor seizure; HC, hemiclonic seizure; MF, multifocal seizure; My, myoclonic seizure; LEV, levetiracetam; TC, tonic-clonic seizure.
Fenfluramine has demonstrated robust efficacy for seizure reduction in DS and has shown encouraging results in other genetically mediated syndromes, such as Lennox-Gastaut syndrome, CDKL5 epilepsy, and sunflower syndrome.9 The mechanisms of action are under active investigation; however, it is known that fenfluramine is a potent serotonin-releasing agent with activity at several 5-HT receptor subtypes.5 Reports in zebrafish models of DS specifically show a significant reduction in dendritic branching of GABAergic neurons. In these experiments fenfluramine restores dendritic arborization, suggesting its potential for reducing seizure activity.5 It is generally a well-tolerated medication, and the literature supports fenfluramine's favorable risk-benefit profile.5
Our patient's quality of life markedly improved with the combination of fenfluramine and ketogenic diet. The severity of DEE can be burdensome to patients and their families. The addition of therapies that are safe, effective, and cost-friendly can be largely impactful. Such therapies may reduce the incidence of complications such as cognitive dysfunction, prolonged hospitalizations, status epilepticus, and sudden unexpected death in epilepsy.
Our patient's findings contribute to the literature suggesting that segments near and within the sodium channel gene cluster may have important clinical implications in patients with complex epilepsy phenotypes. This individual's positive response to fenfluramine and the ketogenic diet suggests that this dual therapy may benefit patients with DEE involving the 2q24.2-q24.3 chromosome region, beyond just DS.
Author Contributions
Rima Madan and Fiorella Guido: Acquisition and interpretation of the data, writing the manuscript, and creating figures. Nicole Brescia: Supervising physician, responsible for interpretation of data, editing manuscript, and overseeing case report. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.




