Hereditary sensory autonomic neuropathy type VI in the age of genetic testing
Abstract
Background
Hereditary sensory and autonomic neuropathy type VI (HSAN VI) is a rare recessive genetic disorder caused by mutations in the human dystonin (DST) gene. We report a novel homozygous alternate transcript mutation in the DST gene causing a severe neonatal form of HSAN VI.
Patient Description
This baby boy was born with severe hypotonia, respiratory distress, dysmorphic features, and bilateral club feet. Imaging, karyotyping, Prader–Willi assay, spinal muscular atrophy genetic panel and myotonic dystrophy genetic panel were all negative. A comprehensive neuropathy panel detected a homozygous pathogenic variant in the DST gene—alternate transcript NM_015546.4:c.1357G>A (p.Trp4525*). Nerve conduction studies revealed mixed axonal and demyelinating sensorimotor neuropathy, suggesting the possibility of motor involvement in severe forms of this rare condition. The infant ultimately developed sepsis and died from cardiorespiratory arrest. Neuropathological findings of focal and mild spinal nerve axonal degeneration were nonspecific.
Conclusion
Collective analysis of these patients would help to further characterize the spectrum of disease pathology and could provide insight into the neurophysiology and neuropathology of this rare condition.
Introduction
Hereditary sensory neuropathies, also known as hereditary sensory and autonomic neuropathies (HSANs), are a group of disorders with a variable spectrum of involvement of sensory, motor, and autonomic disturbances.1 These are genetically and clinically heterogeneous types of disorders and are classified into eight groups.2
HSAN VI is a recessive genetic disorder caused by mutations in the human dystonin gene (DST, previously known as bullous pemphigoid antigen 1) located on the short arm of chromosome 6. It is now recognized as a spectrum disorder. Studies show that DST mutations affect the actin cytoskeleton organization and function, thereby delaying cell adhesion, spreading, and migration, possibly interfering with the neuronal outgrowth and guidance processes.3 There are three major neuronal isoforms of dystonin: dystonin-a1, -a2, and -a3.4 Each isoform dictates cellular localization and function.5 The heterogeneity of HSAN VI is believed to be related to the number of dystonin isoforms that the mutation affects. However, the absence of isoform dystonin-a2 is thought to be the universal determinant of HSAN VI.3 Compensatory mechanisms by other intact dystonin-a isoforms may play a role in the heterogeneity of this rare disorder, but this has yet to be determined.3
We report for the first time in literature a homozygous alternate transcript mutation in the DST gene causing HSAN VI. This article may provide insights into the genetic mutation, clinical manifestation, neurophysiology, and neuropathology of HSAN VI.
Patient Description
A neonate with severe hypotonia, dysmorphic features, and club feet
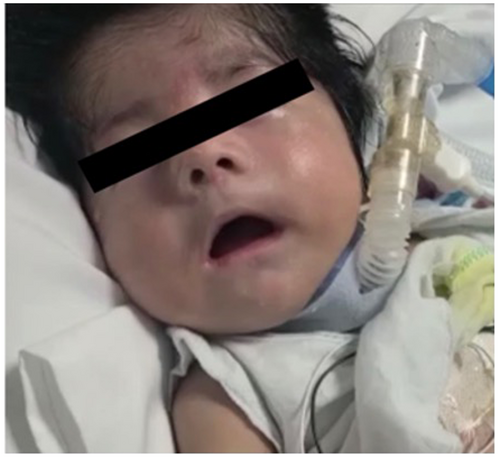
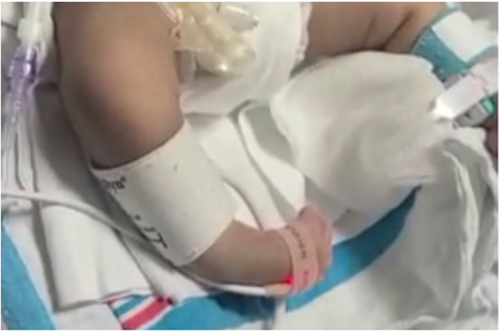
This boy of Hispanic descent was born via spontaneous vaginal delivery with no cry, severe hypotonia, respiratory distress, dysmorphic features, and bilateral club feet. There is distant consanguinity between parents. He was admitted to the neonatal intensive care unit for hypotonia and respiratory support. On evaluation (Figures 1 and 2), the baby had frog-leg posture, fish-mouth appearance, bilaterally flattened nasolabial folds, high arched palate, hypertrichosis on his extremities and trunk, synophrys, long philtrum, and dysmorphic ears. Examination also revealed wide-spaced nipples, bilateral club feet, and a contracted right thumb. On neurological exam, the baby had severe hypotonia and weakness, minimal spontaneous movement of his extremities, diffusely absent reflexes, mute plantars, poor suck reflex, and a poor plantar and palmar grasp. His mother's examination was negative for percussion and grip myotonia. Head ultrasound and magnetic resonance imaging of the brain without contrast were both unremarkable. Spinal muscular atrophy genetic panel (Invitae) was negative. Karyotyping, Prader–Willi assay, and a myotonic dystrophy genetic panel were also negative. New York state newborn screening was also normal. A comprehensive neuropathy panel detected a homozygous pathogenic variant in the DST gene—alternate transcript NM_015546.4:c.1357G>A (p.Trp4525*). The variant detected in our patient creates a premature translational stop signal (p.Trp4525*) that is expected to result in an absent or disrupted protein product. Parental testing confirmed autosomal recessive inheritance pattern.
Nerve conduction studies (NCS)/needle electromyography (EMG) were performed in both arms and the left leg. NCS revealed severely reduced left median compound motor action potential with prolonged onset latency. There were no elicitable motor responses from right median, left tibial, peroneal, and ulnar nerves; absent sensory response was observed on the right and left median and left superficial peroneal nerves. EMG testing was normal except for large motor units for the left flexor pollicis, which is suggestive of neurogenic changes. The pattern of absent response in NCS is suggestive of sensorimotor neuropathy, which could be due to either neuropathy with severe axonal loss or demyelination with secondary axonal loss.
The infant remained unstable during the 2.5-month hospital stay and had multiple daily episodes of apneas, bradycardia, and blood pressure instability. The infant ultimately developed sepsis and died from cardiorespiratory arrest. Neuropathological findings at autopsy demonstrated mild focal spinal nerve axonal degeneration. Some neurons in the spinal cord gray matter revealed ballooning with adjacent mild vacuolization. Focal muscle fiber atrophy was also evident. Otherwise, there was no significant decrease of neuronal populations in the ganglia.
Discussion
HSAN VI typically presents at birth; however, onset may be delayed depending on the genetic expression. In newborns, the disease is characterized by hypotonia, respiratory and feeding difficulties, and autonomic abnormalities, including labile cardiovascular function.6 In our review of the literature (Table 1), newborns have postural contractures and limb abnormalities at birth, which could be due to in utero hypotonia and weakness. The paucity of facial expressions and open-mouth appearance is another common finding. Absent deep-tendon reflexes, alacrimia, and absent corneal reflexes also occur. Additional findings reported in neonates are recurrent unexplained hyperpyrexia, bilateral vocal cord paresis, erythematous skin blotching, and absence of an axon flare with intradermal histamine. Later in infancy and childhood, HSAN VI is commonly associated with peripheral neuropathy, with decreased sensation to pain, touch, and vibration. Seizures are not common. Death in neonates is most often due to cardiopulmonary arrest.
| Edvardson et al.4 | Manganelli et al.5 | Cappuccio et al.6 | Fortugno et al.1 | Sakaria et al.7 | Present case | |
|---|---|---|---|---|---|---|
| Age of onset | At birth | Infancy | 4 months | Second to third decade | At birth | At birth |
| Ethnicity | Ashkenazi Jew | Southern Italy | Caucasian | Italian | Unknown | Hispanic |
| Clinical features (neurological and associated) | Hypotonia, flexion contractures, and autonomic instability | Sensory neuropathy, progressive mutilating distal ulcerations, and joint deformities | Pain insensitivity of limbs, skin blistering, peeling, and ulcers | Severe pain insensitivity, joint deformities, and autonomic dysfunction | Hypotonia | Dysautonomia, joint deformities, and dysmorphic facies |
| Brain imaging | MRI normal | N/A | MRI: syringomyelia (D3–D8) | N/A | CT: Poor gray–white matter differentiation, suggestive of HIE MRI: Small T2 hyperintense area in the left thalamus |
Normal |
| Genetics/mutation identified | Homozygous c.14865 delA mutation in exon 83 of the DST gene, causing frameshift | Compound heterozygous mutations in exons 4 and 5 of DST gene: c.616C>T c.687+1G>A | Compound heterozygous for two variants in the DST gene: c.3886A>G (p.R1296X) in exon 29 and c.806C>T (p.H269R) in exon 7 |
Compound heterozygous for two variants of DST gene: c.608C>A, p.(Ala203Glu) and c.12988A>T, p.(Lys4330*) |
Homozygous missense variant c.1118C>T (p.Pro373Leu) in DST (NM_001144769.2) | Homozygous alternate transcript NM_015548.4:c.13574G>A (p.Trp4525*) |
| Parental mutation | Heterozygous mutations of DST gene in parents | Unknown | Father heterozygous for p.R1296X and mother heterozygous for p.H269R variant | Unknown | Unknown | One copy of the variant was paternally inherited and one copy was maternally inherited |
| Age of death | 2 years | N/A (alive) | N/A (alive) | N/A (alive) | N/A (alive) | 3 months |
| Autopsy findings | N/A | N/A | N/A | N/A | N/A | Neuropathology findings were consistent with axonal loss and neuronal degenerative changes in spinal nerve roots and spinal cord gray matter |
- Abbreviations: DST, dystonin; HIE, hypoxic-ischemic encephalopathy; HSAN VI, hereditary sensory and autonomic neuropathy type VI; MRI, magnetic resonance imaging; N/A, not available.
In terms of ancillary tests, imaging and EEG are usually normal. Nerve conduction tests may be helpful in inherited neuropathies, but with the latest advances in genetics, there is less utility, especially in the neonatal critical care setting. Nevertheless, the test may help in crafting a clinical picture for new genetic DST variants. Another case series found sensory and autonomic degeneration on skin and nerve biopsy.5 Genetic testing, for all cases, appears relevant, as all cases are associated with some type of pathogenic variation in the DST gene. These genotypic variations in DST gene mutations for HSAN VI cases likely explain the heterogeneity of this rare condition. The variant in our patient is expected to result in an absent or disrupted protein product.
Interestingly, we noted motor involvement in addition to sensory involvement in NCS/EMG testing for our patient. One case series found motor abnormalities (absent/reduced amplitude of compound muscular action potentials) limited to intrinsic foot muscles.5 A mouse model featured severe dorsal root ganglia degeneration for sensory and late stages of motor neuron disease.5 Our data add the possibility of motor involvement in severe phenotypes of this rare condition.
Our patient's neuropathological findings were mild and considered nonspecific. Compared with the more commonly described HSAN III (Riley-Day syndrome),8 HSAN VI appears to involve more nerve roots and neurons, as seen in neuropathology as well as electrophysiology (Table 2); this may explain the prominent findings of hypotonia and peripheral neuropathy in our patient.
| Our patient | HSAN III |
|---|---|
Spinal nerve roots with axonal loss and degeneration Neurons show ballooning changes with mild vacuolization in the adjacent neuropils |
Decreased number of small myelinated and unmyelinated neurons |
| Normal dorsal ganglions | Decreased number of dorsal root ganglion Small superior cervical and thoracic ganglia (cardiac dysfunction) |
| Paraspinal muscle and diaphragm with focal atrophy | Not observed |
| Few neurons in spinal gray matter with neurodegenerative changes | Reduced primary substance P axons in substantia gelatinosa (dorsal spinal cord involved with pain/temperature/touch) Intermediolateral gray columns: reduced neurons |
- Abbreviations: HSAN III, hereditary sensory and autonomic neuropathy type III.
Conclusion
We report a novel homozygous alternate transcript mutation in the DST gene causing HSAN VI in a neonate. Collective analysis of these patients would help to further characterize the spectrum of disease pathology and could provide insight into the neurophysiology and neuropathology of this rare condition.
Author Contributions
Lekshmi Peringassery Sateesh: Conceptualization; data curation; formal analysis; writing—original draft. Pavani Chitamanni: Conceptualization; data curation; formal analysis; writing—review and editing. Danielle Akinsanmi: Data curation; formal analysis; writing—review and editing. Suman Ghosh: Writing—review and editing. Steven G. Pavlakis: Writing—review and editing. Alexandra Reznikov: Conceptualization; resources; supervision; writing—review and editing.
Acknowledgments
We thank our neonatal intensive care unit team for taking care of this infant with utmost care. We also thank our neuropathologist Dr. Jianying Zeng for providing us with a comprehensive neuropathology report.
Conflicts of Interest
Dr. Suman Ghosh is an editorial board member of the Annals of Child Neurology Society. The remaining authors declare no conflicts of interest.




