Axonal pathfinding during the development of the nervous system
Abstract
Guidance of axons sprouting from maturing neuroblasts, during intermediate trajectories and in seeking target neurons for synaptogenesis, is a fundamental developmental process in central nervous system maturation. Axons but not dendrites sprout from neuroblasts during migration. The growth cone of the axonal tip projects constantly changing multiple veils and spikes (lamellipodia and filopodia) that contain microtubules, actin microfilaments, mitochondria, endosomes, and membrane receptor proteins. They are sensitive to changes in ionic calcium flux and may be impeded by perinatal hyper- or hypoglycemia. The growth of axonal membranes occurs mainly at the growth cone. Neurofilaments appear in the axonal tip as it approaches its target. Growth cones are attracted to or repelled by various extracellular matrix molecules that guide them, such as netrins and glycoproteins. Numerous genes are involved, some specific for only certain projections. Neurotransmitters later to be secreted are recognized in growing axons before their synthesis. Axonal fascicles are enveloped by extracellular keratan sulfate that ensures that fascicles contain axons of similar origin and destination and whose neurons secrete the same transmitter. Near their targets, axonal tips may ramify to form synapses on more than one neuron. Transitory pioneer axons provide supplementary mechanical guides to permanent axonal trajectories. Thalamus and olfactory bulb contain axonless neurons with dendrodendritic synapses. Chromaffin neurons of neural crest origin develop no neurites. Most cerebral malformations involve aberrant axonal pathfinding; in holoprosencephaly, keratan sulfate abnormally ensheathes individual axons. Axonal pathfinding to near or distant target neurons is primordial for synaptic circuitry subserving normal and abnormal neurological functions including epilepsy.
I consider primitive nerve fibres to be protoplasmic outgrowths of the central nerve cell.
R. Albert Kölliker (1886)
Introduction
Synaptic circuitry is a fundamental basis of nervous system function, both local synaptic plexi and widespread synaptic networks. Guidance of axons emanating from immature neurons to their near or distant programmed targets is known as axonal pathfinding, a developmental process essential for developing correct synaptic relations and circuitry. Pathfinding involves both intermediate trajectories and the final neuronal site of synapse formation at the end of that trajectory. Molecules that guide growing fetal axons within the central nervous system (CNS) are identical or similar to those that guide migratory cells. The term axon is shortened from axis cylinder, so named by the late-nineteenth-century German anatomist Otto Karl Friedrich von Dieter, who also coined the term dendrite, derived from the Greek word dendr meaning tree.
The entire process of axonogenesis in the developing CNS may be classified into sequential phases: (1) sprouting of the initial axon from the polarized soma of the neuroblast, which often occurs before the migration of the neuroblast is even completed, unlike dendritogenesis, which does not occur until the neuroblast is in its final position after completing migration; (2) intermediate projection toward the target; (3) selection of target neurons and rejection of others with which to form synapses; (4) ramification of the axonal tip to innervate more than one neuron in some cases. These small terminal branches were named collaterals by Ramón y Cajal; (5) synaptogenesis, over time accompanied by deletion of old synapses no longer needed, called synaptic pruning.
Timing of developmental processes, including temporal relations to other simultaneous developmental processes, is essential for nervous system ontogenesis.1 In short, local connections, axonal pathfinding, and synaptogenesis may occur in brief periods in the human fetus, such as the intrinsic axonal bundles of the globus pallidus and pencil fibers of Wilson in the corpus striatum, as well as connections within the brainstem and short intracortical interneuronal connections. Long tract function may be delayed months or years until the completion of both terminal axonal ramifications and then myelination. For example, this process in the corticospinal tract is not complete in humans until two years of age, but much sooner in many four-legged animals, which helps explain why newborn horses can stand and walk with difficulty but young human infants cannot do so. The human corpus callosum is not complete in this regard until late adolescence. Axonal pathfinding is not just a process of embryonic and early fetal life, but is still in progress in the neonate and especially in the preterm infant, hence of practical importance, not just academic interest. For example, because growth cones are highly sensitive to calcium ion concentrations and flux, neonatal hypocalcemia can impair axonal growth and synaptogenesis, resulting in clinical manifestations.
Growth Cone of the Axonal Tip
The growing presynaptic axonal tips en route to their destinations, named by Ramón y Cajal as the growth cone (cône de accroisement),2 are multiple spike-like extensions or filopodia at the immature axonal tip that continuously change in morphology by extending and retracting. Between the multiple filopodia are veil-like membranous lamellipodia. The principal components of these growth cone structures are microtubules and microfilaments of actin. Microfilaments are randomly oriented and mesh-like within the lamellipodia, but are in parallel as tightly packed bundles within the filopodia (Figure 1). Actin microfilamentous connections to the plasma membrane of the growth cone link the filament network to adhesive contacts of filopodia and lamellipodia with other surfaces.4 Neurofilaments are not yet part of the cytoarchitecture of the immature growth cone, but become a late component when the target is approached or reached.5
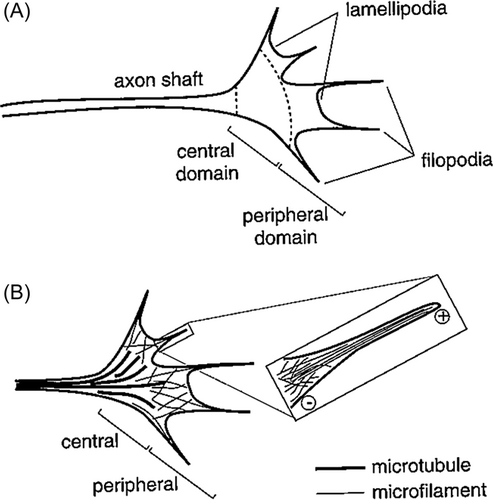
Growth cones also contain mitochondria, multiple small endocytotic vesicles, and numerous surface receptors for guidance molecules. Endocytes in the initial axonal segment maintain neuronal polarity by recognizing and engulfing polarized transmembrane proteins.6 Their function probably is similar in the growth cone. Neurotrophin binding to their receptors stimulates endocytosis into signaling endosomes, contributing to the growth cone motility.7 Endocytosis and downstream signaling from endosomes help enable rerouting of axons, which is spatially and temporally regulated by adaptor proteins or coreceptors, for example, if a barrier lesion appears.8 The growth cone recycles CAMs from its rear to its leading front through these endosomes, thereby polarizing growth cone adhesiveness.9
Axonal growth occurs mainly at the growth cone because it is at the tip that a new axonal membrane is added and moves backwards toward the neuronal soma.3, 5, 10 Axons generally grow in straight lines within the neuraxis, but when an attractant causes the growth cone to deviate to one side, the actin microfilaments accumulate asymmetrically toward the side to which the growth cone encounters the attractive signal, which causes the growth cone to deviate to that side (Figure 2).3, 5, 10, 11 Microtubules also shift position within the filopodia, toward the side to which the growth cone deviates.12, 13 Asymmetrical growth toward the side of an attractant depends upon the ability of the growth cone to detect and respond to differences in attractant concentrations or gradients encountered by the different sides of the growth cone.5, 14-16 Microtubule–actin microfilament coupling or cross-linkage, in which actin arcs exert compressive forces to surround and bundle microtubules in the core of the growth cone, coordinates the two cytoskeletal structures.15, 17 The same principle applies to axonal regeneration after injury.18, 19 Brain-derived neurotrophic factor (BDNF) is well characterized as promoting axonal regeneration and functional recovery.20-22
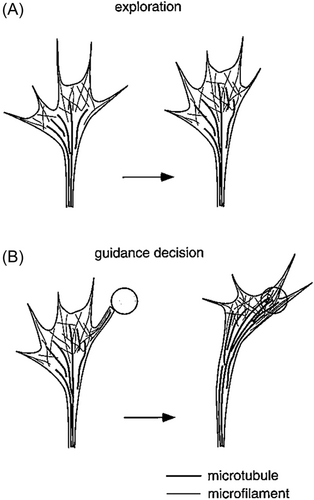
Not only the growth cone but also the shaft of the growing axon is highly sensitive to changes in calcium ion concentration and flux. Pyramidal neurons in vitro exposed to Ca2+ influx exhibit shortening of the axonal shaft and lengthening and extension of growth cone filopodia.23 Ca2+ is important not only for growth cone and axonal growth, but also for turning as it approaches its target because calcium regulates rearrangements of the growth cone cytoskeleton and voltage-gated calcium channels can perturb mobility toward a source of netrin-1.4, 24, 25 The polarity of growth cone guidance is determined by Ca2+ release from the endoplasmic reticulum.26 In vitro light absorption from lasers' near-infrared wavelength activates temperature-sensitive membrane receptors, which results in an influx of calcium and growth cone collapse.27 This importance of calcium ion concentration in the growth cone may have clinical implications for human neonates, particularly preterm infants, in whom hypo- or hypercalcemia might affect or impede axonal pathfinding and synaptogenesis.
Growth cone membrane receptors for guidance molecules and adhesion molecules attach growth cones to the extracellular matrix and enable them to pull themselves forward as they seek their target.4, 24, 28-30 Tissue culture studies demonstrate that growth cones advance best when they can adhere strongly to substrate surfaces.29 Even when the length of a filopodium is constant, its microfilaments are not static but exist in a state of dynamic equilibrium with their (+) polymerized distal ends balanced by their (−) depolymerized proximal end.3 The drug cytochalasen deletes growth cone filopodia and impairs axonal pathfinding in vitro by preventing actin polymerization.31, 32 The same polymerization is true of the microtubules as well, which also are essential for bending the growth cone filopodia toward its target.11 These data underscore the critical importance of filopodia in axonal pathfinding.
Postnatally, the infant and child continue to grow and the neuroaxis lengthens with body growth. Axons, therefore, must continue to lengthen even after synapse formation is established. They do so by adding material to all parts of the axon. The growth cone no longer exists after synaptogenesis but the axonal shaft, under the stress of tension, incorporates new material for axonal lengthening and widening.33 Sustained axonal growth depends upon the synthesis of components in the neuronal soma, which supplies the materials for the assembly of neurofilaments and microtubules that are transported distally by the axoplasmic flow.5 Synthesis of growth-associated proteins (GAPs), including GAP-43, diminishes or ceases after axonal growth is completed.34 Actin and tubulin synthesis also stops but can be reactivated for regeneration, but there is not a high physiological turnover rate.5
Chemotaxis in Guiding the Growth Cone
Chemotaxis or the synonym chemotropism is the attraction or repulsion of growth cones by certain molecules that guide axons in their intermediate trajectories and final approaches to targets.5, 35-37 Extracellular chemical signals that so attract or repel specific axons is a process known since the early and mid-twentieth century. The type of axon is recognized not so much by neuroanatomical orientation such as longitudinal growth (e.g., corticospinal, spinothalamic, spinocerebellar tracts) or commissural growth as by the type of neurotransmitter produced by its neuron (glutamatergic, GABAergic, cholinergic, dopaminergic, serotonergic). Even though transmitter substances are not yet even synthesized in these immature neurons, their future identities are already evident to chemotaxis molecules.
In long fascicles, axonal attraction or repulsion is guided not by orientation in the neural tube (e.g., longitudinal projections of the spinothalamic and corticospinal tracts; horizontal commissural axons) as by the type of neurotransmitter they will secrete at maturity. Remarkably, these future transmitters are recognized during intermediate pathfinding and at terminal targets before the immature neuron is even synthesizing or secreting the chemical substance; the genetic programming is already in place for the specific neuronal type and its neurotransmitter and is evident to axonal guidance molecules. It is important, therefore, to know the transmitter associated with the axons of each tract.
Growth cones can advance either on the surfaces of other cells (cell adhesion molecules, or CAMs) or on the extracellular matrix substrate (substrate adhesion molecules). Most molecules on the surfaces of other cells that enable such movement, such as N-CAM, are members of an immunoglobulin superfamily, but some such as N-adherin are not. They mediate their effects by providing temporary attachment sites for filopodia and acting as ligands and receptors in a signaling pathway. Integrins within the receptor cell can bind to material in the extracellular matrix, including laminin and collagen, and activate intracellular signaling pathways.5
Axonal guidance cues belong to four major classes: netrins, semaphorins, slits, and ephreins.37-39 Transmembrane proteins of the growth cone are similar to those of most other neural plasma membranes, including cell-adhesion molecules, ion channels, and cell surface receptors.3 GAP-43 is specifically associated with growth cones.40 Some receptors, such as β-1-integrin, are specifically localized at the tips of filopodia.36, 41 Netrin and its receptor are primary attractants of commissural axons at all levels of the neuroaxis, from the spinal cord to the forebrain.42-47 Netrin is generated by progenitor cells rather than by floor plate cells through which ventral commissural axons pass in the embryonic spinal cord.43, 47 Anterior commissure formation in the telencephalon is markedly reduced when netrin is knocked out in experimental animals.42 Semaphorins and their binding membrane receptors, particularly the large family of plexins, contribute to axonal guidance at the receptor neuron for synapse formation.48, 49 Some semaphorins, such as the glycoprotein collapsing-1, are mainly repulsion molecules.50 Slit is another family of genes and their transcription products that are important in commissural axonal guidance, but slit expression is influenced by other genes, suppressed by Wnt and in turn suppressing netrin but activating Robo2.42, 51 The Eph family of receptor tyrosine kinase and their ligands, the ephrins, is another example of repulsive molecules to growth cones, especially in the retina.52 Tyrosine phosphorylation is important for the early transduction of external growth cone guidance signals. Cyclic nucleotides such as cyclic adenosine monophosphate modulate growth cone attraction and can change the signal to growth cones from attractive to repulsive or vice versa.3, 53 A formin family member, Fmn2, is identified as another regulator of growth cone motility by mediating the coupling of the actin cytoskeleton to adhesion molecules in the substrate matrix.54
Neurotrophic factors also serve as guidance cues to growth cones. In particular, nerve growth factor (NGF) reacts to TrkA receptor and RhoA kinase and attracts growing axons.22, 55-58 BDNF and glial-derived neurotrophic factor are others that influence growth cone navigation.56, 59
Additional important primary factors in axonal guidance are extracellular phosphorylation60 and, especially, axonal glycosylation and glycolysis, which are closely related to mitochondrial oxidative phosphorylation.39, 61 Glycolytic enzymes are found throughout the axoplasm including within the growth cone of growing axons. Inhibition of glycolysis by NGF impairs axonal extension and growth cone dynamics.61 Many proteins in axonal guidance pathways are glycoproteins (see keratan sulfate [KS], below); glycosylation modulates the regulation of axonal motility.38 More than half of all mammalian proteins undergo glycosylation, and glycosylating enzymes and their substrates are highly conserved in evolution.62, 63 Congenital disorders of glycosylation produce several distinctive human metabolic diseases and thus aberrant axonal projections and targets may underlie some of the neurological deficits of affected children.61 The chemotropic theory of Ramón y Cajal2 was confirmed in numerous studies showing the key role of extracellular guidance molecules.39, 46, 64, 65
Electrical currents for axonal guidance
In the early and mid-twentieth century, enthusiasm prevailed for a hypothesis, called neurobiotaxis or galvanotropism, that electrical signals or electrical fields guide migratory axons,66-71 but these data were weak.5 Early attempts to confirm the role of electrical currents on neurons in tissue culture also yielded equivocal results, in part due to the primitive nature of the instruments available at that time.72, 73 More modern electrical current generators and cellular recording devices in vitro produced some evidence that the orientation and growth of neurites indeed do respond to electrotonic signals,74, 75 but extrapolation to in vivo responses poses further uncertainty and is difficult to study in animal embryos and fetuses in vivo. Electrical fields are mediated by the influx of calcium ions that can alter the polymerization of microfilaments and link microtubules by cross-bridges.10 Whereas electrical fields or electrotonic signaling might influence axons during their growth in vivo, its role probably is minor and perhaps mediated by activating attractant molecules.
Pioneer axons
The earliest axons to establish tracts were named pioneer axons by Harrison in 1910.76 In many centripetal and centrifugal pathways to and from the cerebral cortex, these initial axonal projections are transitory. Such temporary efferent axons of the corpus callosum, corticospinal, and corticothalamic tracts emanate from precocious neurons of the subplate zone,77, 78 which is a transitory fetal cortical layer that disappears before term. Intermediate links from the marginal zone, even before the cortical plate forms, connect the future cerebral cortex with the ganglionic eminence and primordial thalamus.78 Gli-3 is essential for the differentiation of subplate neurons that emit pioneer axons79 as well as crucial to permanent callosal axons. Permanent axons of the forebrain commissures in the mature brain arise from the small pyramidal neurons of layer 3, those of the corticospinal tract from larger pyramidal neurons of layer 5, and corticothalamic axons from intermediate-sized neurons of Layer 6. Horizontal lamination of the cortex, as seen histologically, is not established until 22 weeks; however, the permanent axonal projections during the first half of gestation come from the cortical plate with a microcolumnar architecture so that one can only state at that age that they arise from the middle or deep parts of the early fetal cortical plate. The pioneer axons of these important white matter structures first appear at 7–10 weeks gestation. The purpose of pioneer axons would appear to be another guide for the permanent axons, perhaps as a mechanical guide as suggested by Weiss in 1941.80 Extracellular tunnels between neuroepithelial cells or glial cells to guide outgrowing axons had been proposed by Wilhelm His in 1887.81 Mechanical axonal guides by transitory pioneer axons in the peripheral nervous system also was demonstrated in amphibians during limb regeneration.82 Furthermore, durotaxis, the response to variations in substrate rigidity, is another mechanical factor influencing the motion of axonal bundles.83 Axon-to-axon signaling within a fascicle is recently recognized as yet another factor in pathfinding,84 though whether it plays a role between pioneer and permanent axons is unknown. The role of pioneer axons in central tract formation in mammals including humans thus remains incompletely understood.
Genetic programming
Many developmental genes are involved in axonal guidance, but most of these genes provide other functions as well, not only in the embryonic and fetal nervous system but also in many other organs where their functions are quite different. Some genes are intact with others, sometimes sequentially, such as the intimate relation between Slit1 and Netrin1.85 Mutations or deletions of specific genes may lead to aberrant axonal projections to heterotopic sites not normally intended.
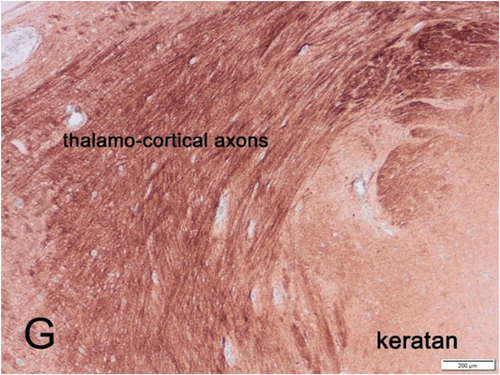
Among the best-studied genes are those regulating specific axonal projections and targets but not necessarily involved in all axonal path-seeking. Thalamocortical and corticothalamic connections are established in the human fetus between 14 and 27 weeks gestation and are mainly guided by Slit-1, Netrin-1, Robo-1 and -2, Tbr1, Gbx2, Mash-1, Lhx2, and Pax6.85-94 The zinc-finger gene and its transcription factor Gli-3 is especially important for thalamocortical connections because of its early expression before the cortical plate even forms and its role in subplate zone development,79, 92, 95 and because its reduced expression upregulates Sonic hedgehog (Shh) and Wnt genes in the ventral telencephalon, impairing pathfinding by thalamocortical axons.92, 96 The ciliogenic transcription factor of Rfx3 is expressed in the primordial thalamus and the medial ganglionic eminence of the germinal matrix as well for the formation of thalamocortical connections.97 RFX3 also is essential for the formation of the corpus callosum98 and ependymal cell differentiation.99 As noted earlier in this review, netrins are essential for the passage of commissural axons in the ventral part of the spinal cord.42-47, 85, 100 The guidance of commissural axons across the ependymal floor plate of the embryonic spinal cord requires other specialized gene expressions.101, 102 Focal adhesion kinases modulate some genetic expressions downstream of axonal guidance cues.101 Many genes thus are relatively specific for certain central pathways and not for others, and many functions in cooperation or sequential expression of others.
KS proteoglycan
A recently discovered axonal guidance molecule is KS, a glycosaminoglycan that attaches to larger proteoglycan molecules, sometimes hundreds of KS chains on one proteoglycan. KS is synthesized in the CNS by astrocytes and secreted into the extracellular spaces (Figure 3). Keratan should not be confused with keratin or cytokeratin, an entirely different molecule that forms fingernails, part of the epidermal cells of human skin, the nasal horn of the rhinoceros, and the soft shell of shrimp. KS is widely distributed in the human body in many organs, with the highest concentrations in the cornea, cartilage, and brain. The metabolism of KS is well studied.104-109 Because KS is not electron-dense, it is invisible by transmission electron microscopy. It also is not demonstrated by magnetic resonance imaging (MRI) or other neuroimaging techniques.103
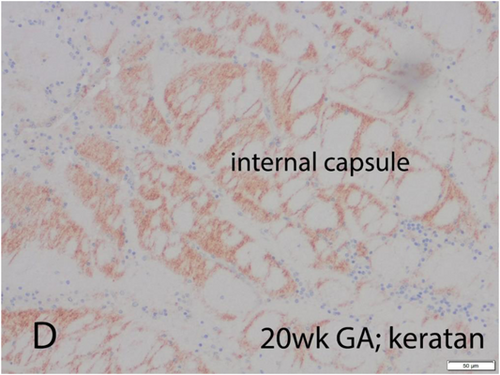
KS proteoglycan is present in the dorsal and ventral median septa of the spinal cord and repels the glutamatergic axons of the dorsal columns to prevent an aberrant crossing of the midline but facilitates the GABAergic commissural axons that pass through the spinal cord ventral median septum.110 In the human cerebral cortical plate, KS appears in the second half of gestation (Figures 4 and 5), first in the molecular zone and then in the deep layers of the cortex and the subcortical U-fiber layer (but not in the deep white matter), and finally throughout the cortex before term, but remains in highest concentration in the deep layers and U-fibers into adult life.103
Axonal Projections in Cerebral Dysgenesis
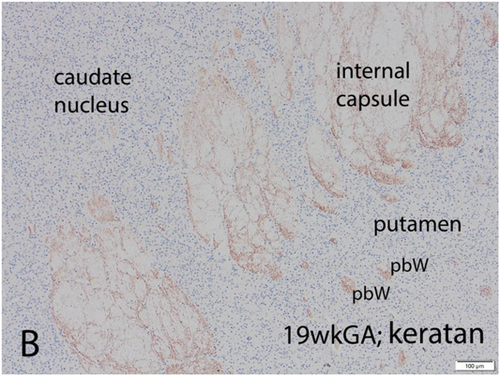
Most cerebral malformations involve alterations of multiple developmental processes, including axonal pathfinding of either short connections or long projections or both. The most frequent major malformation in the brain of liveborn neonates is agenesis of the corpus callosum and sometimes also of the anterior commissure, depending upon the timing of onset of the genetic expression or of a teratogenic influence.1 In lobar and semilobar holoprosencephaly, KS ensheathes individual axons as well as envelops their fascicles (Figure 6), an insulation that may protect against epilepsy.111 This KS ensheathement of axons occurs many weeks or months before myelination of axons ensues, both pre- and postnatally. Side-by-side duplication of the spinal central canal can occur due to upregulation of a gene expressed in the dorsoventral gradient in the neural tube during embryonic life, for example, in Walker (type 2; “cobblestone”) lissencephaly.112 Such central canal duplication also involves failure of development of the dorsal median septum from roof plate ependymal cell processes, which secrete KS; rostrally extending glutamatergic axons of the dorsal columns may, therefore, aberrantly cross the midline since they are not repulsed and the brain becomes confused about the laterality of sensory stimuli.103
The present generation of neuroimaging techniques, including fetal and postnatal MRI, cannot yet detect KS or demonstrate axonal growth cones. They can, however, define most cerebral malformations including aberrant axonal projections, particularly diffusion tensor imaging (DTI; tractography).113 The DTI sequence in MRI enables the demonstration of aberrant axonal projections in hemimegalencephaly.114, 115 Intravital imaging of neocortical heterotopia reveals aberrant axons around heterotopic neurons.116 Microscopic examination of tissue sections of the brain, using immunocytochemical antibodies to demonstrate neuronal maturation and synaptogenesis, provides another means of demonstrating axonal projections in fetuses postmortem.117, 118 Genetic correlations are essential. The genetic defects in callosal agenesis are multiple in both isolated and syndromic cases. In a form of human familial lissencephaly with many abnormal axonal pathway abnormalities as well, mutations in MACF1 encoding zinc-binding residues of the microtubule-binding GAR domain are the etiology.119 Many more examples could be cited.
Axon-Less Neurons
Throughout the neuroaxis are a population of neurons that lack axons and form dendrodendritic synapses. They are most prevalent in the thalamus and the deep granular cell core of the olfactory bulb, which is an olfactory thalamic equivalent with dendrodendritic synapses between granular neurons and also granulomitral cell synapses.120-122 Another type of axon-less and also dendrite-less neuron is the chromaffin cell of the adrenal medulla, carotid body, and a few other small peripheral nervous system chromaffin structures, which secrete neurotransmitters into the blood and do not require neurites, though these cells express synaptophysin and surface glutamate receptors. Chromaffin cells are of neural crest origin and migration and thus do not require growth cones. Other neural crest-derived neurons of the peripheral autonomic nervous system also migrate without axonal sprouting or growth cones, but at their destination, they form axons and dendrites. An example is the parasympathetic ganglion cells of the submucosal and myenteric plexi of the intestine for peristalsis.
Author Contributions
Harvey B. Sarnat: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; writing – original draft; writing – review and editing.
Acknowledgments
I am grateful to Drs. Shuhong Liu and Young Ou of the University of Calgary Research Immunohistochemistry Laboratory and Ms. Patricia McInnis of the Alberta Children's Hospital Anatomical Pathology Laboratory for their meticulous preparations of tissue techniques. I thank Dr. Laura Flores-Sarnat for her reading of the manuscript and for offering many useful suggestions. This work was supported by Alberta Children's Hospital Research Institute, Grant No. 60-28450, Project No. 10027949.
Conflict of Interest
The author declares no conflict of interest.




