Investigate of LOC101928988 Regulatory Effect on the DAPK2 Transcription in Breast Tumors
Funding: This work was supported by the Hormozgan University of Medical Sciences provided financial assistance for this study (grant no. 4020047).
ABSTRACT
Background
The World Health Organization has mentioned breast cancer holds the highest incidence rate among all types of cancer globally. Death-associated protein kinase 2 (DAPK2) is a serine/threonine kinase linked to various forms of malignancy, such as breast cancer. This protein assumes a pivotal function in a multitude of cellular mechanisms, including apoptosis, autophagy, and cell migration.
Aims
This study aimed to study the LOC101928988 regulatory effect on the DAPK2 expression in breast cancer.
Methods
In this study, 38 paired tumoral and normal tissues were selected from patients. Quantitative real-time PCR was used to analyze the expression of DAPK2 and LOC101928988. The interactions of DAPK2 and its intermediate elements with LOC101928988 were predicted by docking analysis.
Results
The expression of DAPK2 and LOC101928988 was downregulated in tumor tissues compared to the control group. Further analysis revealed a significant positive correlation between DAPK2 and LOC101928988 levels in tumoral and adjacent normal tissues. A comparison of gene expression between different grades, stages, and HER2 statuses showed significant findings. ROC curve analysis of DAPK2 and LOC101928988 expression revealed 77% and 72% AUC for BC tissue, respectively.
Conclusions
Overall, our results suggest that alterations in the levels of DAPK2 and LOC101928988 may be involved in tumor initiation and progression in breast cancer. It has also been reported that LOC101928988 probably has a role in regulating DAPK2 expression through interaction with transcription factors.
Abbreviations
-
- AUC
-
- area under the curve
-
- cDNA
-
- complementary DNA
-
- COREST
-
- corepressor for element-1-silencing transcription factor
-
- CTCF
-
- CCCTC-binding factor
-
- DAPK2
-
- death-associated protein kinase 2
-
- ER receptor
-
- estrogen receptor
-
- EZ2H
-
- enhancer of zeste homolog 2
-
- HNRNPL
-
- heterogeneous nuclear ribonucleoprotein L
-
- lncRNAs
-
- long non-coding RNAs
-
- ncRNAs
-
- non-coding RNAs
-
- NSCLC
-
- non-small cell lung cancer
-
- PR receptor
-
- progesterone receptor
-
- RBPs
-
- RNA-binding proteins
-
- REST
-
- RE1-silencing transcription factor
-
- ROC curve
-
- receiver-operating characteristic curve
-
- TFs
-
- transcription factors
-
- WHO
-
- World Health Organization
1 Introduction
The World Health Organization (WHO) is alarmed by breast cancer's highest incidence rate among all types of cancer among women worldwide. In 2023, ~297 790 newly detected cases of invasive breast cancer are expected in women residing in the United States [1]. Breast cancer is a complex disease and its exact causes are not fully understood [2]. Scientific investigations have indicated that modifications in genes implicated in apoptotic and autophagy mechanisms play pivotal roles in the progression and advancement of this condition [3-5]. Apoptosis is an innate process that aids in the elimination of damaged or aberrant cells from an organism, whereas autophagy functions as a mechanism for salvaging impaired or dysfunctional cellular constituents [6-10]. The malfunction of apoptotic and autophagy genes can result in the survival and proliferation of cancerous cells, ultimately culminating in the expansion and dissemination of tumors [11-13]. Several essential genes are involved in breast cancer apoptosis, including TP53, BCL2, and BAX [14-16]. In addition, autophagy genes, such as BECN1, ATG5, and ATG7, are dysregulated in breast cancer [17, 18]. Among the various genes involved in autophagy, death-associated protein kinase 2 (DAPK2) has garnered increasing attention because of its regulatory role in both autophagy and apoptosis [19, 20].
DAPK2 is a serine/threonine kinase that has been linked to various malignancies, such as breast cancer [21]. This protein assumes a pivotal function in many cellular mechanisms, including apoptosis, autophagy, and cell migration [22-24]. DAPK2 actively participates in regulating the cytoskeleton, which is responsible for upholding the configuration and composition of cells [25]. Its role in tumor suppression through the modulation of these pathways has been increasingly acknowledged [26]. DAPK2 not only promotes apoptosis through programmed cell death but also augments autophagic activity by interacting with essential proteins including SQSTM1/p62 and beclin-1, hence affecting cellular homeostasis and survival during stress conditions [27]. DAPK2 phosphorylates SQSTM1/p62 at serine 403, which enhances the binding of SQSTM1/p62 to LC3, a protein involved in autophagosome formation. This interaction promotes clearance of damaged or unwanted proteins by autophagy [28, 29]. Moreover, it was found that DAPK2 plays a regulatory role in the mTORC1 pathway, which is a crucial modulator of autophagy [25]. DAPK2 exerts its inhibitory effects on mTORC1 by phosphorylating and activating TSC2, a positive promoter of mTORC1 [30]. It is improbable that DAPK2 possesses the capability to interact with beclin-1, a factor that plays a crucial role in the inception of autophagy, as well as Bcl-XL, a protein that exhibits anti-apoptotic properties and can hinder autophagy [31]. Phosphorylation of serine 90 on beclin-1 by DAPK2 has been demonstrated to augments its interaction with Vps34, a kinase that is important in the initiation of autophagy. This particular phosphorylation event facilitates autophagy [32].
Understanding the functions of DAPK2 is essential, as it reveals not only its roles in autophagy and cytoskeletal regulation but also its significant implications in cancer biology [33]. The research has shown that DAPK2 is a critical factor in various malignancies, including lung, colorectal, and thyroid cancer. In the case of lung cancer, there is a connection between lower levels of DAPK2 expression and heightened tumor proliferation, along with an unfavorable prognosis [26, 34]. Similarly, in colorectal cancer, reduced DAPK2 expression has been associated with tumor advancement and the spread of cancer cells [35]. Moreover, DAPK2 plays a crucial role in the initiation and progression of thyroid cancer, as it contributes to tumor development and the ability to resist TRAIL-induced apoptosis through autophagic degradation of I-κBα [36]. Despite its established roles, the potential of DAPK2 as a therapeutic target in cancer treatment remains an active area of research, underscoring the complexity of its function in cellular processes [37]. Thus, further investigation of DAPK2 is vital for understanding tumor biology and exploring new therapeutic strategies.
ncRNAs are various types of RNAs without protein-coding functions, and regulation of gene expression has been identified as the most important function of them [38]. They are recognized as important regulators of genes involved in various physiological processes [39]. Long noncoding RNAs (lncRNAs) are a subtype of ncRNAs that are typically characterized as transcripts exceeding 200 nucleotides in length that do not undergo protein translation [40]. RNA-binding proteins (RBPs) belong to a category of molecules that can bind to distinct RNA sequences and thereby contribute to various processes involving RNA, such as processing, transport, stability, and translation [41]. The interaction between RBPs and lncRNAs takes place through diverse mechanisms, governing the regulation of their stability and function, and ultimately, gene expression [42, 43]. A notable instance of this phenomenon is observed in the interaction between RBP HuR and lncRNA NEAT1, where HuR stabilizes NEAT1, consequently facilitating the formation of paraspeckles and leading to the alteration of gene expression [44]. This intricate interplay between RBPs and lncRNAs underscores the critical role of ncRNAs in fine-tuning gene expression and cellular responses.
A lncRNA named LOC101928988 with accession code NR120344.1 in NCBI was transcribed antisense from the DAPK2 gene. The expression effects of this gene and lncRNA in breast cancer have not yet been investigated. The objective of this study was to examine the expression profile of DAPK2 and its antisense lncRNA LOC101928988 within the mTORC signaling pathway in breast cancer, focusing specifically on their involvement in autophagy. Our aim was to evaluate the potential impact of these molecules on the regulation of autophagy during the development and progression of breast cancer and to assess their viability as therapeutic targets. By analyzing the expression patterns in breast cancer tissues and their adjacent normal tissues, we aimed to gain a deeper understanding of the molecular mechanisms that cause dysregulation of autophagy in breast cancer and to discover new biomarkers for diagnosing and treating breast cancer patients.
2 Subjects, Materials, and Method
2.1 Ethics Statement
The Hormozgan University of Medical Sciences Ethics Committee authorized the study procedures (IR.HUMS.REC.1402.294), and all enrolled patients provided informed consent. Data related to clinicopathology were obtained from the patient records. This study was conducted in accordance with the Declaration of Helsinki (Association, 2019).
2.2 Sample Collection
A total of 38 tumor samples along with adjacent normal tissues were collected from patients who underwent breast surgery, with their consent, at the MRI hospital located in Shiraz, Iran. Fresh cancerous and adjacent non-tumor specimens were rapidly frozen in liquid nitrogen and subsequently preserved at −80°C immediately after resection to facilitate RNA extraction. It should be noted that the patients had not received any previous treatment, and their carcinoma diagnosis was confirmed by pathologists situated within the hospital premises. The clinical data related to the specimens from the patients are given in Table 1.
| Parameter | Cases (n) |
|---|---|
| Age (average) | |
| ≤ 50 | 22 |
| > 50 | 16 |
| Stage | |
| Ι, II | 29 |
| III | 9 |
| Grade | |
| Ι, II | 28 |
| III | 10 |
| Tumor size | |
| < 2 cm | 17 |
| 2–5 cm | 19 |
| Lymph node metastasis | |
| Yes | 17 |
| No | 21 |
2.3 RNA Extraction and qRT-PCR
Total RNA was extracted using a Total RNA Extraction Kit A101231 (Parstous, Iran). Reverse transcription was performed on RNA samples to produce complementary DNA (cDNA) using the Easy cDNA Synthesis Kit (Parstous, Iran). Finally, gene expression was quantified using real-time PCR with SYBR Green Master Mix (Ampliqon, Denmark). β-Actin served as an endogenous control. The Pfaffl method was used to normalize the gene expression levels. The primer sequences used are listed in Table 2.
| Gene | Primer | Primer sequence |
|---|---|---|
| β-Actin | F | 5′-GCC TTT GCC GAT CCG C-3′ |
| R | 5′-GCC GTA GCC GTT GTC G-3′ | |
| DAPK2 | F | 5′-GACTTTGGTCTGGCTCACGA-3′ |
| R | 5′-GATGACGCCTATGCTCCACA-3′ | |
| LOC101928988 | F | 5′-GAGTCCGAAGACCCAAGTTCA-3′ |
| R | 5′-CTCTCCACCTTTTCCATTCGC-3′ |
- Abbreviations: F, forward; R, reverse primer.
2.4 Analysis of Molecular Docking
Many biological activities depend on interactions between nucleic acids and proteins. Understanding their structure facilitates the development of targeted treatments. Molecular docking is essential for identifying complex structures owing to its cost and complexity [45]. In this study, RNA-protein interactions were predicted by docking analysis. The RNA Alifold web server (http://rna.tbi.univie.ac.at) predicted the secondary structure of lncRNAs. RNA Alifold, designed with user-friendliness in mind, predicts an optimal secondary structure for a set of aligned sequences with maximum limits of 3000 nucleotides and 300 sequences [46]. Then, we obtained the 3D RNA structure from the RNA composer database, available at https://rnacomposer.cs.put.poznan.pl/, which employs a machine translation approach based on the RNA FRABASE database [47]. Next, we used SWISS-MODEL (https://swissmodel.expasy.org/), a powerful program designed for automated protein homology modeling, to design the 3D structure of the protein in PDB format [48]. The HDOCK web server (https://hdock.phys.hust.edu.cn) was used for docking analysis [49].
2.5 Statistical Analysis
REST 2009 software was used for qPCR data analysis. In addition, Graph Pad/Prism 8.0.2 program was used for statistical analysis to compare gene expression levels among the groups under study. Data are presented as mean ± SD. Kruskal–Wallis and Mann–Whitney tests were employed to compare unpaired data after investigating the normality of data distribution using the Kolmogorov–Smirnov test. Spearman's correlation coefficient performed correlation analysis and p < 0.05 was considered a significance level.
3 Results
3.1 DAPK2 Expression
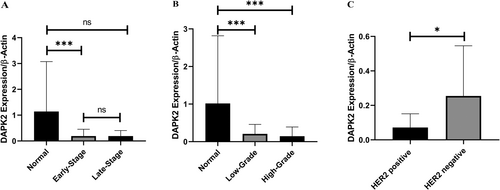
These findings indicated that DAPK2 expression was markedly reduced in tumor samples compared to that in adjacent normal tissues (p < 0.05). Tumoral samples showed a 5.24-fold reduction in DAPK2 expression (p = 0.011) (Figure 1). It was also observed that DAPK2 expression was significantly reduced in early-stage tumors compared to adjacent normal tissues (p = 0.0003). However, in late-stage tumors, the reduction in expression was borderline (p = 0.055). Moreover, DAPK2 expression was significantly decreased in both low- and high-grade tumors compared to adjacent normal tissues (p = 0.0016 and p = 0.0018, respectively). Notably, the expression of DAPK2 was significantly lower in HER2+ tumors than in HER2− tumors (p = 0.016) (Figure 2). Further analyses based on the status of ER and PR receptors, tumor size, lymph node involvement, Ki67 status, and family history did not reveal any significant relationship.
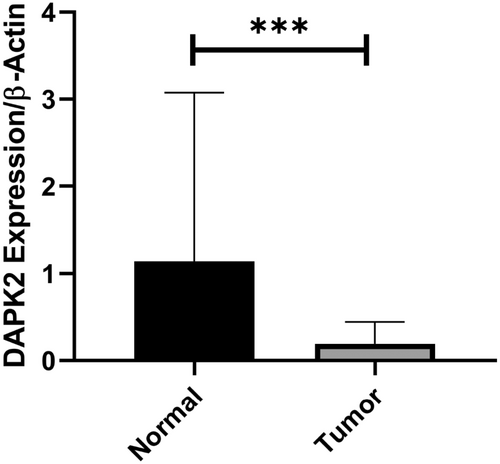
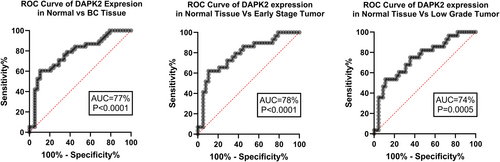
DAPK2 expression ROC curve analysis revealed 77% AUC (p < 0.0001) for BC tissue, 78% AUC (p < 0.0001) for early-stage tumors, and 74% AUC (p < 0.0001) for low-grade tumors (Figure 3).
3.2 LOC101928988 Expression
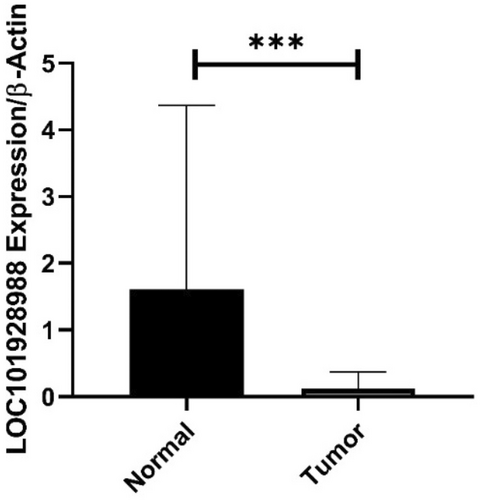
The expression of LOC101928988 was downregulated in tumor samples compared to that in adjacent normal tissues. Tumoral tissues showed a 2.44-fold increase in LOC101928988 expression compared to the control group (p < 0.0001) (Figure 4). The expression of LOC101928988 was significantly downregulated in early-stage tumoral tissues in comparison to control group (p = 0.002), but no significant decrease was found in late-stage tumoral tissues. Similarly, the expression of LOC101928988 was remarkably downregulated in low-grade tumoral tissues compared to that in the control group (p = 0.001), but no significant decrease was reported in expression in high-grade tumoral tissues (Figure 5).
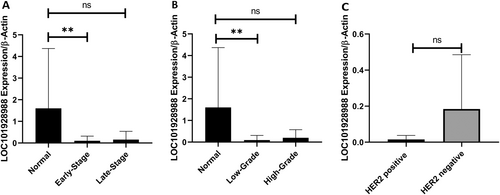
LOC101928988 expression ROC curve analysis revealed 72% AUC (p = 0.0006) for BC tissue, and especially 74% AUC (p = 0.0007) for early-stage and 75% AUC (p = 0.0004) for low-grade tumors (Figure 6). Further analyses based on the status of HER2, ER, and ki67 status, lymph node involvement, tumor size, and family history did not show any significant relationship.
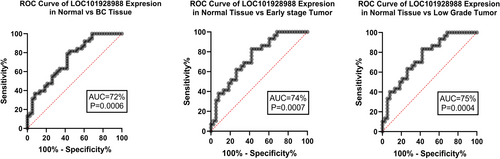
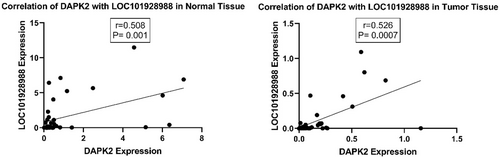
Further analysis showed a meaningful positive correlation between DAPK2 and LOC101928988 expression levels in tumoral and adjacent normal tissues (Figure 7).
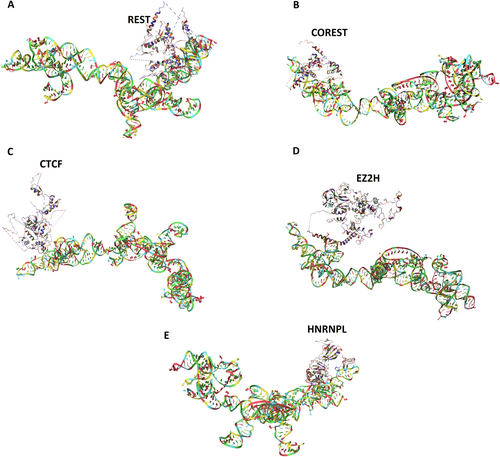
3.3 LOC101928988 and Transcription Factors (TFs) Interactions
Docking analysis showed that LOC101928988 could interact with, HNRNPL, CTCF, EZ2H, REST, and COREST that have binding sites in DAPK2 Promoters (chromosome 15: 64044400–64 047 201), with a docking energy score of −408.05, −316.24, −373.84, −332.14, and −385.68, respectively (Figure 8).
4 Discussion
Genetic mutations, chromosomal instability, epigenetics, and ncRNAs are the major components contributing to tumor development in breast cancer [50, 51].
Our results revealed that the expression of DAPK2 and LOC101928988 was downregulated in tumoral tissues compared to that in the control group. Further analysis indicated a meaningful positive correlation between DAPK2 and LOC101928988 expression in tumoral and adjacent normal tissues. Our results revealed that the expression of DAPK2 and LOC101928988 was downregulated in tumoral tissues compared to that in the control group, indicating their potential roles in breast cancer pathogenesis. The significant positive correlation between DAPK2 and LOC101928988 expression levels suggests that these genes may interact in regulatory pathways critical for tumor development, which could be a therapeutic target. Furthermore, considering the multifactorial nature of breast cancer, strategies such as repurposing drugs and administering various vitamins, including vitamin E, as prophylactic measures may have a tumor-modulatory effect with a positive impact on breast cancer outcomes [52].
The DAPK2's expression level has been found to be considerably downregulated in early-stage tumors compared to adjacent normal tissues. Moreover, the expression level of DAPK2 was notably decreased in both low- and high-grade tumors compared to that in adjacent normal tissues. HER2-positive tumors exhibited significantly lower expression level of DAPK2 than HER2-negative tumors. Similarly, when compared with adjacent healthy tissues, the expression of LOC101928988 was considerably lower in early-stage tumor tissues. Low-grade tumoral tissues also exhibited a significantly lower expression of LOC101928988 than adjacent normal tissues. ROC curve analysis of DAPK2 expression revealed an AUC of 77% for BC tissues, with a particularly high AUC of 78% for early-stage tumors and 74% for low-grade tumors. In contrast, ROC curve analysis of LOC101928988 expression revealed an AUC of 72% for BC tissues, with a particularly high AUC of 74% for early-stage tumors and 75% for low-grade tumors.
Research has shown that DAPK2 plays a dual role in cellular processes, particularly autophagy and apoptosis [26]. Nevertheless, it is recognized to function as a tumor suppressor in various types of cancers, including breast, ovarian, colorectal, bladder, gastric, NSCLC, and squamous cell carcinoma [22, 24, 53-56]. Certain signaling mediators, such as mTORC1, TP73, and Beclin-1, are crucial for the proper tumor suppressive activity of DEPDC2 [22, 57, 58].
Previous studies have described the function of ncRNAs, particularly miRNAs, in regulating the expression of DAPK2. For instance, a study by Su et al. [59] revealed the upregulation of miR-520h in breast cancer cell lines and tissues compared with normal cases. It was found that miR-520h interferes with DAPK2 expression post-transcriptionally by directly binding to the 3′-UTR of DAPK2 mRNA, thereby protecting cancer cells from apoptosis [60]. Moreover, miR-520h can inhibit paclitaxel-induced apoptosis in breast cancer by suppressing the expression of DAPK2 and caspases [59]. Hence, DAPK2 could be considered as a crucial gene that triggers cell death and suppresses cell proliferation in breast cancer cells. Similarly, another study reported that DAPK2 upregulation induces cell arrest and apoptosis in colorectal cancer. In their study a miR-1285 binding site was identified in DAPK2 mRNA. miR-1285, an oncogenic miRNA, targets DAPK2 mRNA and inhibits its expression in colorectal cancer. Therefore, inhibition of miR-1285 activity can stimulate apoptosis and lead to cell cycle arrest [55]. In another study, c-myc was shown to regulate miR-106 expression in T24 cells. A significant decrease in DAPK2 levels has been reported in bladder cancer cells because miR-106 efficiently binds to the 3′-UTR of DAPK2. Consequently, miR-106 suppresses DAPK2 transcription and inhibits apoptosis in bladder tumor cells [61]. Another study showed that lncRNA MIAT could function as an opposing endogenous RNA to increase DAPK2 levels by sponging miR-22-3p, ultimately resulting in the apoptosis of cardiomyocytes, a critical process involved in the development of diabetic cardiomyopathy [62].
The contribution of lncRNAs to the progression and development of cancer has been established in numerous studies [63]. These molecular regulatory functions are based on their sequences and structures. These can form secondary and tertiary structures with different domains, with the potential to interact with proteins, chromatin, and other transcripts [64]. For instance, the nuclear lncRNA HOTAIR function has been well investigated, and it was found that it inhibits the transcription of HoxD cluster genes by binding to the PRC2 complex of the epigenetic mechanism to regulate histone modifications in trans. HOTAIR has been demonstrated to modify the chromatin state during tumor metastasis and is increased in metastatic breast carcinomas, leading to a modified distribution of PRC2 activity from embryonic fibroblasts to breast epithelial cells [65, 66].
LOC101928988 is a type of lncRNA known as antisense lncRNA, which is located on the DAPK2 gene region. Antisense lncRNAs can manage the transcription and expression of different genes and regulate various signaling pathways. LncRNAs perform these functions through cis or trans actions. In cis action, an lncRNA interacts with the promoter region with precise sequence complementarity, while in trans action, it interacts with defective sequence complementarity [67]. These lncRNAs were identified in both the nucleus and the cytoplasm. Compared with those present in the nucleus, antisense lncRNAs are more common in the cytoplasm. These cytoplasmic lncRNAs function as regulators of the mRNA degradation and protein synthesis processes [68, 69].
Nuclear lncRNAs regulate the transcriptional landscape by interacting with chromatin remodelers, like polycomb complex's members. These lncRNAs frequently serve this purpose in cancer [70]. Recently it was indicated that upregulation of lncRNA PANDAR, leading to the conduction of G1/S transition and promoting cell proliferation in breast cancer cells. Further experiments revealed that interaction of PANDAR with the Bmi1, a member of PRC1 complex, decrease the p16INK4A level [71, 72]. Also, it has been discovered that oncogenic lncRNA, linc00511, is overexpressed in ER− breast cancer. This lncRNA inhibits cell death and accelerates the G1/S transition, leading to a worse patient prognosis. Linc00511 recruits EZH2 to the CDKN1B promoter. Notably, EZH2 acts as the catalytic subunit of PRC2 complex [73, 74].
In another study, the nuclear lncRNA EGOT1 was found at lower levels in breast cancer tissues. It is transcribed in an antisense direction from the ITPR1 gene's intronic regions. Its association with microtubule function suggests its possible influence on the response to paclitaxel, a chemotherapeutic drug. Further studies showed the direct binding ability of EGOT1 to IPTR1 pre-mRNA, which led to the formation of double-stranded RNA and regulation of ITPR1 expression. This finding demonstrates that EGOT1 plays a role both within and beyond the ITPR1 gene, which enhances the efficiency of paclitaxel treatment in breast cancer cells [75].
The results of our study revealed that changes in the expression of DAPK2 and LOC101928988 could be correlated with the initiation and progression of breast cancer. It seems that LOC101928988 has a regulatory effect on DAPK2 expression. Complementary studies using RNAInter and GeneCards databases revealed that LOC101928988 may interact with a number of TFs and RBP that have a target site on the promoter of DAPK2. Docking analysis showed that LOC101928988 could interact with HNRNPL, CTCF, EZ2H, REST, and COREST. These factors contribute to epigenetic changes, especially DNA methylation. It seems that LOC101928988 acts as a scaffold for these factors and plays a role in the assembly of the complex that mediates methylation of the DAPK2 promoter. The abovementioned hypothesis was further underscored by the docking analysis results, which unveil distinct interaction positions for each element within LOC101928988. Crucially, the binding of each factor did not impede the subsequent binding of other factors, thereby fortifying the hypothesis. Confirmation of this hypothesis requires functional and supplementary studies. According to the results of the ROC curve analysis, LOC101928988 has the potential to be further investigated as a diagnostic and prognostic panel. Further studies with larger statistical populations and functional tests, such as RIP, are recommended to validate the performance of LOC101928988. Although our analysis offers predictive insights into the interactions between LOC101928988 and DAPK2, computational predictions necessitate empirical validation to confirm their biological significance. To resolve this, it is proposed to undertake more research, including co-immunoprecipitation assays to verify direct contacts between LOC101928988 and DAPK2, as well as luciferase reporter assays to evaluate the regulatory influence of LOC101928988 on DAPK2 promoter activity.
Moreover, the found predictive efficacy of 74% for LOC101928988's target, DAPK2, prompts significant issues concerning its therapeutic relevance. Although our analysis offers significant insights into the regulatory link between these two substances, it is essential to recognize that the sample cohort was predominantly classified according to pathological phases rather than molecular subtypes. This methodological decision may restrict the generalizability of our results, given the heterogeneity and diverse genetic features associated with breast cancer. Subsequent research should focus on stratifying patient samples based on recognized molecular subtypes, including luminal A, luminal B, HER2-enriched, and triple-negative breast cancer. This approach enables us to clarify the exact settings in which LOC101928988 may regulate DAPK2 expression and potentially identify patient subgroups that could benefit most from focused therapy efforts employing this lncRNA. These investigations would not only clarify LOC101928988's function in breast cancer biology but also augment its potential as a predictive biomarker in personalized medicine.
5 Conclusion
This study includes a well-defined objective centered on understanding the regulatory relationship between LOC101928988 and DAPK2 in breast cancer, which addresses a critical gap in the current cancer research landscape. Our findings revealed that dysregulation of DAPK2 and LOC101928988 might play a role in the initiation and progression of breast cancer. Moreover, it was proposed that LOC101928988 may be involved in regulating DAPK2 gene expression through interaction with TFs. The use of paired tumoral and normal tissue samples from breast cancer patients allows for a robust comparison of gene expression in a relevant clinical setting, enhancing the ecological validity of the findings. However, the relatively small sample size may restrict the generalizability of the results. Further extensive functional validation of the mechanisms by which LOC101928988 regulates DAPK2 is required and, investigations aimed at employing LOC101928988 as an integral part of a diagnostic and prognostic panel could be beneficial.
Author Contributions
Mohammadreza Saberiyan: conception and design of the study, data analysis and writing the manuscript. Nazila Ghasemi, Negar Jafari, Mahboubeh Sadeghi, Abbas Ghaderi: acquisition of data, data analysis, and writing the manuscript. Pegah Mousavi: contributed to the study's conception and design as well as its writing. The final manuscript version was reviewed by all authors and approved.
Acknowledgments
We express our sincere gratitude for the technical help provided by the Vice Chancellor for Research at Hormozgan University of Medical Sciences. Hormozgan University of Medical Sciences provided financial assistance for this study (grant no: 4020047).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Upon request, the corresponding author will provide the data supporting the study's conclusions. The statistics are not accessible to the general public because of ethical and privacy concerns.




