Rat subthalamic nucleus and zona incerta share extensively overlapped representations of cortical functional territories
ABSTRACT
The subthalamic nucleus (STN) and the zona incerta (ZI) are two major structures of the subthalamus. The STN has strong connections between the basal ganglia and related nuclei. The ZI has strong connections between brainstem reticular nuclei, sensory nuclei, and nonspecific thalamic nuclei. Both the STN and ZI receive heavy projections from a subgroup of layer V neurons in the cerebral cortex. The major goal of this study was to investigate the following two questions about the cortico-subthalamic projections using the lentivirus anterograde tracing method in the rat: 1) whether cortical projections to the STN and ZI have independent functional organizations or a global organization encompassing the entire subthalamus as a whole; and 2) how the cortical functional zones are represented in the subthalamus. This study revealed that the subthalamus receives heavy projections from the motor and sensory cortices, that the cortico-subthalamic projections have a large-scale functional organization that encompasses both the STN and two subdivisions of the ZI, and that the group of cortical axons that originate from a particular area of the cortex sequentially innervate and form separate terminal fields in the STN and ZI. The terminal zones formed by different cortical functional areas have highly overlapped and fuzzy borders, as do the somatotopic representations of the sensorimotor cortex in the subthalamus. The present study suggests that the layer V neurons in the wide areas of the sensorimotor cortex simultaneously control STN and ZI neurons. Together with other known afferent and efferent connections, possible new functionality of the STN and ZI is discussed. J. Comp. Neurol. 522:4043–4056, 2014. © 2014 Wiley Periodicals, Inc.
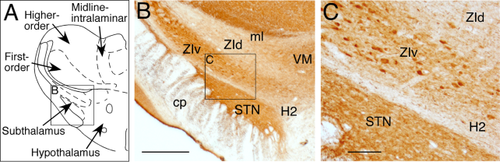
The subthalamic nucleus (STN) and the zona incerta (ZI) are two major structures of the subthalamus, which is also known as the ventral thalamus. The STN is a small, dish-shaped, and very cell dense nucleus located dorsomedial to the cerebral peduncle. The ZI is a much larger dish-shaped area than the STN and extends across the entire rostrocaudal and mediolateral axis of the subthalamus. At the level of the STN, the ZI can be subdivided into the dorsal ZI (ZId) and ventral ZI (ZIv) based on cellular composition and efferent and afferent projection sites (Nicolelis et al., 1992; Mitrofanis, 2005).
The STN and ZI may have different functional roles. The STN receives main inputs from the frontal cortex (Cx), globus pallidus external segment (GPe), thalamus, and brainstem and projects mainly to basal ganglia nuclei (Kita, 1994). The ZI has much wider afferent and efferent projection sites and is considered to be involved in various functions including arousal and attention control and sensory gating by integration of wide area brain activity and control of thalamic and brainstem activity (Ricardo, 1981; Shammah-Lagnado et al., 1987; Mitrofanis, 2005; Bartho et al., 2007; Urbain and Deschenes, 2007), although ZI may also play other roles (Mitrofanis, 2005).
The Cx projects heavily to both the STN and ZI. A common feature of these projections is that the Cx layer V pyramidal cells projecting to the STN and ZI provide extensive collateral projects to other brain areas including the dorsal thalamus, midbrain, pontine nuclei, and spinal cord (Levesque et al., 1996; Veinante et al., 2000; Kita and Kita, 2012). Some of the Cx neurons project to both the STN and ZI (Kita and Kita, 2012). However, the Cx areas projecting to the ZI are wider than those projecting to the STN. Major Cx–STN projections originate from the motor and prefrontal Cx and some from the anterior limbic Cx (Canteras et al., 1990; Degos et al., 2008; Haynes and Haber, 2013). There are no clear data to indicate the existence of projections from the parietal and occipital Cx to the STN. The ZI receives projections from all the STN projecting areas plus somatosensory and other parietal association Cx (Mitrofanis and Mikuletic, 1999). These overviews lead to an important question: whether Cx projections to the STN and ZI have independent functional organizations or a global organization encompassing the subthalamus as a whole.
In the monkey, the prefrontal, the motor, and the limbic Cx form extensively overlapped projection sites in the STN (Haynes and Haber, 2013). The projections from the monkey primary and secondary motor Cx to the STN also form overlapped projection sites (Nambu et al., 1996). However, it is not known how the functional and subfunctional cortical topography are represented in the ZI or the subthalamus as a whole. To investigate these issues and to clarify or confirm some ambiguous Cx projections to the subthalamus described in previous studies using classical tracing methods, we performed anterograde tracing studies using a highly sensitive lentivirus-based expression of green fluorescent protein (GFP) (Grinevich et al., 2005).
MATERIALS AND METHODS
Virus preparation and injections
Lentiviruses were produced as previously described by Dittgen et al. (2004) using the FCK(1.3)GW vector containing 1.3 kb recombinant promoter of the mouse calcium/calmodulin-dependent protein kinase II gene and GFP as a reporter gene. The vector backbone is based on a construct FUGW, described in Lois et al. (2002). Lentiviruses were injected into adult male Sprague–Dawley rats (270–320 g; Charles River, Wilmington, MA) in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the University of Tennessee Health Science Center Guide for the Use and Care of Laboratory Animals in Research. The rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (60 mg/kg; Pfizer, New York, NY) and xylazine (10 mg/kg, Agri Lab. St. Joseph, MO) and placed on a stereotaxic apparatus. For lentivirus injection, each rat received a craniotomy over the intended area, and the tip of the virus-containing glass micropipette (tip diameter ∼30 μm) glued to the needle of a 10-μl Hamilton syringe was placed approximately 1 mm below the cortical surface. Approximately 0.1 μl of viruses (∼1 × 106 infectious particles/μl) for the frontal Cx or 0.2 μl for the parietal and occipital Cx were pressure-injected by a pulse motor-driven actuator.
After 3 weeks of survival, the rats were deeply anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) and were perfused through the heart with 10–20 ml of isotonic saline followed by 200–300 ml of a fixative (mixture of 4% formaldehyde and 0.2% picric acid in a 0.12 M sodium phosphate buffer, pH 7.4). After perfusion, the brains were removed and postfixed overnight at 4°C and then equilibrated in a 10% followed by a 30% sucrose phosphate buffer (pH 7.4). The brains were cut into 40-μm coronal sections on a freezing microtome.
Immunohistochemistry
Immunostaining for GFP was performed for amplification and transmitted light microscopic visualization of GFP-labeled axons and boutons. The sections were rinsed with phosphate-buffered (pH 7.6) saline (PBS) and incubated in PBS containing 0.2% Triton X-100 and 0.5% dry milk for 5 hours. Then, the sections were incubated overnight in PBS containing rabbit anti-GFP (Invitrogen, Temecula, CA, Cat# A-11122, RRID: AB_221569) and 0.5% dry milk, followed by biotinylated anti-rabbit antibody (0.5 μg/ml, BA-1000; Vector, Burlingame, CA) for 1.5 hours and avidin–biotin–peroxidase complex (ABC; 1:100, Vector) for 1.5 hours. The peroxidase was then visualized by incubating the sections in PBS containing 3,3′-diaminobenzidine (DAB; 0.05%), NiCl (0.001%), and H2O2 (0.003%). This process stained the GFP-labeled axons a dark blue color. Some of these sections were further processed for immunostaining for neuron-specific nuclear protein (NeuN) or the calcium binding protein parvalbumin (PV). The sections were incubated overnight in PBS containing anti-NeuN (Millipore, Temecula, CA, Cat# MAB377, RRID: AB_2298772) or anti-PV monoclonal antibody (Swant, Bellinzona, Switzerland, Cat# PV235, RRID: AB_10000343), followed by biotinylated anti-mouse antibody (0.5 μg/ml, BA-2000, Vector) for 1.5 hours and avidin–biotin–peroxidase complex (ABC, 1:100, Vector) for 1.5 hours. The peroxidase was then visualized by incubating the sections in PBS containing DAB (0.05%) and H2O2 (0.003%). The immunostaining for NeuN stained the nuclei neurons a yellow–brown color and stained the cytoplasm a light yellow color. After several rinses, immunostained sections were mounted on gelatin-coated slides, air-dried, dehydrated in graded alcohols to xylene, and coverslipped.
The slides were observed under a microscope (Olympus BX50) with a drawing tube. Five sections with rostrocaudal separations of 160 μm (i.e., ones in every 4th consecutively cut section) covering the rostrocaudal extent of the STN were drawn, and the labeled boutons in the subthalamus were plotted. Original drawings were made under a 40× objective, marking one dot for every approximately five boutons. Many landmarks such as blood vessels and fiber bundles were also drawn. For the final computer drawings, each dot represents 10 markings (i.e., approximately 50 boutons) from the original. Microscopic images were acquired by a digital camera (Nikon D70). Images were assembled in Adobe Photoshop (CS4) and Deneva Canvas-X, with adjustments for contrast, brightness, and color balance to obtain optimal visual reproduction of data.
Antibodies
The primary antibodies used in this study are listed in Table 1. The GFP antibody is a rabbit polyclonal raised directly against GFP isolated from Aequorea victoria. It was purified by ion-exchange and has been used previously in Drosophila to label genetically expressed GFP by comparing localization of expression in specimens with and without GFP expression (Kamikouchi et al., 2006). The NeuN monoclonal antibody recognizes NeuN in most central nervous systems of vertebrates (information provided by Millipore). The staining is primarily in the nucleus of neurons (Mullen et al., 1992). The PV monoclonal antibody labels a subpopulation of neurons in normal brain with high efficiency, but does not stain the brain of parvalbumin knockout mice (manufacturer's datasheet). It produced a pattern of labeling similar to previous findings in the rat brain (Celio and Heizmann, 1981; Kita and Kita, 2001).
| Name, RRID | Immunogen | Manufacturer, species, cat. no. | Dilution |
|---|---|---|---|
| Green fluorescent protein (GFP), AB_221569 | Purified full-length native protein | Invitrogen, rabbit polyclonal, A-11122 | 1:2,000 |
| Parvalbumin (PV), AB_10000343 | Parvalbumin purified from carp muscle | Swant, mouse monoclonal, PV235 | 1:40,000 |
| Neuronal nuclear antigen (NeuN), AB_2298772 | Purified cell nuclei from mouse brain | Millipore, mouse monoclonal, MAB377 | 1:10,000 |
Delineations of STN and ZI
The STN contains abundant PV-immunoreactive (PV+) fibers and terminals and could be clearly delineated from surrounding structures (Fig. 1). The PV-stained sections were also used for subdividing the ZI into the ZId and ZIv: the former is pale with scattered PV+ neurons, whereas the latter is darker due to abundant PV+ fibers and terminals and numerous oval-shaped PV+ neurons. The Forel field H2 (H2) is a pale, narrow border area between the STN and ZIv and contains small PV-negative neurons surrounded by fibers.
RESULTS
General features of injection sites and GFP-labeled axons in the subthalamus
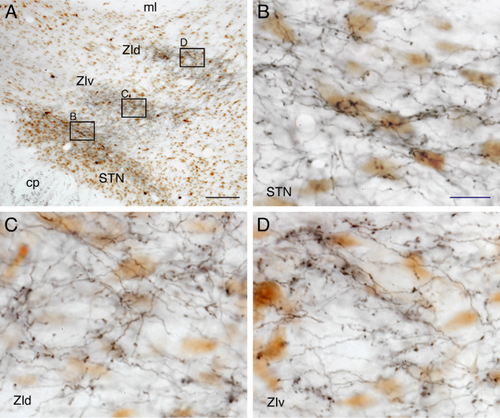
The present results were obtained from two to three selected injection cases for each of the targeted Cx areas. Either 0.1 or 0.2 μl virus injection in the Cx infected pyramidal neurons in an area 0.5–1.0 mm in diameter. In all the injections, infected neurons were found only in the injection sites. The infection of the neurons was in an all-or-none manner, and therefore, clear delineation of the injection sites was possible. The morphological integrity of the neurons observed under the light-microscope suggested that the viruses we used did not damage the infected neurons. Many neurons, probably γ-aminobutyric acid (GABA)ergic, in the injection sites were not infected (Fig. 2). In the subthalamus, the axons of the infected neurons, even small-caliber axons and thin collaterals, expressed abundant GFP. The light microscopic features of the GFP-labeled axons in the STN and ZI were the same. The terminals were small to medium and were unevenly distributed along thin axons. Some terminals were also found on short stalks, which were very similar to the previous studies using different anterograde tracers (e.g., see Figs. 3 and 8) (Mitrofanis and Mikuletic, 1999; Bartho et al., 2007; Kita and Kita, 2012).
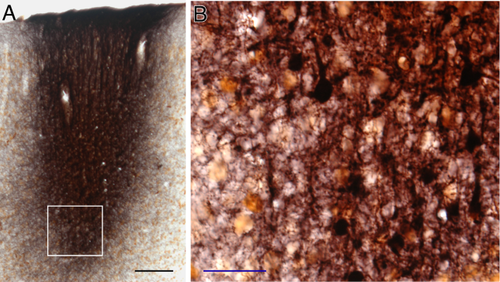
Agranular and anterior cingulate Cx
Lentiviruses were injected into different rostrocaudal levels of the lateral or medial agranular Cx (AGl and AGm, respectively). Labeled axons originating from the injection sites descended through the striatum, the pallidum, the internal capsule, and the cerebral peduncle (not shown). Then, thin collateral axons entered from the cerebral peduncle to the subthalamus through its rostral or ventral border and formed en passant or terminaux boutons. Some of the axons innervating the STN traveled further in dorsomedial and caudal directions to innervate the ZI (Fig. 3).
Figure 4 shows the labeling of large terminal areas in the STN, ZIv, and ZId after virus injections into different parts of the AGl of three rats. In a global view, the labeled terminal fields were in very similar locations, although the labeling density and distributions of heavily labeled patchy areas differed from case to case. In the STN, a disk-shaped heavily labeled area expanded from the ventral part of the rostral STN to the dorsal part of the middle STN. The orientation of the disk was parallel to the dorsal border of the STN. The ventromedial part of the caudal STN was free of labels or only sparsely labeled by any of the AGl injections. In the ZI, virus injections in the rostral part of the AGl resulted in the labeling of dense terminal areas in H2, medial parts of caudal ZIv, and medial parts of caudal ZId (Fig. 4A,B). The terminal areas in the STN and ZI were continuous along the trajectory of axons traversing from the internal capsule in the dorsomedial caudal direction, as mentioned above (Fig. 3). Injections in the caudal part of the AGl resulted in only sparse labeling in the ZI, although the labeling in the STN was dense (Fig. 4C). The labeling density of these labeled areas was heterogeneous, with very dense patchy areas to sparse areas (Fig. 3).
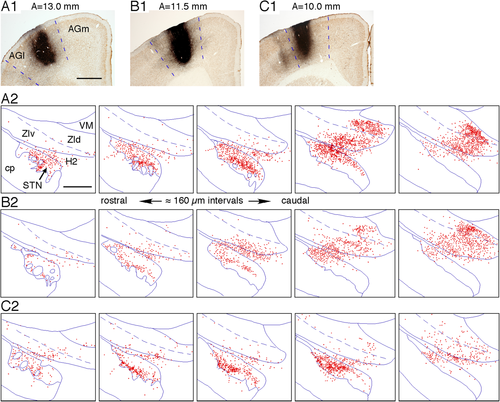
Figure 5A–C shows results of virus injections into different rostrocaudal levels of the AGm of three rats. In the STN, labeled areas diffusely covered a large part of the STN except the most lateral part of the STN and a medial part of the caudal STN. In the ZI, the labeled areas diffusely covered a large part of both the ZIv and ZId, with heavier labeling in the ventromedial parts than the dorsolateral (Figs. 5A–C). Overall, the heavily labeled locations were very similar to those seen after AGl injections, although AGm injections in general resulted in more diffuse labeling compared with those seen after AGl injections.
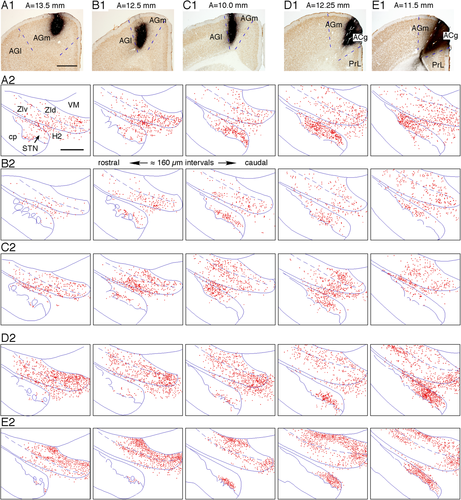
Lentivirus injections into the anterior cingulate Cx (ACg) were performed in four rats. The two rats with the most infected neurons in the ACg were selected for analysis (Fig. 5D,E). The other two rats had injection sites extending more into the AGm and were not chosen for this study. The GFP-labeled axons from the ACg descended through the striatum, the rostrodorsal part of the globus pallidus, and the internal capsule. From the cerebral peduncle, two separate groups of thin axons innervated the subthalamus. One group of thin axons in the internal capsule descended to the cerebral peduncle and then entered the STN and formed a dense terminal field in the ventromedial part of the middle to caudal STN (Fig. 5D,E). Another large group of medium-sized axons entered from the internal capsule to the rostrolateral part of the ZId, traversed the ZId and ZIv in the caudomedial direction, and emitted thin collaterals with boutons in these areas. The terminal field with dense patches and surrounding moderate to sparse areas covered most of the ZId and the medial part of the ZIv (Fig. 5D,E). The terminal areas in the STN were located slightly more medially compared with those labeled after AGm injections. In the ZI, labeled terminals were found in the medial two-thirds of the ZI and greatly overlapped with the AGl and AGm terminal areas. Because these ACg virus injections infected some AGm neurons, some of the labeling could belong to AGm neurons. The group of axons innervating the ZI traveled further medially and dorsally to innervate the ventromedial nucleus, intralaminar nuclei, and posterior thalamic nuclei.
Orbital and medial limbic Cx
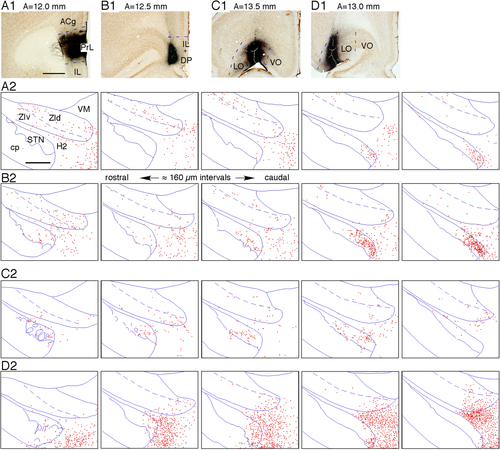
Virus injections into the frontal prelimbic (PrL), the infralimbic (IL), the dorsal peduncular Cx (DP), and the lateral part of the lateral orbital Cx (LO) resulted in sparse to moderate labeling of boutons in the medial part of the caudal STN, adjacent H2, and lateral hypothalamus (Fig. 6A,B,D). Virus injections into the medial part of the LO resulted in sparse labeling of boutons in the rostral two-thirds of the STN (Fig. 6C). Thin axons innervating these areas entered from the medial part of the cerebral peduncle. The ZI contained only occasional axons and very sparse boutons after the orbital and the medial limbic Cx injections (Fig. 6A–D).
Primary somatosensory and parietal Cx
Lentivirus injections were made into the anterior and posterior parts of the primary somatosensory Cx (S1) of two rats. GFP-labeled axons from the anterior S1 traveled through the striatum and pallidum and reached the cerebral peduncle. Thin collateral axons emitted in the cerebral peduncle entered the subthalamus through its rostral or ventral border. A moderate density of GFP-labeled boutons was observed in the rostrolateral part of the STN, the medial parts of the caudal ZIv, and the medial parts of the caudal ZId (Fig. 7A). These labeled locations were very similar to those observed after virus injections into the AGl.
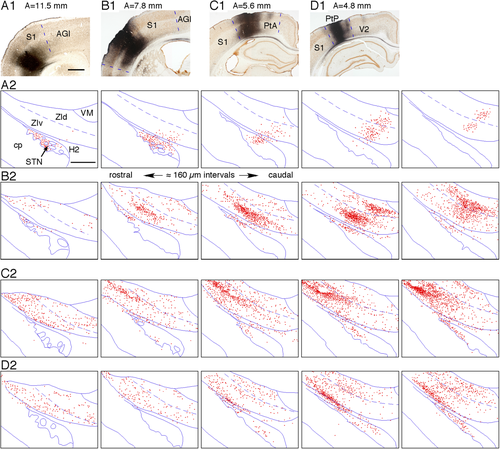
For the virus injections into more posterior regions of S1, such as the whisker barrel field, and also for the injections into the parietal Cx described below, most of the thin axons innervating the ZI and STN emerged from the axons that innervated the dorsal thalamus (Levesque et al., 1996). The axons innervating the dorsal thalamus branched out from parent axons in the internal capsule and traversed in a dorsomedial direction through the rostrolateral part of the ZI and emitted thin secondary collaterals innervating the ZI and STN (Fig. 8). Virus injections into the whisker barrel field resulted in the labeling of very heavy terminal fields in the mid-mediolateral caudal two-thirds of the ZIv and ZId and moderate density terminal fields in the lateralmost part of the STN (Fig. 7B). These labeled areas were located slightly dorsolateral to the AGl projection sites.
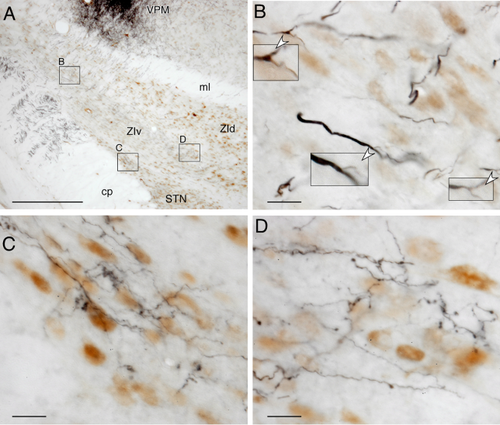
Viruses were injected into the anterior and posterior parietal Cx of two rats. Labeled axons originating from the injection sites entered the most dorsal part of the caudal striatum at the level of the internal segment of the pallidum (approximately 6 mm from the interaural line), traversed a short distance medially through the striatum, and entered into and descended through the internal capsule. Some of the axons in the internal capsule emitted collaterals innervating the dorsal thalamus, and thin collateral axons innervating the ZI and STN emerged from those collateral axons, as described above.
Virus injections into the anterior parietal Cx heavily labeled the lateral part of the ZIv and moderately labeled the adjacent lateral part of the ZId (Fig. 7C). These labeled areas were slightly lateral to the S1 projecting areas. Virus injections into the posterior parietal Cx heavily labeled the ventrolateral part of the middle ZIv and moderately labeled the rest of the ZIv and the caudal two-thirds of the ZId (Fig. 7D). Virus injections into both anterior and posterior parietal Cx labeled a moderate density of boutons in the narrow lateral part of the STN. In all the S1 and parietal injections, the projection sites of the STN were laterally continuous to the labeled areas in the ZIv.
Visual and auditory Cx injections
Lentivirus injection into the visual Cx approximately 4 mm interaural and 4 mm lateral from the midline and into the auditory Cx approximately 4 mm interaural and 7 mm lateral from the midline resulted in sparse terminal labeling at the lateralmost part of the rostral ZId and ZIv (data not shown). Axons innervating these areas were collaterals of axons traversing from the subcortical white matter through the caudodorsal striatum and the internal capsule to the dorsal thalamus at the level of the STN. No labeled axons were found in the STN after visual and auditory Cx injections.
DISCUSSION
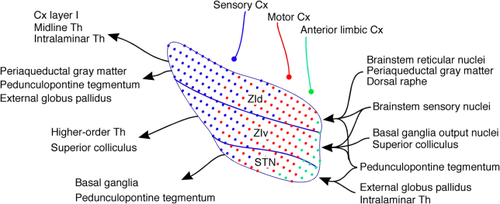
The most significant finding of this study is that the Cx–subthalamic projections have a large-scale organization encompassing all subdivisions of the subthalamus (the STN, ZIv, and ZId) and that a group of cortical axons originating from a particular area of the Cx sequentially innervates and forms terminal fields in each subdivision. This study also revealed that the representation of cortical functional zones encompasses the subthalamus as a whole, and that from the lateral to medial direction, the sensory, motor, and limbic Cx projections form large cloud-like terminal zones with fuzzy borders and extensive overlaps (Fig. 9). The functional implications of these cortical afferents together with other projections are discussed in more detail below.
Methodological considerations
The most significant advantage of the lentivirus tracing method is that there is absolutely no labeling of fibers of passage. Both high molecular weight (10 k) biotinylated dextran amines (BDA) and Phaseolus vulgaris leucoagglutinin (PHA-L) can be taken up by fibers of passage, although to a lesser degree than low molecular weight BDA and horseradish peroxidase (HRP) (Reiner et al, 2000). The viruses we used appeared to do no damage to neurons at the injection sites, as judged by the morphological integrity of the injection sites observed under the light microscope. The viruses infected neurons in an all-or-none manner, and the somata and axons of all the infected neurons expressed abundant GFP. Therefore, the extent of the injection site and the projection sites can be clearly identified. In the projection sites, the axons of infected neurons, even small-caliber axons and thin collaterals from a long distance away from the injection sites, expressed abundant GFP, and Golgi-like GFP staining characteristics allowed clear visualization of fine axons with boutons. Visualization of thin axons containing small amounts of PHA-L and BDA was often difficult in our experience (Kita and Kitai, 1987; Kita and Kita, 2012).
We know of only three anterograde tracing studies of Cx–subthalamic projections that systematically covered large cortical areas. There were two studies on Cx–STN projections in the monkey (Nambu et al., 1996; Haynes and Haber, 2013) and one on Cx–ZI projections in the rat (Mitrofanis and Mikuletic, 1999), and all three studies used the BDA method. This is the first study to analyze both the STN and ZI using the most advanced method existing today. This study also systematically covered functionally diverse cortical areas.
Functional zones
The large-scale organization of the functional zones found in this study is as follows: The parietal sensory association and somatosensory Cx projecting zones occupy a large part of the dorsolateral ZId and ZIv. The locations of the parietal association and the somatosensory Cx projecting zones in the ZI were similar to but much larger than those shown using the BDA method (Mitrofanis and Mikuletic, 1999; Mitrofanis, 2005). This study confirmed the observation made by classical HRP and WGA–HRP methods in rats, cats, and monkeys that the STN has small somatosensory Cx projecting zones at the dorsolateralmost part of the STN, which was located adjacent to the somatosensory zone in the ZIv (Carpenter et al., 1981; Canteras et al., 1988; Noda and Oka, 1993), whereas studies using autoradiographic methods did not find this projection (e.g., Afsharpour, 1985). This study revealed that the parietal association Cx also projects to a similar area of the STN.
The motor Cx zone includes a large middle part of the STN and a large medial two-thirds of the ZI. In general, the locations of the zones were similar to previous reports on STN projections (Afsharpour, 1985; Canteras et al., 1990; Nambu et al., 1996; Haynes and Haber, 2013) and on ZI projections (Mitrofanis and Mikuletic, 1999). However, the areas seen in the present study were much larger than previously implied. We also noted that the STN of primates and rodents have slightly different topographies of the functional zones. The primate has a large dorsolateral prefrontal association Cx that projects to the mid-mediolateral part of the STN, and the motor Cx innervates to the lateral part of the STN (Parent and Hazrati, 1995; Haynes and Haber, 2013), whereas in rodents, the area equivalent to the primate dorsolateral prefrontal Cx may be very small or nonexistent (Vertes, 2004; Preuss, 1995), and projections from the rat motor cortex occupy the mid-mediolateral part of the STN.
The ACg has been often considered as a prefrontal structure because of its involvement in various motor-related cognitive functions (Harrison and Mair, 1996; Dalley et al., 2004). However, a microstimulation study in the rat suggested that the dorsal part of the ACg includes the eye and nose areas of the primary motor Cx (Brecht et al., 2004). We found that the dorsal part of the ACg projects heavily to the STN and ZI. The ACg projection areas included the ventromedial part of the caudal half of the STN and the medial two-thirds of both the ZId and ZIv. These areas greatly overlapped with AGm projecting areas. ACg and AGm projections to other brain sites were also very similar (Gabbott et al., 2005). Mitrofanis and Mikuletic (1999) reported that BDA injections into the caudal part of the dorsal ACg densely and diffusely labeled the medial two-thirds of the ZId. These observations suggest that the ACg may have dual functions, primarily motor and motor-related cognitive, and in the latter, eye and nose movements may play essential roles.
The anteromedial limbic Cx projection zone includes the ventromedial part of the STN, adjacent H2, and lateral hypothalamus. No STN projection was seen from the visual Cx. The limbic, orbital, and visual Cx only sparsely project to the ZI (Power et al., 2001).
The functional zones described above extensively overlapped with each other. The overlap may be not unique to the rodent STN. The primate prefrontal, premotor, motor, and limbic projections to the STN also have extensive overlaps (Nambu et al., 1996; Haynes and Haber, 2013). Also, the extensive overlap in the STN may be not unique to cortical projections. Bevan et al. (1997) showed that projections from the external segment of the globus pallidus and the ventral pallidum, sensorimotor, and limbic structures, respectively, to the STN converge onto the same neurons. There are currently very few data on the primate Cx–ZI projections, and therefore, it is not possible to compare them at this time. The neurons in the STN and ZI have dendritic arbors extending a long distance in the dorsolateral to ventromedial direction (Kita et al., 1983; Bartho et al., 2007). Both the projection patterns and the morphological characteristics described above suggest that each neuron in the subthalamus may receive inputs from wide areas of the Cx.
Somatotopic representations
Previous studies suggested that projections from the primate motor Cx to the STN and rat somatosensory Cx projections to the ZI have somatotopic representations (Nambu et al., 1996; Nicolelis et al., 1992; Shaw and Mitrofanis, 2002). It was evident in this study that axons originating from different locations in the somatosensory or motor Cx resulted in the labeling of terminal fields in slightly different but largely overlapping locations in the STN and ZI. It was also evident that axons originating from a small area in the Cx formed large terminal fields in the STN and ZI. These observations suggest that the projections from the motor and somatosensory Cx have extensively overlapped, fuzzy somatotopic representations in the STN, ZId, and ZIv.
Other afferent and efferent projections of the subthalamus
The STN receives strong afferent projections from the GPe, ventral pallidum, intralaminar thalamic nuclei, and pedunculopontine tegmental nucleus (PPTg). These projections have extensively overlapped topographical organizations similar to Cx projections (Groenewegen et al., 1993; Maurice et al., 1998; Kita, 2007). The STN sends glutamatergic outputs mainly to other basal ganglia nuclei (Ricardo, 1980; Kita and Kitai, 1987). Thus, the STN may be involved in an associative function by integrating motor, prefrontal, and limbic related inputs that are required to perform complex behaviors. This assumption is supported by the observation that the STN is more active during complex movement tasks (Lehericy et al., 2006).
The ZId receives afferent projections from several brainstem nuclei, including the dorsal raphe, periaqueductal gray matter, PPTg, and midbrain and pontine reticular nuclei (Ricardo, 1981; Watanabe and Kawana, 1982; Shammah-Lagnado et al., 1985; Border et al., 1986; Kolmac et al., 1998). The sensory trigeminal nuclear complex and the dorsal column nuclei also project to the ZId (Nicolelis et al., 1992; Simpson et al., 2008). These projections are diffuse and have no clear topography in the ZId. Thus, projections from various functional domains converge on the ZId (Ricardo, 1981; Shammah-Lagnado et al., 1985, 1987; Kolmac et al., 1998).
The GABAergic neurons in the ZId project to the layer I of a very wide area of the Cx in a diffuse and rough topographical manner, to midline and intralaminar thalamic nuclei, to the pretectal area, and to pontine reticular nuclei (Shammah-Lagnado et al., 1987; Lin et al., 1990, 1997; Nicolelis et al., 1992, 1995; Power et al., 1999). Known projection sites of glutamatergic neurons in the ZId include the globus pallidus, periaqueductal gray, and PPTg (Beitz, 1989; Heise and Mitrofanis, 2004).
The ZIv receives topographically organized projections from the deep layers of the superior colliculus, the sensory trigeminal nuclear complex, and the dorsal column nuclei (Nicolelis et al., 1992; Kolmac et al., 1998). Other afferents known to project to the ZIv include the PPTg, the deep cerebellar nuclei, and the basal ganglia output nuclei globus pallidus interna (GPi) and substantia nigra pars reticulata (SNr). The topographical arrangements of the afferent zones from the brainstem sensory and motor related nuclei roughly match those Cx afferent zones (see also Roger and Cadusseau, 1985; Shammah-Lagnado et al., 1985).
The ZIv contains mostly GABAergic neurons. These neurons project to the superior colliculus and the higher order thalamic nuclei such as the posterior and anterior pretectal nuclei and contralateral ZI (Watanabe and Kawana, 1982; Foster et al., 1989; Kolmac et al., 1998; Bartho et al., 2002). These afferent and efferent projections of the ZIv appear to show clearer topography than ZId projections. There are heavy mutual intra-incertal connections between the ZId and ZIv and also within the each subdivision.
Deep brain stimulation of the motor zone of subthalamus
Deep brain stimulation (DBS) of the STN and other brain areas has been used for the treatment of parkinsonisms. DBS sites effective for parkinsonisms include STN and ZI motor zones. Plaha et al. (2006) suggested that DBS of the ZI motor zone might produce more beneficial effects than DBS of the STN in some cases. DBS of the ZI is also effective for essential tremor and parkinsonian tremor (Xie et al., 2012). We speculate that the DBS of the STN and ZI motor zones may exert different effects because these areas have different connections and may have different functional roles, as discussed above.
In vivo unit recordings show that stimulation of the motor Cx induces a short latency excitation in STN and ZI neurons (Fujimoto and Kita, 1993; Bartho et al., 2007). Thus Cx stimulation may be used to locate effective DBS sites. In order to distinguish STN and ZI motor zones, the short latency response should be combined with additional observations such as unique response and firing patterns of neurons in these nuclei (Bartho et al., 2007; Lavallee et al., 2005). It should also be noted that because of the extensive overlap of different functional zones and extensive local collateral connections, an activation of only motor related circuits by DBS of the subthalamus is not possible.
Functional implications
Based on the present and previous studies, we suggest that the subthalamus integrates and analyzes a broad spectrum of inputs and performs two modes of control. One mode of control is to integrate diverse cortical and brainstem inputs and outputs that control appropriate arousal, attention, sleep–waking cycle, nociceptive behavior, and posture–locomotive responses through the direct and thalamus-mediated projections to the Cx and brainstem, as postulated by several authors (Ricardo, 1981; Steriade et al., 1982; Shammah-Lagnado et al., 1985; Berry et al., 1986; Kolmac et al., 1998; Power and Mitrofanis, 2001; Mitrofanis, 2002, 2005). The global projection patterns of the ZId described above may be well suited for this mode of control (Kolmac et al., 1998; Power and Mitrofanis, 2001). We postulate that integration of coincidentally arriving weak multimodal inputs from brainstem nuclei or multiple areas of the Cx can drive ZI neurons. Several behavioral studies showed that electrical or chemical stimulation of the ZI generates locomotor activity, some of which is associated with defense orientation (Mogenson et al., 1985; Milner and Mogenson, 1988; Murer and Pazo, 1993; Supko et al., 1992; Pèrier et al., 2002). For the general sensory function, activation of the ZI with glutamate has antinociceptive effects in the rat (Petronilho et al., 2012). Bartho et al. (2007) showed that activity of both ZId and ZIv neurons is synchronous to layer V neurons in the somatosensory cortex. They suggested that the widespread projections from the Cx drive the ZI activity and that the GABAergic efferent signals from ZI neurons may play a critical role in synchronizing thalamocortical and brainstem rhythms. The DBS effects discussed above may be due to a suppression of abnormal oscillations in the motor zones of the subthalamus.
The second mode is to control specific events through more functionally specific input–output channels. Nicolelis et al. (1992) found that individual ZId and ZIv neurons have multi-whisker receptive fields with low-threshold activation to whisker movements. Ma (1996) found that the firing of some ZIv neurons pauses prior to the beginning of and during saccades. The pauses were related to the duration of but unrelated to the direction of saccades. These observations suggest that the ZI may be involved in the generation of orientative head and eye movements probably through the superior colliculus. We have often tested neurons in the motor zones of the ZId and ZIv by electrical stimulation of the motor cortex in rats and monkeys during recording of electrode penetrations to the STN. We observed that single low-intensity stimulation could excite most of the neurons with a very short latency, suggesting that direct inputs from a small area of the motor Cx could activate the ZI neurons (Kita, unpublished observations). Urbain and Deschenes (2007) showed that the projection from the motor Cx to the ZI gates vibrissal responses in a thalamocortical pathway. For the gating, the motor Cx activates GABAergic neurons in the motor zone of the ZI, which then inhibits thalamic projecting neurons in the ZIv sensory zone through intra-incertal connections. An opposite direction of control, from the ZI sensory zone to the ZI motor zone through intra-incertal connections, is also possible. Trageser et al. (2006) proposed that PPTg–ZIv–posterior thalamic projections are involved in the behavioral state–dependent gating of sensory inputs. These recent studies suggest that the ZI is involved in the control of specific sensorimotor functions. The present study suggests that the layer V neurons in the wide areas of the sensorimotor Cx simultaneously control basal ganglia, sensory gating, and, arousal and attention functions, respectively, through the STN, ZIv, and ZId.
ACKNOWLEDGMENTS
We thank B. Chism for technical assistance and R. Kita for editing the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ROLE OF AUTHORS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. HK designed the research. TK and HK performed the research and analyzed the data. TK, PO, and HK wrote the paper.