Central pupillary light reflex circuits in the cat: II. Morphology, ultrastructure, and inputs of preganglionic motoneurons
ABSTRACT
Preganglionic motoneurons supplying the ciliary ganglion control lens accommodation and pupil diameter. In cats, these motoneurons make up the preganglionic Edinger-Westphal population, which lies rostral, dorsal, and ventral to the oculomotor nucleus. A recent cat study suggested that caudal motoneurons control the lens and rostral motoneurons control the pupil. This led us to examine the morphology, ultrastructure, and pretectal inputs of these populations. Preganglionic motoneurons retrogradely labeled by introducing tracer into the cat ciliary ganglion generally fell into two morphologic categories. Fusiform neurons were located rostrally, in the anteromedian nucleus and between the oculomotor nuclei. Multipolar neurons were found caudally, dorsal and ventral to the oculomotor nucleus. The dendrites of preganglionic motoneurons within the anteromedian nucleus crossed the midline, providing a possible basis for consensual responses. Ultrastructurally, several different classes of synaptic profiles contact preganglionic motoneurons, suggesting that their activity may be modified by a variety of inputs. Furthermore, there were differences in the synaptic populations contacting the rostral vs. caudal populations, supporting the contention that these populations display functional differences. Anterogradely labeled pretectal terminals were observed in close association with labeled preganglionic motoneurons, particularly in the rostral population. Ultrastructural analysis revealed that these terminals, packed with clear, spherical vesicles, made asymmetric synaptic contacts onto motoneurons in the rostral population, indicating that these cells serve the pupillary light reflex. Thus, the preganglionic motoneurons found in the cat display morphologic, ultrastructural, and connectional differences suggesting that this rostral preganglionic population is specialized for pupil control, whereas more caudal elements control the lens. J. Comp. Neurol. 522:3978–4002, 2014. © 2014 Wiley Periodicals, Inc.
Abbreviations
-
- AM
-
- anteromedian nucleus
-
- APt
-
- anterior pretectal nucleus
-
- At
-
- axonal terminal
-
- At*
-
- anterogradely labeled axon terminal
-
- Den
-
- dendrite
-
- Den*
-
- retrogradely labeled dendrite
-
- EWpg
-
- preganglionic Edinger-Westphal nucleus
-
- EWcp
-
- centrally projecting Edinger-Westphal nucleus
-
- H
-
- habenular nucleus
-
- INC
-
- interstitial nucleus of Cajal
-
- III
-
- oculomotor nucleus
-
- IIIn
-
- oculomotor nerve
-
- MLF
-
- medial longitudinal fasciculus
-
- MPt
-
- medial pretectal nucleus
-
- MRF
-
- midbrain reticular formation
-
- MV
-
- multivesicular body
-
- ND
-
- nucleus of Darkschewitsch
-
- NPC
-
- nucleus of posterior commissure
-
- Nu
-
- nucleus
-
- OPt
-
- olivary pretectal nucleus
-
- PAG
-
- periaqueductal gray
-
- PC
-
- posterior commissure
-
- PPt
-
- posterior pretectal nucleus
-
- PUL
-
- pulvinar
-
- RB
-
- retroflex bundle
-
- rER
-
- rough endoplasmic reticulum
-
- RN
-
- red nucleus
-
- SC
-
- superior colliculus
-
- SOA
-
- supraoculomotor area
-
- Soma
-
- cell body
-
- Soma*
-
- retrogradely labeled cell body
-
- Sp
-
- spine
-
- VTA
-
- ventral tegmental area
As crepuscular animals that hunt primarily at dusk and dawn, cats display a considerable ability to change their pupillary diameter in response to luminance changes. With their frontally placed eyes, they display both direct and consensual pupillary responses. Cats also have excellent stereoscopic vision and can accurately target objects within their near field, as anyone who has “played” with a cat can attest. This stereoscopic vision is supported by the frontal placement of their eyes and fine control over both vergence and lens accommodation. These capabilities have made them a favored model for those studying the pupillary light reflex and for those examining the neuronal control of the lens and pupil as part of the near response (for review see Loewenfeld, 1993; Gamlin, 2000). However, anatomical examination of the parasympathetic, preganglionic motoneurons projecting to the ciliary ganglion for control over these functions has revealed that this population has a relatively disorganized distribution in cats (Sugimoto et al., 1977; Loewy et al., 1978; Toyoshima et al., 1980); these cells are not confined to a well-circumscribed nucleus like that found in primates (Akert et al., 1980; Burde and Loewy, 1980; Clarke et al., 1985; Sun and May, 1993; May et al., 2008a,b) and birds (Gamlin et al., 1984; Gamlin and Reiner, 1991; Reiner et al., 1991). Instead, the feline organization appears more similar to that of lateral-eyed animals, with relatively limited oculomotor capabilities such as those of rodents (Vann and Atherton, 1991; Smeraski et al., 2004; Kozicz, 2007) and rabbits (Johnson and Purves, 1981).
The preganglionic parasympathetic motoneurons in the cat are scattered dorsal, rostral, and ventral to the oculomotor nucleus proper (III) and are not confined to the cytoarchitectonically defined Edinger-Westphal nucleus (EW). Instead, evidence has mounted that the cytoarchitectonically defined EW in cats and rodents contains cells that project centrally (Loewy et al., 1978; Loewy and Saper, 1978; Burde et al., 1982; Maciewicz et al., 1983; Strassman et al., 1987; Klooster et al., 1995a) and that this nucleus contains mainly peptidergic neurons, including urocortin 1-positive cells, as opposed to cholinergic motoneurons (Maciewicz et al., 1983; Strassman et al., 1987; Kozicz et al., 1998; Bachtell et al., 2002a, 2002b). The fact that the peptidergic centrally projecting and cholinergic peripherally projecting populations have largely separate distributions was further underscored by comparative studies in cat, macaque, and humans (Horn et al., 2008; May et al., 2008a). It is evident that the arrangement of these populations with respect to a cytoarchitectonically defined EW varies across species. Consequently, the terminology has been recodified by designating the population of cholinergic motoneurons supplying the ciliary ganglion as the preganglionic EW population (EWpg) and the peptidergic interneurons as the centrally projecting EW population (EWcp; Kozicz et al., 2011). In cats, the EWcp neurons lie primarily within a well-circumscribed, midline nucleus dorsal to III, whereas the EWpg neurons have a scattered, perioculomotor location.
Our interest in the cat EWpg population was piqued by a study that used transsynaptic retrograde tracers to discriminate parasympathetic populations related to control of the ciliary muscle that regulates lens accommodation, as opposed to those controlling the sphincter pupillae muscle, which regulates pupil diameter (Erichsen and May, 2002). This study indicated that lens control motoneurons were located primarily caudally: dorsal to III, in the supraoculomotor area (SOA) and to a lesser extent in EWcp, and ventral to III, among the exiting oculomotor nerve fiber bundles. In contrast, pupil control motoneurons were located primarily rostral to III, in and lateral to the anteromedian nucleus (AM), even though the AM is continuous with the EWcp and also contains numerous peptidergic neurons (May et al., 2008a). In light of these findings, we decided that a more detailed examination of the morphology and ultrastructure of the cat preganglionic motoneurons was warranted. The morphology of the preganglionic motoneurons supplying the ciliary ganglion was defined by injecting the ciliary ganglion with retrograde tracers. The types of synapses terminating on these identified motoneurons were then characterized with the electron microscope. To distinguish pupillary from lens motoneurons, an anterograde tracer was injected into the olivary pretectal nucleus of animals that also had ciliary ganglion injections. In this way, we directly determined the targets of the pupillary light reflex pathway, allowing us to gain more insight into the anatomical substrate for controlling the pupil and lens.
MATERIALS AND METHODS
Surgical procedures
Nine adult cats of both sexes, weighing 1.5–3.5 kg, were used in this study. Animal procedures were performed in accordance with NIH guidelines, using protocols approved by the local Institutional Animal Care and Use Committee. Prior to surgery, animals received an injection of steroid (dexamethasone, 2.5 mg/kg, SC) to limit tissue swelling and atropine (0.1 mg/kg, SC) to reduce respiratory secretion. Animals were anesthetized by administration of sodium pentobarbital (35 mg/kg, IV). Anesthesia was supplemented, as necessary, to maintain an adequate surgical level throughout the operations. Analgesics (butorphanol 0.3 mg/kg or buprenex 0.01 mg/kg) were administrated to alleviate postoperative discomfort. After a 24-hour survival period for transport of tracers, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) before transcardial perfusion. Sodium nitrite (1.0 ml, 1.0%, IV) was administered to facilitate vascular dilation, and the animals were perfused with 0.1 M phosphate-buffered saline (pH 7.2), followed by fixative containing 1% paraformaldehyde and 1.5% glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.2).
Ciliary ganglion injection (n = 4, ganglion alone)
The ciliary ganglion was injected in order to define the distribution, morphology, and ultrastructure of preganglionic motoneurons in the cat. The ciliary ganglion was exposed through either a dorsal or a lateral approach to the orbit in animals held in a stereotaxic apparatus. For the dorsal approach, a hole was drilled through the frontal sinus. The superior rectus muscle and optic nerve were retracted laterally to allow visualization of the ganglion. For the lateral approach, a horizontal skin incision about 2.5 cm long was made 1–1.5 cm posterior to the lateral angle of eye. The temporalis muscle was retracted to allow access to the orbit through the incomplete bony orbit of the cat. The lateral rectus muscle was exposed and then cut and retracted. A small portion of the zygomatic bone was drilled away to access the posterior portion of the orbit better. The eyeball was temporarily collapsed by removing the aqueous humor from the anterior chamber to enhance the exposure. The ciliary ganglion was exposed by blunt dissection. The lateral approach, although more technically demanding, produced less trauma and was adopted in the later experiments. The neuronal tracer was introduced into the ganglion by penetration with several insect pins, the tips of which were covered by a recrystallized solution of 2% wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP; Sigma, St. Louis, MO) and 20% horseradish peroxidase (HRP; Sigma). We attempted to avoid contaminating the nerve trunk by which the ciliary ganglion is attached to the inferior division of the oculomotor nerve, or the adjacent extraocular muscles. In two animals, the contralateral ciliary ganglion was also exposed, but the inferior branch of the oculomotor nerve distal to the ciliary ganglion was injected with the WGA-HRP and HRP tracer as a control. After injections, the skin over the site was closed with suture.
Pretectal injection (n = 5, pretectum plus ganglion)
To access the olivary pretectal nucleus, a midline incision was made in the scalp, and the temporalis muscle was disinserted and retracted laterally so that a craniotomy could be made just off the midline. The cortex overlying the superior colliculus and posterior thalamus was aspirated. The tracer, biocytin (Sigma), was injected into the olivary pretectal nucleus by use of a 1.0-μl Hamilton syringe. The tip of the needle was inserted 0.8–1.2 mm into the brainstem at the tectopulvinar junction, between 2.5 and 2.8 mm off the midline, and 0.1–0.2 μl of 4.0% biocytin was pressure injected. Gelfoam was used to fill the aspiration void, and the muscle and skin were reapproximated and secured with suture. These animals then received a ciliary ganglion injection of WGA-HRP and HRP, as described above.
Histochemical procedures
After perfusion, the brains were blocked in the stereotaxic plane, postfixed for 1 hour in the same fixative with which they were perfused, and then stored overnight in 0.1 M PB (pH 7.2) at 4°C. The appropriate blocks were sectioned in a 50-, 50-, 100-μm-thick repeating series with a vibratome (Technical Products International, Inc.). For light microscopic (LM) observations, 50-μm sections were used, and 100-μm sections were used for electron microscopic (EM) procedures.
WGA-HRP protocol
Labeling with WGA-HRP was demonstrated by use of a tetramethylbenzidine (TMB; Fisher, Fair Lawn, NJ) procedure. This is a modification of the TMB-ammonium molybdate method of Olucha and colleagues (1985). Sections were incubated in 0.1 M PB (pH 6.0) that contained 0.25% ammonium molybdate and 0.005% TMB. For the reaction, hydrogen peroxide was added to achieve a final concentration of 0.01%. The reaction period was extended overnight at 4°C, followed by stabilization in a 5.0% ammonium molybdate solution in 0.1 M PB (pH 6.0). Those sections used for LM study were mounted onto gelatinized slides, air dried, counterstained with cresyl violet or neutral red, dehydrated, cleared, and coverslipped. Labeled cells were drawn using an Olympus BH-2 microscope equipped with a drawing tube. They were photographed with a Nikon E600 microscope equipped with a Nikon DS-Ri1 digital camera. Images were obtained in Nikon Elements software, and the contrast and illumination were adjusted in Photoshop (Adobe) to emulate their appearance to the eye. Those sections intended for EM examination were reacted further with diamidinobenzidine HCl (DAB; Sigma) as described by Chen and May (2007).
Combined WGA-HRP and biocytin protocol
To demonstrate the WGA-HRP labeling and biocytin labeling in the same sections, they first underwent the ammonium molybdate-TMB procedure, and the TMB reaction product was protected with DAB as described above. After rinsing in 0.1 M PB (pH 7.2), sections were processed to visualize biocytin as follows. Sections were rinsed with a solution of 0.1% Triton X-100 in 0.1 M PB (pH 7.2) and subsequently incubated in a solution of 1:500 avidin-HRP in 0.1% Triton X-100 in 0.1 M PB (pH 7.2) for 14 hours at 4°C. The sections were again rinsed with 0.1 M PB (pH 7.2), followed by preincubation with 0.05% diaminobenzidine (DAB) in 0.1 M PB (pH 7.2). Solutions of 1% nickel ammonium chloride and 1% cobalt acetate were added at a concentration of 0.5 ml/100 ml. The reaction was initiated with 3.0% hydrogen peroxide at a concentration of 0.3 ml/100 ml of DAB solution. Those sections used for light microscopic study were rinsed, mounted, counterstained with cresyl violet, dehydrated, cleared, and coverslipped. Those used for EM were prepared as described below.
EM procedures
The 100-μm sections were rinsed in PB after the reaction steps and examined with a stereoscope (Olympus SZ40). Sample areas containing retrogradely labeled preganglionic motoneurons were cut out of the section and separately processed. The samples included one of the following three regions: the area of the anteromedian nucleus; the supraoculomotor area, including EWcp; and the region ventrolateral to III. The EM samples were prepared and examined using previously described methods (Chen and May, 2007; Sun and May, 2014).
RESULTS
Preganglionic motoneuron distribution and morphology
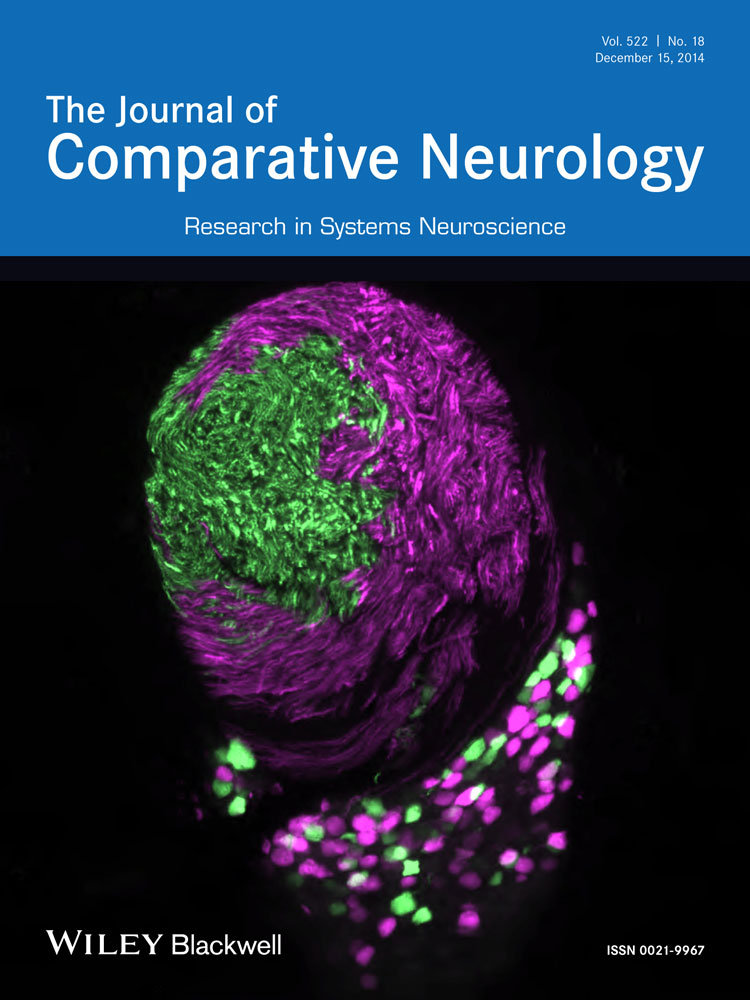
Injections of WGA-HRP into the ciliary ganglion always labeled preganglionic motoneurons at three perioculomotor sites: 1) rostrally, in and ventrolateral to the anteromedian nucleus (AM); 2) dorsally, in the supraoculomotor area (SOA), in EWcp and between III; and 3) ventrally, among the exiting fibers of III. We will refer to these as the rostral, dorsal, and ventral populations, respectively. To characterize these preganglionic motoneurons better, we sought to maximize the extent of their retrograde labeling. However, those injections that produced reasonably complete labeling of this population also produced retrograde labeling of large motoneurons in III and among the fascicles of the medial longitudinal fasciculus. The labeling of these cells was presumed to be due mainly to spread of tracer from the ciliary ganglion. To confirm this view, the distribution of preganglionic motoneurons following a left ciliary ganglion injection (Fig. 1, left) was compared with a control injection of WGA-HRP and HRP into the right inferior branch of the oculomotor nerve distal to the ciliary ganglion (Fig. 1, right). The most rostral group of labeled presumed preganglionic motoneuron (large dots) was located within and ventrolateral to AM (Fig. 1A–C). These sometimes extended into the periaqueductal gray. More caudally, labeled cells were present in the area ventral to III (Fig. 1D–G) as well as dorsal to III, including SOA and a few cells in EWcp (Fig. 1D–F). A small population of cells was sandwiched between the rostral poles of III (Fig. 1E,F). More lightly labeled, presumed somatic division motoneurons (small dots) were also present (Fig. 1D–H). On the right side, a control injection of the nerve did not label cells in the three locations rostral, ventral, and dorsal to III described above. However, numerous large multipolar neurons (30–50 μm) were labeled (diamonds) in the oculomotor nucleus proper and within the adjacent medial longitudinal fasciculus (MLF; Fig. 1D–H).
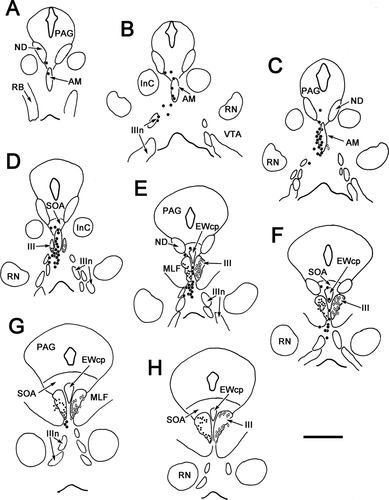
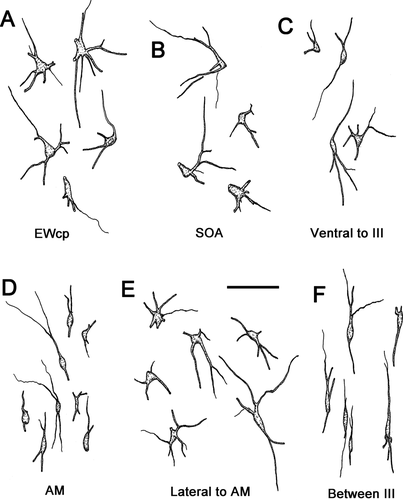
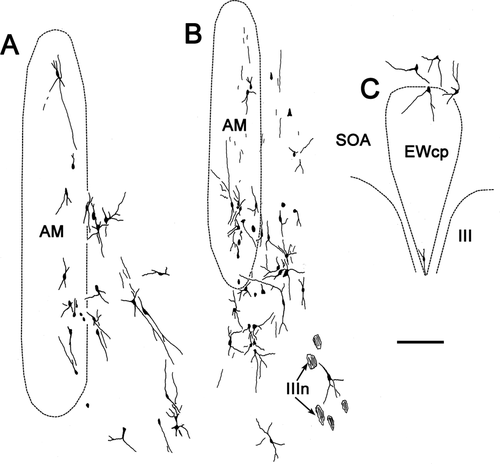
The morphology of these preganglionic motoneurons is demonstrated in Figure 2. Most of the cells in the caudal locations, EWcp (Fig. 2A), SOA (Fig. 2B), and ventral to III (Fig. 2C), were multipolar neurons with somata averaging 15 × 22 μm (short × long axis). The somata of AM cells (Fig. 2D) were mainly fusiform in shape and averaged 6 × 25 μm (short × long axis). Their long somatic axes and dendritic fields were vertically oriented. Fusiform cells with dorsoventrally oriented dendrites were also found on the midline between the somatic division nuclei (Fig. 2F). These may represent a caudal extension of AM. The cells found lateral to AM (Fig. 2E) often had fusiform cell bodies, but their multipolar dendritic fields do not show the degree of orientation seen in AM. Further appreciation of the dendritic field distribution with respect to the nuclear boundaries is provided in Figure 3. Some of the labeled motoneurons in AM had dendrites that crossed the midline (Fig. 3A,B). Dendrites often crossed the border between AM and ventrolateral to AM as well. The dendrites of preganglionic motoneurons located in and around the EWcp were observed to cross the SOA/EWcp border (Fig. 3C). The dendrites of the cells dorsal to III sometimes crossed the midline as well (Fig. 3C), but this tendency lessened at more caudal levels. The dendrites of cells ventral to III were not observed to send dendrites into III or to cross the midline (not illustrated).
Ultrastructure of the preganglionic motoneurons
The retrogradely labeled motoneurons were observed to have electron-lucent cytoplasm, so the clusters of crystalline, electron-dense TMB reaction product were easily identified. However, labeling was completely confined by the cell membrane so that the relationships of presynaptic terminals to these HRP-labeled elements were clearly visible. The reaction product was present not only in motoneuron somata (Figs. 4D,E, 5A), but also in proximal dendrites (Fig. 4A,B) and even in small, presumably distal dendrites (Figs. 4C, 5B). Terminal contacts on spines were occasionally observed (Fig. 5C). Consequently, the characteristics of profiles terminating on these preganglionic motoneurons could be observed throughout the somatodendritic tree. Axodendritic contacts were the most common element. Labeled preganglionic motoneurons and their dendritic processes were found to be exclusively postsynaptic to vesicle-containing profiles.
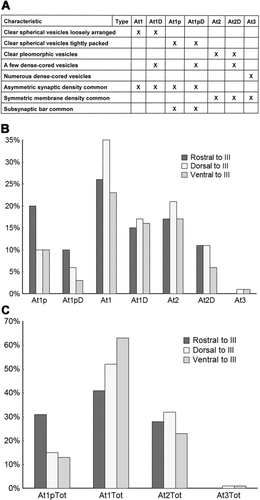
The presynaptic elements contacting labeled preganglionic motoneurons are ultrastructurally heterogeneous with respect to their vesicular and synaptic characteristics. We have provisionally divided them into three main classes: type 1 terminals contained clear spherical vesicles, type 2 terminals contained clear pleomorphic vesicles, and type 3 terminals were characterized by a large number of dense-cored vesicles. We have further subdivided these three classes into seven distinct subclasses of terminals (Fig. 6A). There appeared to be subclasses of type 1 terminals based on the organization of the vesicles. In one subclass, the vesicles were very densely packed within the profile, so we have specified these terminals as type 1p, in contradistinction to a subclass with fewer, more dispersed vesicles for which we have reserved the term type 1. Scattered dense-cored vesicles were sometimes observed in type 1, type 1p, and type 2 terminals. These profiles have been specified with a D, e.g., type 1D.
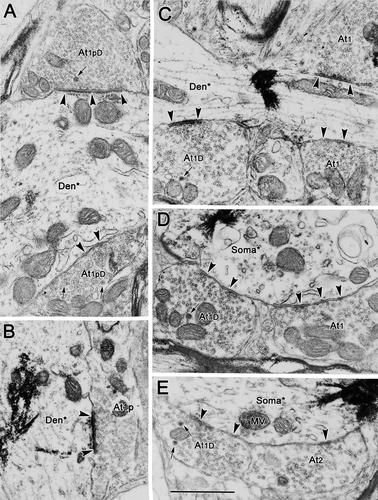
Type 1p synaptic profiles with dense-cored vesicles (At1pD; Fig. 4A) and without them (At1p; Fig. 4B) were common presynaptic components in our samples. They contacted both labeled somata and dendrites, and they were particularly common on proximal dendrites, such as those shown here. These profiles ranged from 0.85 to 2.08 μm in size (long axis) and contained tightly packed, clear, spherical vesicles (25–40 nm in diameter) that evenly filled the terminal profiles. In the type 1pD examples, a few dense-cored vesicles (70–90 nm in diameter) were scattered within the profiles. Type 1p terminals frequently formed distinctly asymmetric synaptic densities that were often associated with a dense, periodic subsynaptic bar (Fig. 4A,B).
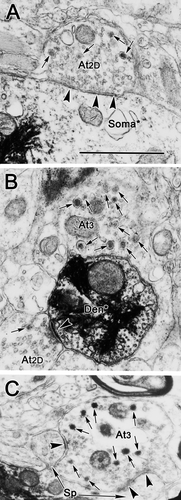
Type 1 synaptic profiles (At1), which were also commonly observed, are shown in Figure 5C,D. These profiles were somewhat larger overall (1.05–2.65 μm, long axis) than type 1p profiles. Like type 1p profiles, they also contained clear, spherical vesicles. However, the vesicles were a little larger than those found in type 1p profiles, measuring 30–50 nm in diameter. Vesicles were less closely packed in these profiles, although they were more concentrated in the vicinity of the synaptic density. These terminals sometimes displayed prominent synaptic membrane densities, but, in most samples surveyed, they were less asymmetric than those seen in the type 1p profiles. Type 1D terminals containing dense-cored vesicles, in addition to the clear spherical vesicles, were also commonly seen (Fig. 4D,E).
An example of a type 2 synaptic profile (At2) is shown in Figure 4E. These profiles contained clear pleomorphic or flattened vesicles (20 × 60 nm, short × long axes) in addition to spherical vesicles (30–50 nm in diameter). The example shown is an axosomatic contact, but axodendritic contacts were also very common. In some cases, profiles with clear pleomorphic vesicles also contained a few dense-cored vesicles (70–90 nm in diameter) and were identified as type 2D terminals (At2D). Again, the example shown (Fig. 5A) is axosomatic, but axodendritic contacts were also common. Both type 2 and type 2D synaptic profiles made synaptic contacts in which the degree of electron density is less prominent than that of type 1 profiles, placing these within the category of symmetric contacts.
Type 3 synaptic profiles (At3) were quite rare (Fig. 5B,C). In type 3 profiles, large, dense-cored vesicles were the dominant vesicle type. These dense-cored vesicles were generally larger than those seen in other profile types, measuring 70–120 nm in diameter. The limited numbers of clear vesicles found in these terminals were pleomorphic. These terminals synapsed on dendrites (Fig. 5B) and the spinous appendages of the labeled cell somata (Fig. 5C). Their membrane densities are prominent, but clearly symmetric.
The classification scheme described above is admittedly arbitrary. Some of these different types may reflect only different points on a spectrum. In particular, the differences between type 1, 1p, and 2 profiles, in which dense-cored vesicles were not found, and the type 1D, 1pD, and 2D profiles, in which they were found, may rest on where the terminal was sectioned. However, the type 1p terminals are qualitatively different from type 1 terminals; they have smaller, clear vesicles and often display subsynaptic bars.
To provide further detail to our ultrastructural analysis, the labeled preganglionic motoneurons in the different regions (rostral, dorsal, and ventral to III) were examined. At all three sites, the preganglionic motoneurons contained pale nuclei, which were often indented, and displayed prominent nucleoli (Figs. 7D, 8B, 9B). The cytoplasm contained considerable amounts of rough endoplasmic reticulum and mitochondria, and the Golgi complex was well developed. Multivesicular bodies, lysosome-like dense bodies, and lipofuscin granules were occasionally present. In all three regions, the amount of somatic surface area covered by vesicle-containing profiles was relatively small (Figs. 7A–E, 8B,E, 9A–D). A higher density of terminals was present on the dendrites (Figs. 7F,G, 8A–D,F,G, 9E–G). As would be predicted from the light microscopic observations, the shapes and sizes of the labeled somata differed. In the samples from rostral to III (Fig. 7D), the soma of the labeled motoneurons often had a fusiform shape, with an oval nucleus. The motoneurons from the areas dorsal and ventral to III (Figs. 8B, 9B) often displayed larger, more circular nuclei, and the somatic profiles had the randomly emerging dendrites characteristic of multipolar neurons.
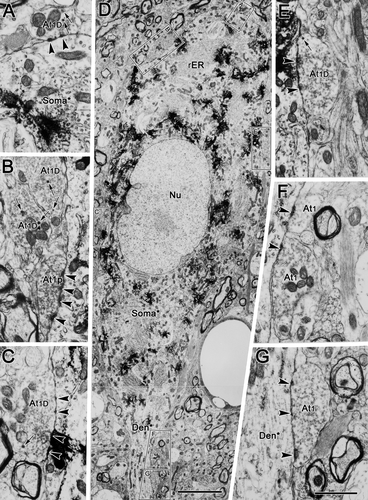
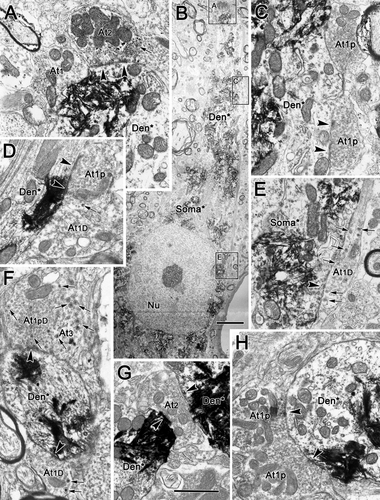
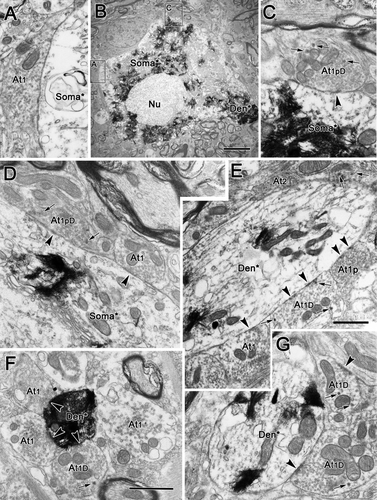
The number of contacts by each terminal type varied between regions. As can be seen in Figure 7, these three sorts of type 1 profiles were particularly evident contacting somata and proximal dendrites in samples from rostral to III. Type 1 contacts (At1) were present on the proximal dendrites (Fig. 7F,G), whereas type 1D (Fig. 7A–C,E) and type 1p (Fig. 7B) terminals were in synaptic contact with the cell's soma. In the samples dorsal to III (Fig. 8), a variety of different terminal types was observed. All subclasses of type 1 terminal were present: type 1p terminals (At1p; Fig. 8C,D,H), type 1pD terminals (At1pD) with dense-cored vesicles (Fig. 8F), type 1 terminals (At1; Fig. 7A) and type 1D terminals (At1D) with dense-cored vesicles (Fig. 8D–F). In addition, type 2 contacts (At2) were well represented (Fig. 8A,G). Type 3 terminals (At3) dominated by dense-cored vesicles were occasionally encountered (Fig. 8F). Similar findings were made with respect to labeled motoneurons ventral to III, as shown in Figure 9. All the subclasses of type 1 terminals were present, including type 1 terminals (At1; Fig. 9A,D–F), type 1D terminals with dense-cored vesicles (At1D; Fig. 9E–G), type 1p terminals packed with vesicles (At1p; Fig. 9E), and type 1pD terminals (At1pD) that also contained dense-cored vesicles (Fig. 8C,D). Type 2 terminals (At2) were also commonly observed (Fig. 9E).
Our qualitative impression was that type 1p and 1pD terminals were more common rostrally in the samples that included AM and that type 3 terminals were found mainly caudally in the samples taken dorsal and ventral to III. To explore these possibilities, we undertook a quantitative analysis of our material. The results are shown in the graphs of Figure 6B,C. In total 788 contacts were classified, including 373 from the samples rostral to III, 142 from samples taken dorsal to III, and 212 from samples taken ventral to III. Among these, roughly 30% were axosomatic contacts, and the rest were axodendritic contacts, but the more limited labeling of distal dendrites likely skews these results toward the soma. The classes of type 1 contacts appear to be the dominant terminal type ending on preganglionic motoneurons compared with the classes of type 2 ending. Type 3 contacts were not only rare but appeared to be nearly absent from the samples rostral to III, even though more contacts were counted in these samples. The most striking finding is that the subclasses packed with small clear vesicles, type 1p and 1pD, are twice as prevalent, making up 30% of the terminals, in the rostral samples than in either of the two caudal regions sampled (dorsal 15% and ventral 13%).
Relationship between olivary pretecal terminals and preganglionic motoneurons
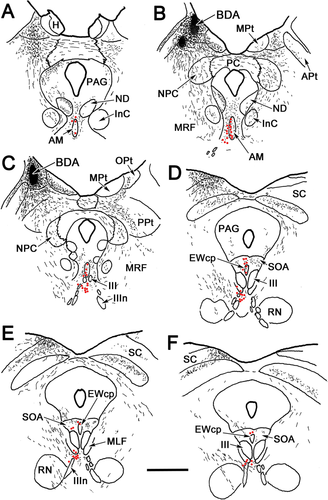
The relationship between pretectal premotoneuron axons and preganglionic motoneurons was examined by combining injections of biocytin into the olivary pretectal nucleus (OPt) and injections of WGA-HRP into the ciliary ganglion. Figure 10B,C shows a biocytin injection site largely confined to OPt. It led to axonal (lines) and terminal arbor (stippled) labeling in the midbrain (Fig. 10A–F), which was distributed as follows. On the ipsilateral side, labeled axons were observed rostrally, crossing through the nucleus of the posterior commissure (NPC), posterior pretectal nucleus (PPt), midbrain reticular formation (MRF), and periaqueductal gray (PAG; Fig. 10A–F). There was some evidence of termination in all these nuclei. The fibers in the periaqueductal gray terminated along its lateral edge (Fig. 10C–E) and in the nucleus of Darkschewitsch (ND; Fig. 10A,B). These fibers also terminated in and ventrolateral to AM (Fig. 10A–C), in the ipsilateral SOA, and in EWcp (Fig. 10D–F). Some fibers observed in the midbrain reticular formation produced a few terminals ventrolateral to III, whereas others decussated (Fig. 10E,F). Numerous labeled fibers crossed in the posterior commissure (PC) to supply the contralateral side (Fig. 10A–C). These divided into one group that turned dorsally and terminated in the contralateral OPt and one group that turned ventrally. The latter terminated in or passed through the contralateral NPC and posterior pretectal nucleus (Fig. 10B,C). Fibers passing through NPC terminated in, and ventrolateral to, AM on the contralateral side (Fig. 10A–C) and in the contralateral SOA and EWcp (Fig. 10D–F). At more caudal levels, far fewer terminal arbors were present in the region around III (Fig. 10F). Preganglionic motoneurons (dots) labeled from ciliary ganglion injections were found in and lateral to AM (Fig. 10A–D), in the SOA, in EWcp, and between III (Fig. 10D–F) and ventral to III (Fig. 10D–F). Thus, their distribution overlapped sites where biocytin-labeled, pretectal terminals were found.
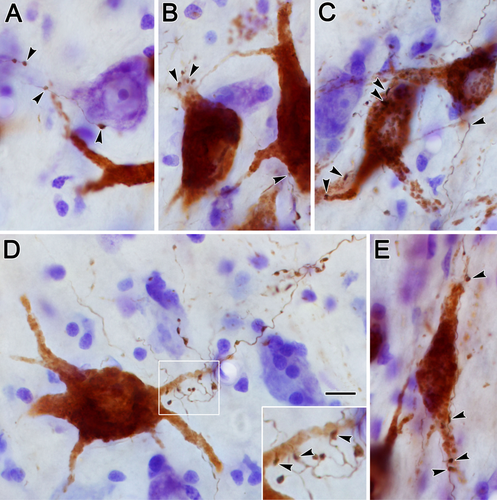
Biocytin-positive axon terminals were often observed in close association with the labeled preganglionic motoneuron somata or their dendrites. Examples of these close associations (arrowheads) are shown in the photomicrographs of Figure 11. Both en passant and termineaux type boutons were observed on the labeled pretectal axons, the former being far more numerous and the latter usually found on a short branch extending from the main axon. The close associations were observed mainly on the proximal dendrites of the retrogradely labeled motoneurons (Fig. 11A–E), although axosomatic associations were also present (Fig. 11B,C,E). Other terminals were not associated with labeled cells (Fig. 11A,D). The overall pattern of the biocytin labeling in relationship to the retrogradely labeled preganglionic motoneurons from the case illustrated in Figure 10 can be appreciated in Figures 12 and 13. Labeled cells displaying close associations with labeled terminals are indicated by arrows. These were numerous within and lateral to the anteromedian nucleus (Fig. 12A). As shown in Figure 12B, such associations were almost as common at the rostral pole of III, where AM and EWcp are in continuity. However, they were relatively rare caudally, where the preganglionic motoneurons lie ventral to III, among the exiting oculomotor nerve bundles, and dorsal to III, in SOA and EWcp (Fig. 13A).
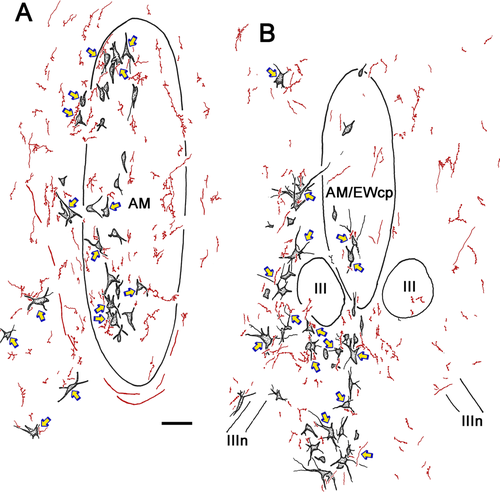
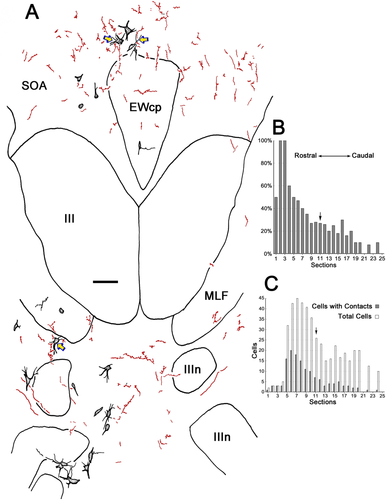
The extent of the difference in presumptive contacts between the region rostral to III and the caudal regions above and below III was quantitatively analyzed. The labeled preganglionic motoneurons with and without associated biocytin-labeled pretectal axon terminals were counted in every third section from the case that displayed the most midbrain terminal labeling. A much higher percentage of motoneurons displays close associations rostrally (Fig. 13B). The distribution of motoneurons with terminal associations is rostrally displaced relative to rostrocaudal distribution of the preganglionic population as a whole (Fig. 13C). Indeed, about 70% of all the motoneurons with associated terminals are found in the AM area, in front of the rostral pole of III (left of arrow). The remaining 30% is about evenly divided between the regions dorsal and ventral to III, although these caudal areas contain nearly half the preganglionic motoneurons between them (right of arrow).
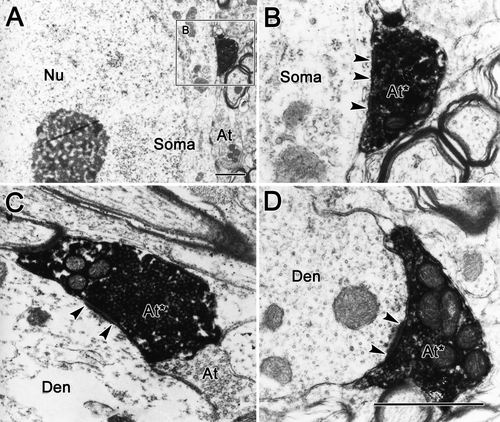
Electron microscopic analysis was performed to establish definitively the presence of synaptic contacts between the pretectal terminals and the preganglionic profiles. The DAB-biocytin reaction product is an amorphous, electron-dense material. It attaches to the outer membranes of the mitochondria, synaptic vesicles, and inner surface of the plasmalemma, and it can fill the cytosol in heavily labeled examples (Fig. 14). Labeled terminals were observed in synaptic contact with both somata (Fig. 14A,B) and dendrites (Fig. 14C,D). Most of the biocytin-labeled pretectal terminals may be classified as type 1p profiles, because they displayed asymmetric synaptic membrane densities with a periodic subsynaptic bar and contained tightly packed, spherical vesicles and several mitochondria (Fig. 14C,D). Biocytin-labeled terminals were observed in samples taken from rostral, dorsal, and ventral to III. However, the rostral samples that contained AM had, by far, the most labeled terminals (n = 61), and the region dorsal and ventral to III contained far fewer examples (n = 16 and 7, respectively).
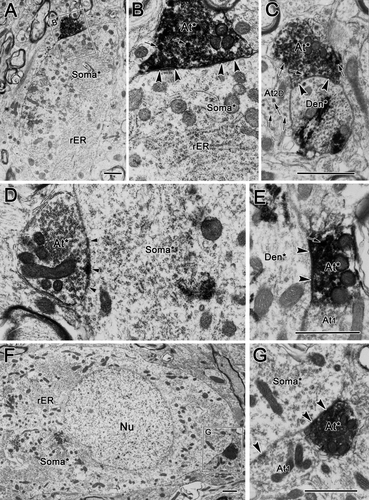
In many cases, biocytin-labeled pretectal terminals were found to make synaptic contacts on WGA-HRP-labeled preganglionic motoneuron profiles. The WGA-HRP label, which presents as clustered electron-dense crystals, is easily distinguished from the fine-grained biocytin label. In the present study, the biocytin label was observed exclusively in terminal profiles, whereas WGA-HRP label was confined within cell somata and dendritic profiles. Figure 15 shows some examples of labeled terminals contacting labeled cells. Labeled olivary terminals were found in association either with labeled somata (Fig. 15A,B,D,F,G) or with small and large labeled dendrites (Fig. 15C,E, respectively). Biocytin-labeled pretectal terminals contacted labeled dendritic profiles more often than labeled somata. Most labeled terminals synapsing on labeled motoneurons appeared to be type 1p profiles, but a very small number of biocytin-labeled terminals contained dense-cored vesicles, making them more similar to type 1pD terminals (Fig. 15B,C). All but one of the 15 observed examples in which labeled contacts terminated on labeled cells were from samples taken rostral to III.
DISCUSSION
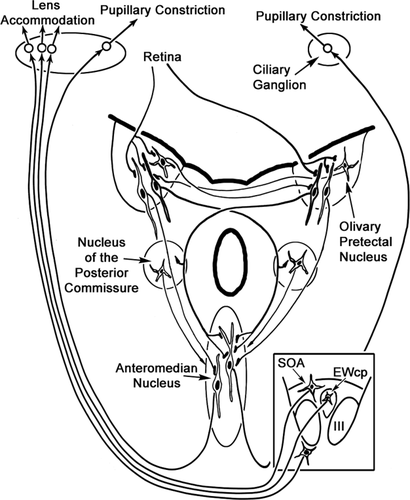
The present results confirm and extend previous descriptions of the distribution of preganglionic motoneurons supplying the ciliary ganglion in the cat (Sugimoto et al., 1977; Loewy et al., 1978; Toyoshima et al., 1980; Strassman et al., 1987; May et al., 2008a). Specifically, the results show that these motoneurons are not confined to EWcp, but are instead distributed rostral, dorsal and ventral to III. This is the first detailed ultrastuctural analysis of preganglionic motoneurons supplying the ciliary ganglion. This EM analysis indicates that these motoneurons receive synaptic input from a variety of terminal types. This might be expected, because motoneurons are likely to be controlled by excitatory inputs for the pupillary light reflex; by excitatory and inhibitory inputs from near triad premotor neurons related to convergence and divergence, respectively; and perhaps by inhibitory inputs that dilate the eye in darkness or in response to sympathetic arousal. The present results confirm and extend the findings of Erichsen and May (2002), who suggested that pupillary control is located more rostrally in the population and lens control is located more caudally, based on the numbers of cells labeled transsynaptically following anterior chamber or ciliary body injections. Specifically, morphological and ultrastructural differences were seen between rostral neurons located in and lateral to AM compared with caudal populations located dorsal and ventral to III. The rostral population was also characterized by more dendrites that crossed the midline, providing an additional potential anatomical basis for consensual pupillary responses. This is the first study to demonstrate directly that the pupillary light reflex pathway is served by synaptic contacts between OPt axon terminals and EWpg motoneurons. Furthermore, these OPt terminals were demonstrated, at the LM and EM level, to terminate preferentially on preganglionic motoneurons in and lateral to the anteromedian nucleus, indicating that the pupillary light reflex circuit in the cat works through this rostral population (Fig. 16).
Technical considerations
Because the ciliary ganglion is a small target containing considerable collagen, it is difficult to inject it without involving the nerve trunk to which it is attached or the muscles in the vicinity. However, the cell labeling from diffusion away from the ganglion can be differentiated in several respects. First, the locations of the labeled cells from the ciliary ganglion injection were different from those commonly presumed for somatic motoneurons, with the exception of medial rectus motoneurons, which sometimes extend beneath the medial longitudinal fasciculus (MLF; Akagi, 1978; Miyazaki, 1985). The distribution of retrogradely labeled preganglionic motoneurons was fairly consistent in all the cases in our study. Furthermore, the control experiments indicate that somatic motoneurons projecting in the inferior branch of the oculomotor nerve are exclusively confined within III and among the fascicles of the MLF (Fig. 1). Second, the preganglionic motoneurons were generally smaller than somatic motoneurons and displayed fewer primary dendrites. Although it is known that some smaller somatic motoneurons, which supply multiply innervated fibers in the extraocular muscles, are present (Miyazaki, 1985; Wasicky et al., 2004), they are located within III in the cat (Bohlen et al., 2012) and so should not have confused the present results. Finally, the label in the somatic motoneurons was usually weaker than that of the preganglionic motoneurons, because the former receive tracer via diffusion rather than direct injection. Thus, the possibility that some of the labeled cells identified as preganglionic motoneurons were actually somatic motoneurons has been minimized.
The EM sample areas did not include the oculomotor nucleus proper, excluding the possibility that we sampled somatic motoneurons. However, this meant that we did not sample the preganglionic motoneurons lying between the rostral poles of III. Labeling of the dendrites of the preganglionic motoneurons was incomplete. This reduced our ability to characterize fully the dendritic morphology of these neurons and the composition of labeled terminals contacting them. In terms of the ultrastructural characterization, we were able to identify small, presumably distal dendrites that contained retrogradely label, but this labeling was less prominent than labeling of proximal dendrites, so we refrained from further quantifying the distribution of the terminal types with respect to their location on the dendritic tree.
The pretectal injection spread slightly beyond the boundaries of OPt, but, as shown by Sun and May (2014), there are few premotor neurons projecting to AM in these adjacent regions. Although pretectal terminals were observed in regions outside those containing preganglionic motoneurons, we analyzed only those regions that contained motoneurons. Close associations were observed between pretectal terminals and preganglionic motoneurons, and the electron microscopic evidence demonstrates that these close associations were indeed synaptic contacts.
Preganglionic motoneuron distribution
The distributions and morphological characteristics of retrogradely labeled preganglionic motoneurons observed following the ciliary ganglion injections in the present study agree with those of prior studies in the cat (Loewy et al., 1978; Sugimoto et al., 1978; Toyoshima et al., 1980; Maciewicz et al., 1983; Erichsen and May, 2002). Specifically, these cells are located ipsilaterally, within and lateral to AM, between the rostral poles of III; in SOA, including EWcp; and in the area ventral to the oculomotor complex, sometimes intermingling with the oculomotor nerve bundles. This is quite different from the organization seen in monkeys (Akert et al., 1980; Burde and Loewy, 1980; Clarke et al., 1985; May et al., 2008a, 2008b; Horn et al., 2008) and galago (Sun and May, 1993). In these primates, the preganglionic motoneurons are generally confined within EWpg and a small portion of AM. The differences in organization of the preganglionic motoneurons between cats and monkeys, both of which are frontal-eyed species, may reflect a more refined control over the lens in primates. Thus, a well-organized and discrete population of preganglionic motoneurons may be a hallmark of the oculomotor complex in the foveate primate in which fine control of the near triad subserves the use of the hands in near space. Such a capability may be a prerequisite for tool preparation and use.
Preganglionic motoneuron morphology
We found cat preganglionic motoneurons to be heterogeneous in terms of their morphology, in agreement with a previous report (Toyoshima et al., 1980). The rostrally placed cells, especially those close to the midline, are mainly fusiform in shape with their axes vertically oriented, whereas more caudally placed cells located in EWcp, in SOA, and in the area ventral to III are mainly multipolar in shape. The cells located between the rostral poles of III are also fusiform in shape, which may indicate that they represent a caudal continuation of the population in AM. In support of this, Sillito and Zbrožyna (1970a, 1970b) recorded pupilloconstriction neurons primarily on the midline between the rostral poles of III in the cat. In addition, the dendrites of cells in and lateral to AM freely cross the border of this nucleus, suggesting that these two sets of neurons may, in fact, be part of a single functional group that receives common inputs. The morphological difference between the rostral population and the caudal population is in line with the rostrocaudal functional difference suggested by transsynaptic retrograde labeling: the rostral group is enriched with cells controlling the sphincter pupillae muscle, whereas the caudal group is enriched with cells controlling the ciliary muscle (Erichsen and May, 2002).
The tendency for dendrites to cross the midline may be in agreement with this analysis. In frontal-eyed species, pupil size is balanced between the two eyes, and illumination of either eye influences pupil diameter. Thus, this pattern of dendritic arborization may serve as an anatomical substrate for the consensual pupillary response that complements other circuit features for balancing consensual and direct pupillary responses (Sun and May, 2014; Fig. 16). This tendency for dendrites to cross the midline lessened caudally. Although the accommodative drive to the two eyes is generally balanced, it may not be perfectly equal under all conditions. When targets are viewed away from the midline, the distance between each eye and the target will not be the same. Thus, it may be necessary to tune the level of accommodation in each eye independently. The lateralization of the dendritic fields in the presumptive, caudal lens-control population would allow such modulation.
Preganglionic motoneuron ultrastructure
The labeled preganglionic neurons and their dendrites are exclusively postsynaptic elements. In fact, the present experiment revealed that the preganglionic neurons receive as many as seven morphologically distinct types of axon terminals. A previous investigation of cat preganglionic motoneuron ultrastructure had simply divided terminals into those containing spherical and pleomorphic vesicles and allowed identification only of terminals on the cell soma (Ichinohe et al., 1996). The existence of several types of terminals on labeled preganglionic motoneurons indicates that preganglionic neurons receive input from sources other than from pretectal premotor neurons. The sources of these different types of terminals may include the deep nuclei of cerebellum and the hypothalamus, since there is anatomical evidence establishing these structures as inputs to the SOA and AM (Sugimoto et al., 1982; Breen et al., 1983; May et al., 1992). Sympathetic centers in the hypothalamus appear to inhibit the pupillary light reflex, perhaps as part of descending hypothalamospinal circuits that activate the dilator pupillae muscle (Hodes and Magoun, 1942; Sillito and Zbrožyna, 1970a, 1970b; Schreyer et al., 2009). These might contribute type 2 and/or 2D terminals with pleomorphic vesicles and symmetric synaptic contacts to motoneurons located in AM. There is also evidence that the fastigial nucleus of the cerebellum modulates the pupillary light reflex and so might provide excitatory (type 1, 1D, 1p, or 1pD) inputs (Tsukahara et al., 1973; Ijichi et al., 1977). Furthermore, cells whose activity increases when viewing far targets have been recorded in the interposed nucleus (Zhang and Gamlin, 1998). These may provide one of the terminal types observed contacting preganglionic motoneurons in SOA and EWcp. The rare type 3 terminals, which are dominated by dense-cored vesicles, are likely to come from sources providing nonspecific input to the brain, such as the locus coeruleus, which has known projections to the region (Breen et al., 1983).
Type 1p and 1pD terminals, which were packed with small, clear spherical vesicles and made asymmetric synaptic contacts onto the preganglionic motoneurons, were more prevalent in the rostral samples. If we are correct in our presumption that these rostral neurons subserve pupilloconstriction, then they most likely represent an excitatory input from the OPt. The double-labeling experiments described here support this contention. The type 1 and 1D terminals may represent excitatory drives from premotor near-response cells, which would be distributed to both pupillary and lens-related motoneurons.
A previous ultrastructural examination of the cat preganglionic motoneurons (Ichinohe et al., 1996) was also directed at identifying differences between pupil- and lens-related preganglionic motoneurons. However, these authors divided their samples differently, into an anterior–dorsal group and a ventral group. The anterior–dorsal group included AM, EWcp, and SOA, whereas the ventral group included cells ventral to III and those ventrolateral to AM. The authors found differences in the somatic shape and synaptic density of terminals contacting these two groups. In view of the present results, it is likely that the large number of cells located in AM caused this population to dominate their anterior–dorsal group. Indeed, Ichinohe et al. describe these motoneurons as being fusiform in shape. Their ventral group contained primarily mulitipolar cells, which agrees with our morphologic data (Figs. 2 and 3). Our conclusion is somewhat different, however, in that we have evidence that the pupil- and lens-related cells are separated on a rostrocaudal, as opposed to a dorsoventral, axis. Nevertheless, our results may be viewed as being mostly in agreement, because AM tends to dominate both our rostral and their anterior dorsal samples, which we both presume contain pupil-related cells.
Pretectal–preganglionic connections and functional segregation
The distribution of terminals seen following OPt biocytin injections parallels that seen with WGA-HRP injections of OPt in the companion to this study (Sun and May, 2014) and supports previous findings suggesting that the OPt projects to the EWpg as part of the pupillary light reflex pathway (monkey: Pierson and Carpenter, 1974; Benevento et al., 1977; Büttner-Ennever et al., 1996; cat: Graybiel, 1974; Itoh, 1977; rat: Itaya and Van Hoesen, 1982; Campbell and Lieberman, 1985; Klooster et al., 1995a). However, the scattered nature of preganglionic motoneurons in most species, including the cat, has in the past made a definitive statement on the circuit difficult. The present study provides clear evidence of extensive close associations between anterogradely labeled OPt terminals and retrogradely labeled preganglionic motoneurons. It further demonstrates that these associations represent synaptic contacts through EM confirmation. This compliments an earlier short report with the monkey (May et al., 2008b). The ultrastructural analysis presented here supports the contention that this projection is an excitatory one, insofar as these type 1p terminals make asymmetric synaptic contacts and are packed with clear, spherical vesicles. These ultrastructural features are similar to those seen in rats (Klooster et al., 1995b). Taken together, these data indicate that the classic view of the pupillary light reflex circuit as an excitatory pathway connecting the retina to preganglionic motoneurons via OPt (Fig. 16) is correct.
Pupil constriction and lens accommodation are presumed to be controlled by functionally separate subgroups of preganglionic motoneurons. Some evidence for this comes from studies in birds in which EWpg controls choroidal blood flow in addition to pupillary constriction and lens accommodation. The avian EWpg contains two separate groups of cells: larger cells control the ciliary muscle and pupillary constrictor, whereas the small cells control choroid capillaries (Reiner et al., 1983; Gamlin et al., 1984; Erichsen and Evinger, 1985; for review see Reiner et al., 1991; Gamlin and Reiner, 1991). In mammals, however, there has been no definitive way to distinguish anatomically between pupil and lens motoneurons. The present findings represent an important step toward resolving this issue in that they show a differential distribution of pupil and lens motoneurons. However, given the relatively loose organization of preganglionic motoneurons in the cat, it seems unlikely that the pupil and lens populations are absolutely segregated.
In agreement with our findings, physiological experiments in cats suggested that AM may contain pupillary constriction neurons, because olivary pretectal neurons could be antidromically activated by stimulating at the level of AM, but not at more caudal levels (Sillito and Zbrožyna, 1970a, 1970b). On the other hand, the results from stimulation studies in primates conflict with this. These groups have reported that AM is involved in lens accommodation and that the EWpg, or a portion of it, produces pupillary constriction when stimulated (Jampel and Mindel, 1967; Clarke et al., 1985; Crawford et al., 1989). The physiological evidence from chronic recording studies is not as definitive on this point. In agreement with the present findings, there is evidence from single-unit recording studies that accommodation-related motoneurons in the cat are located primarily in the area above III (Bando et al., 1981, 1984) and that pupil-related cells lie on the midline between the rostral poles of III (Sillito and Zbrožyna, 1970b). Studies in monkeys indicate that the motoneurons controlling lens accommodation are located in the EWpg (Zhang et al., 1991; Gamlin et al., 1994), but the location of pupil-related neurons has not been specified (Gamlin, 2000).
In summary, based on transsynaptic transports studies (Erichsen and May, 2002) and the present findings, it seems reasonable to assume that in the cat, but not necessarily in other species, the preganglionic motoneurons controlling pupillary constriction are generally more rostrally located and lie in or around AM (Fig. 16). Presumably, preganglionic motoneurons controlling lens accommodation are distributed more caudally, scattered in areas dorsal and ventral to III (Fig. 16). The morphological differences among preganglionic motoneurons at these locations, as revealed in the present study, may reflect these different functions. We have also observed ultrastructural differences between these two populations that underscore the likelihood that they receive different sources of input to support their different functions. Finally, this study provides evidence that most of the preganglionic motoneurons that receive synaptic input from OPt luminance neurons lie rostrally. This rostral concentration of pretectal terminals is in agreement with that observed in axonal degeneration studies in the cat (Itoh, 1977). Taken together, these data provide strong evidence that pupil and lens preganglionic motoneurons are functionally distributed in cats (Fig. 16), with pupil control centered rostrally and lens control centered caudally.
ACKNOWLEDGMENTS
We are grateful to Malinda Danielson for her help with the histology and Glenn Hoskins for his assistance with preparing the material for EM examination.
CONFLICT OF INTEREST STATEMENT
Neither of the authors has any known or potential conflicts of interest to declare with respect to the publication of this work.
ROLE OF AUTHORS
Both authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: PJM. Acquisition of data: WS, PJM. Analysis and interpretation of data: WS, PJM. Drafting of the manuscript: WS. Critical revision of the manuscript for important intellectual content: WS, PJM. Statistical analysis: WS, PJM. Obtained funding: PJM. Study supervision: PJM.