Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain
Abstract
The human brain is often considered to be the most cognitively capable among mammalian brains and to be much larger than expected for a mammal of our body size. Although the number of neurons is generally assumed to be a determinant of computational power, and despite the widespread quotes that the human brain contains 100 billion neurons and ten times more glial cells, the absolute number of neurons and glial cells in the human brain remains unknown. Here we determine these numbers by using the isotropic fractionator and compare them with the expected values for a human-sized primate. We find that the adult male human brain contains on average 86.1 ± 8.1 billion NeuN-positive cells (“neurons”) and 84.6 ± 9.8 billion NeuN-negative (“nonneuronal”) cells. With only 19% of all neurons located in the cerebral cortex, greater cortical size (representing 82% of total brain mass) in humans compared with other primates does not reflect an increased relative number of cortical neurons. The ratios between glial cells and neurons in the human brain structures are similar to those found in other primates, and their numbers of cells match those expected for a primate of human proportions. These findings challenge the common view that humans stand out from other primates in their brain composition and indicate that, with regard to numbers of neuronal and nonneuronal cells, the human brain is an isometrically scaled-up primate brain. J. Comp. Neurol. 513:532–541, 2009. © 2009 Wiley-Liss, Inc.
It is repeatedly stated in the literature and in neuroscience textbooks that the human species is an unusually encephalized primate species, whose brain, five to seven times larger than expected for a mammal of its body size (Jerison,1973; Marino,1998), contains 100 billion neurons and about ten times more glial cells (Kandel et al.,2000; Ullian et al.,2001; Doetsch,2003; Nishiyama et al.,2005; Noctor et al.,2007). The supposedly unusual scaling of the human brain, however, derives from comparisons across orders (Jerison,1973) and, even when restricted to primates, regards only the brain–body relationship (Marino,1998) rather than addressing how its cellular composition compares with that expected from other primates.
Moreover, to our knowledge, the widespread numbers on the cellular composition of the human brain have never been supported by experimental studies. The high anisotropy and large size of the human brain hinder stereological determination of cell numbers and their distribution in the brain as a whole. Estimates of the cellular composition of the human brain are available only for some structures, such as the cerebral cortex (von Economo and Koskinas,1925; Shariff,1953; Pakkenberg,1966; Pakkenberg and Gundersen,1997; Pelvig et al.,2008), cerebellum (Lange,1975; Andersen et al.,1992), and some subcortical nuclei (Pakkenberg and Gundersen,1988). Such studies have estimated the number of cells in the human cerebral cortex as 3, 7, 14, 19–23, or 21–26 billion neurons and, very recently, 28–39 billion glial cells (Pelvig et al.,2008), and the number of cells in the human cerebellum has been estimated as 70 or 101 billion neurons (Lange,1975; Andersen et al.,1992) and fewer than 4 billion glial cells (Andersen et al.,1992). From such studies, the total number of neurons in the human brain might be inferred to fall anywhere between about 75 and 125 billion plus an undetermined number of neurons in the brainstem, diencephalon, and basal ganglia that may or may not be comparatively small.
Additionally, no evidence is found to support the common quote of ten times more glial cells than neurons in the human brain. The glia:neuron ratio in subcortical nuclei can be as high as 17:1 in the thalamus (Pakkenberg and Gundersen,1988), but, given the relatively small combined number of glial cells reported for the cerebral (Pelvig et al.,2008) and cerebellar (Andersen et al.,1992) cortices, the only possible explanation for the quote of ten times more glial than neuronal cells in the entire human brain would be the presence of nearly one trillion glial cells in the remaining structures.
We have recently determined the cellular scaling rules that apply to the brain of a number of rodent and primate species and found that, whereas the rodent brain increases in mass faster than it gains neurons (defined as NeuN-positive cells) across species, suggesting that the average neuronal cell size increases in larger rodent brains (Herculano-Houzel et al.,2006), the primate brain increases in mass linearly with increases in its number of neurons across species, suggesting that the average neuronal cell size does not increase significantly with brain size (Herculano-Houzel et al.,2007). The power laws relating body mass, brain mass, and number of neurons for rodent and primate species allowed us to predict that, if built according to the cellular scaling rules that apply to rodents, a brain of 100 billion neurons should weigh over 45 kg and belong to a body of 109 tons. In contrast, if built according to the scaling rules that apply to primates, this brain of 100 billion neurons should weigh 1.45 kg and belong to a body of 73 kg, values that approach those observed in humans, suggesting that the human brain is indeed constructed according to the same rules that apply to other primates.
We thus set out to determine the total cellular composition of the human brain with the aid of the same method (the isotropic fractionator; Herculano-Houzel and Lent,2005) and relying on the same criterion of NeuN labeling to identify “neurons” and “nonneuronal cells” in order to evaluate how its composition compares with the expected composition of a primate brain of its size. While supporting several independent stereological estimates, our results challenge the values so often cited in the literature and suggest that, with regard to brain cellular composition, humans are just scaled-up, large primates.
MATERIALS AND METHODS
Human material
All brains were obtained from the Brain Bank of the Brazilian Aging Brain Study Group (Grinberg et al.,2007), located at the University of São Paulo Medical School (FMUSP). The project was approved by the Ethics Committee for Research Projects Analysis (CAPPesq) of FMUSP, Research Protocol number 285/04. Informed consent for removal of the brains was provided by next of kin, who also responded to the Clinical Dementia Rating Scale (CDR) semistructured interview and to the Informant Questionnaire on Cognitive Decline in the Elderly—Retrospective Version (IQCODE; Jorm and Jacomb,1989; Morris,1993). Four brains from 50-, 51-, 54-, and 71-year-old males, deceased from nonneurological causes and without cognitive impairment (CDR = 0, IQCODE = 3.0), were analyzed. The brain of the 71-year-old male was included in the analysis because it contained a similar number of cells and an even slightly higher number of neurons than the other brains. The corpses remained at 4°C until the brains were removed from the cranium less than 24 hours after death and fixed immediately.
Fixation and dissection
Brains were fixed by perfusion with 4 liters of 2% phosphate-buffered paraformaldehyde through the basilar artery and the internal carotids, followed by immersion for 36 hours in the same fixative. Fixation for less than 48 hours was critical to allow for antibody recognition of NeuN, while still being enough to guarantee that the nuclei remained intact throughout the homogenization procedure. The meninges and major blood vessels were removed, and the brains were split sagitally into two hemispheres. After dissecting each cerebellar hemisphere by cutting the cerebellar peduncles at the surface of the brainstem, each hemisphere was cut into 1-cm-thick coronal sections, and the cerebral cortex was separated from the remaining regions (basal ganglia, diencephalon, mesencephalon, and pons, named collectively “rest of brain,” or RoB) by cutting through the white matter along the surface of the striatum in each section. In three of the four brains analyzed, one of the hemispheres of the cerebral cortex had the gray matter dissected away from the underlying white matter by careful shaving of the gray matter around the gyri with a scalpel until the white matter was exposed. The medulla was excluded because of inconsistency in the inferior section level among cases during the autopsy procedure. After fixation, the three main regions of interest (cerebellum, cerebral cortex, and RoB) were stored in phosphate-buffered saline (PBS; pH 7.4) at 4°C and subjected individually to the isotropic fractionator method (Herculano-Houzel and Lent,2005). Each structure was cut into smaller pieces that could be homogenized in a tissue grinder and counted in 1 day, and partial results were added together. Determining the total number of cells in each brain typically required about 4–6 weeks.
Isotropic fractionator
The isotropic fractionator method has been described elsewhere (Herculano-Houzel and Lent,2005). Briefly, it consists of a chemomechanical dissociation of fixed biological tissue in a saline detergent solution (1% Triton X-100, 40 mM sodium citrate) using 40–200-ml glass tissue grinders, followed by intense agitation of the suspension containing all nuclei in the original structure, in order to achieve isotropy. After adding the fluorescent DNA marker 4′-6-diamino-2-phenylindole dihydrochloride (DAPI) to the suspension, the density of nuclei is quantified by use of a hemocytometer under a fluorescence microscope (Fig. 1A,C). The total number of nuclei is calculated by multiplying the density of nuclei by the total suspension volume and heretofore is referred to as “total number of cells” in each structure.

Aspect of the nuclei in the hemocytometer. A,B: Typical low-magnification fluorescent micrographs of the same field of cerebellar cell nuclei in suspension stained with DAPI (A) and for NeuN immunoreactivity (B). The arrowheads indicate nuclei that are NeuN negative and therefore identified as nonneuronal nuclei. All other nuclei are NeuN positive and therefore identified as neuronal. Note that nuclei are intact and well scattered. C,D: High-magnification confocal image of NeuN-negative (arrowheads; arrow, nonneuronal nucleus undergoing cell division) and NeuN-labeled cerebellar cell nuclei. The clear, debris-free preparation of free cell nuclei makes the anti-NeuN immunoreactivity easy to distinguish from the virtually nonnexistent background. Scale bars = 40 μm in B (applies to A,B); 20 μm in D (applies to C,D).
Immunocytochemistry
Neuronal nuclei from an aliquot of the suspension were selectively immunolabeled overnight, at room temperature, with mouse monoclonal anti-NeuN antibody (Chemicon, Temecula, CA; MAB377B clone A60 against murine NeuN; Mullen et al.,1992) at a dilution of 1:200 in PBS. This antibody is increasingly used in the literature as a neuronal marker in qualitative (see, e.g., Eriksson et al.,1998; Cossette et al., 2007; Fajardo et al.,2008) as well as quantitative (Gittins and Harrison,2004; Dawodu and Thom,2005) studies of the human brain. Anti-NeuN clone A60 labels no glial cells and recognizes all neuronal cells of most, though not all, subtypes in a variety of vertebrate species, including humans (Mullen et al.,1992; Wolf et al.,1996; Sarnat et al.,1998; Lyck et al.,2008). Neuronal subtypes in the central nervous system known to present no labeling for NeuN include Purkinje cells, mitral cells of the olfactory bulb, inferior olivary and dentate nucleus neurons (Mullen et al.,1992), neurons in the substantia nigra pars reticulata of the gerbil (but not of the rat; Kumar and Buckmaster,2007) and possibly others, as yet unidentified. Here we identify and count as “neurons” all NeuN-stained nuclei and count as “nonneuronal cells” all nuclei that lack NeuN labeling. Although the numbers of neurons in the cerebellum and RoB (which includes the inferior olive) are thus necessarily underestimated, the number of nonstained neurons included therefore in the population designated “nonneuronal” is likely to be very small and actually insignificant compared with the total numbers of cells in these structures (Andersen et al.,1992). A thorough analysis of adjacent sections of human cerebral cortex stained with cresyl violet or NeuN has shown that both methods give correlated estimates of neuronal density, indicated that NeuN is particularly useful for distinguishing small neurons from glia and confirmed the value of NeuN as a tool for quantitative neuronal morphometric studies in human brain tissue (Gittins and Harrison,2004). Additionally, because we identified labeled nuclei by visual inspection under the microscope and not by automated methods, we could confirm that all NeuN-labeled nuclei in each sample were indeed of neuronal morphology and that all nuclei of a particular labeled morphology were labeled in the sample.
After the nuclei were washed in PBS, they were incubated for 2 hours at room temperature with AlexaFluor 555 anti-mouse IgG secondary antibody (Molecular Probes, Eugene, OR), at a dilution of 1:200 in PBS in the presence of 10% normal goat serum. The neuronal fraction in each sample was estimated by counting NeuN-labeled nuclei in at least 500 DAPI-stained nuclei. NeuN staining is smooth, covers the entire nuclear area, and is crisp and easily identifiable from the very low background (Fig. 1B,D). The total number of neurons in each structure was calculated by multiplying the fraction of nuclei expressing NeuN by the total number of nuclei. The number of nonneuronal nuclei was obtained by subtraction. Photomicrographs for documentation were taken using a Zeiss Axioplan fluorescence microscope or, for high magnification, a Zeiss LSM 510 Multiphoton microscope and were acquired digitally in AxioVision or LSM Image Browser software (all from Carl Zeiss MicroImaging), respectively. For illustrations, contrast and brightness of the micrographs were adjusted in Corel Draw X3.
Data analysis
All statistical analyses and regressions were performed in StatView software (SAS, Cary, NC). All data reported are mean ± SD.
RESULTS
We find that the male human brain, aged ∼50 years (n = 3) or 70 years (n = 1) and weighing 1,508.91 ± 299.14 g, contains on average 170.68 ± 13.86 billion cells. Among these, 85.08 ± 6.92 billion cells are located in the cerebellum, 77.18 ± 7.72 billion cells are in the cerebral cortex (including both gray and white matter), and 8.42 ± 1.50 billion cells are found in the remaining regions (RoB; Fig. 2). Because no significant differences were found in mass or in neuronal, nonneuronal, and total cell numbers between right and left hemispheres (t-test, P values typically well above 0.1), all numbers given refer to the combined hemispheres.

Absolute mass, numbers of neurons, and numbers of nonneuronal cells in the entire adult human brain. Values are mean ± SD and refer to the two hemispheres together. B, billion.
Overall, the nonneuronal/neuronal ratio in the whole human brain is close to 1 (Fig. 2), insofar as half of the cells in the human brain, or 86.06 ± 8.12 billion, are neurons (range 78.82–95.40 billion neurons). The fractional distribution of neurons in the human brain does not correspond to the fractional distribution of mass among brain structures (Figs. 2, 3). Although 82% of brain mass consists of cerebral cortex (including subcortical white matter) and 42% consists of cerebral cortical gray matter alone, the 16.34 ± 2.17 billion neurons found in this structure represent only 19% of all brain neurons. In contrast, the cerebellum, which represents only 10% of total brain mass, contains 69.03 ± 6.65 billion neurons, or 80% of all neurons in the human brain. Fewer than 1% of all brain neurons are located in the RoB, comprising basal ganglia, diencephalon, and brainstem (Figs. 2, 3), although this percentage is necessarily underestimated as a result of the known lack of NeuN staining in at least some structures in the brainstem (see Materials and Methods).

Distribution of mass, numbers of neurons, and numbers of nonneuronal cells in the adult human brain. Each bar represents the percentage mass or percentage number of cells located in the cerebral cortex (Cx, black), cerebellum (Cb, gray), and remaining areas of the brain (RoB, white). Values are mean ± SD.
The other half of all human brain cells, or 84.61 ± 9.83 billion, are nonneuronal cells, yielding a nonneuronal/neuronal ratio of 0.99 for the human brain as a whole (Fig. 2). Nonneuronal cells outnumber neuronal cells in the RoB, with a nonneuronal/neuronal ratio of 11.35 (Fig. 2). In the gray matter of the cerebral cortex, the nonneuronal/neuronal ratio is 1.48. In contrast, in the cerebellum, this ratio is only 0.23 (Fig. 2).
In contrast to the distribution of neurons, the fractional distribution of nonneuronal cells in the brain resembles more closely the fractional distribution of mass in the structures (Fig. 3). The cerebral cortex, including the subcortical white matter, holds 72% (or 60.84 ± 7.02 billion) of all nonneuronal cells in the brain, whereas the cerebellum has 19% (or 16.04 ± 2.17 billion) and the RoB holds 9% (or 7.73 ± 1.45 billion) of all the nonneuronal cells in the brain.
The separate analysis of white matter (WM) and gray matter (GM) of the cerebral cortex shows that the former contains 69.6%, or 19.88 ± 2.83 billion, of the nonneuronal cells of the cerebral cortex of one hemisphere (Fig. 4). The ratio between nonneuronal and neuronal cells is 1.48 ± 0.42 for the GM (Fig. 4) and 3.76 ± 0.55 for the combined GM and WM (Fig. 2).

Absolute mass, numbers of neurons, and numbers of nonneuronal cells in the cortical gray and white matter. Values are mean ± SD and refer to the right hemisphere (RH) only (n = 3).
The numbers of neuronal and nonneuronal cells found in the human brain fall very close to the values expected for a primate brain of human dimensions built according to the linear, isometric cellular scaling rules found to apply to primate brains (Herculano-Houzel et al.,2007; Fig. 5a–c). According to these rules, a primate brain weighing 1,508 g would be expected to have 94 billion neurons, with 22 billion neurons in the cerebral cortex, 78 billion neurons in the cerebellum, and 0.6 billion neurons in the RoB (Table 1). The observed numbers of neurons are actually slightly smaller than expected in the human cortex and cerebellum and larger in the RoB (Table 1). The percentage deviations in the observed values from the numbers of neuronal and nonneuronal cells expected in each structure of the human brain given its mass fall within the same range as the values found for each of the species from which the cellular scaling rules were originally obtained (Herculano-Houzel et al.,2007; Fig. 6). The neuronal and nonneuronal cell densities found in the human brain also fall in the range observed in nonhuman primates in that study (Herculano-Houzel et al.,2007; Fig. 5d,e).
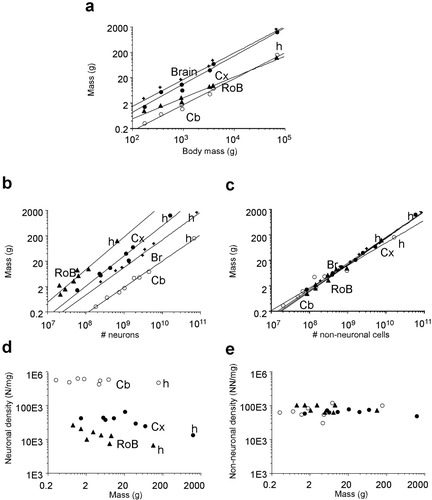
The human brain conforms to the cellular scaling rules that apply to primates. a–c: Mass of the cerebral cortex (Cx, solid circles), cerebellum (Cb, open circles), RoB (triangles), and whole brain (crosses) of six primate species, tree shrews, and humans (h) as a function of body mass (a), number of neurons (b), and number of nonneuronal cells (c). The power functions plotted refer to nonhuman primates only (Herculano-Houzel et al.,2007), and all have exponents close to 1.0. Note that the data points for the human brain fall very close to the plotted functions. d,e: Neuronal (d) and nonneuronal (e) densities in the different brain structures of six primate species, tree shrews, and humans (h) plotted against structure mass.
| Expected | Observed | Difference | |
|---|---|---|---|
| For a primate of 75 kg | |||
| Total brain mass (g) | 1,362 | 1,508 | +10.7% |
| Total number of brain cells | 170.97 | 170.68 | −0.2% |
| Total number of brain neurons | 78.08 | 86.06 | +10.2% |
| Total number of brain nonneurons | 94.28 | 84.61 | −10.2% |
| For a primate brain of 1,508 g | |||
| Total number of neurons | 93.82 | 86.06 | −8.3% |
| Total number of nonneurons | 113.17 | 84.61 | −25.2% |
| For a primate cortex of 1,233 g | |||
| Total number of neurons | 22.36 | 16.34 | −26.9% |
| Total number of nonneurons | 99.02 | 60.84 | −38.6% |
| For a primate cerebellum of 154 g | |||
| Total number of neurons | 77.94 | 69.03 | −11.4% |
| Total number of nonneurons | 11.26 | 16.04 | +42.4% |
| For a primate RoB of 118 g | |||
| Total number of neurons | 0.62 | 0.69 | +11.3% |
| Total number of nonneurons | 7.17 | 7.73 | +7.8% |
- 1 Results are given in billions.
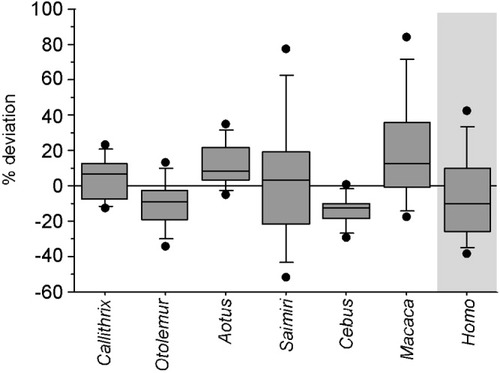
Deviation from the expected cellular composition of the brain structures in six nonhuman primate species and man. Each box depicts the median deviation (expressed as percentage of the expected value) and the 25th and 75th percentiles of the observed numbers of neuronal and nonneuronal cells in the cerebral cortex, cerebellum, and remaining areas from the values expected according to the cellular scaling rules derived from the six first species in the graph. The bars indicate the 10th and 90th percentiles for each species, and the dots indicate the upper and lower extreme deviations. Note that the human brain deviates in its cellular composition from that expected for a primate of its brain size as much as the other species from which the cellular scaling rules for primate brains were derived.
DISCUSSION
It is often stated in the literature that glial cells outnumber neurons in the human nervous system by a factor of 10. Our finding that the human brain has an approximately 1:1 ratio of nonneuronal:neuronal cells implies a necessarily smaller glia/neuron ratio, if endothelial and other mesenchymal cells were removed from the nonneuronal pool. Although this ratio of approximately 1:1 for the whole brain is important counterevidence to the common overestimation of numbers of glial cells in the brain, it conceals the fact that specific structures of the human brain can have maximal glia/neuron ratios (if all nonneuronal cells were glial cells) as small as 0.23, such as the cerebellum, and as large as 11.35, such as the RoB. The maximal glia/neuron ratio of 1.48 that we observed in the total GM of the cerebral cortex is close to the ratio of 1.65 observed by Sherwood et al. (2006) in layer II/III of human prefrontal area 9L and similar to the ratios between 1.2 and 1.6 that Pelvig et al. (2008) encountered in the whole human neocortical GM. These similarities corroborate our findings.
According to the common view in the literature, the glia:neuron ratio increases with brain size (Reichenbach,1989), leading to a predominance of glial cells in large brains (Nedergaard et al.,2003) that would be compatible with a 10:1 ratio in humans. We have shown that, although the average nonneuronal cell size is relatively invariant across brain structures and species, an increasing predominance of glial cells with brain size is indeed found in rodents (Herculano-Houzel et al.,2006), in which average neuronal size increases together with neuronal number, but not in the primates examined so far (Herculano-Houzel et al.,2007), in which average neuronal size, as with average nonneuronal cell size, is estimated to remain relatively stable as numbers of neurons increase. All primates we have analyzed until now (Herculano-Houzel et al.,2007) exhibit ratios of nonneuronal/neuronal cells that are similar to the approximately 1:1 ratio found in the human brain as a whole.
The glia/neuron ratio has been considered to be of great relevance because of the multiple functional relationships recently demonstrated between these cell types (Nedergaard et al.,2003; Shaham,2005) and has been hypothesized to reflect neuronal activity (Reichenbach,1989). Alternatively, the glia:neuron ratio might simply follow the ratio between average neuronal and average glial cell mass. We have proposed (Herculano-Houzel et al.,2006) that this occurs as the nearly all-neuronal parenchyma is invaded in early postnatal development by glial progenitors that divide until the newly formed glial cells, of a relatively constant average size, reach confluence (Zhang and Miller,1996). In this scenario, a neuronal parenchyma of a given volume built of a large number of small neurons is invaded by progenitors that will give rise to the same number of glial cells as a parenchyma of the same volume built of a smaller number of larger neurons, but the latter, with larger neurons, will have a much larger glia:neuron ratio than the former. It is noteworthy that the low glia:neuron ratio in the cerebellum is due not to a conspicuous lack of glial cells but rather to a very large number of very small neurons, because its nonneuronal cell density is even somewhat larger than that in other structures. The relative constancy of glial cell densities observed across rodents and primates, humans included, may therefore be more physiologically meaningful in terms of their role in the metabolic maintenance and functional support of brain tissue than their numeric ratio to neurons.
Our estimate of an average total of 86 billion neurons in the human brain is compatible with previous stereological determinations for individual structures such as the cerebral cortex (von Economo and Koskinas,1925; Pakkenberg and Gundersen,1997; Pelvig et al.,2008) and the cerebellum (Lange,1975; Andersen et al.,1992). Exact numbers are probably highly variable among humans, particularly given the variation of over 50% in the number of cortical neurons among individuals of the same sex described recently in the literature (Pelvig et al.,2008). Although the numbers of neurons that we report for the RoB (which includes NeuN-negative neurons in the inferior olive and possibly other structures as well) are necessarily underestimated, the number of NeuN-negative neurons included in the “nonneuronal” population of the RoB is likely to be negligible compared with the 85 billion neurons found in the ensemble of cerebral cortex and cerebellum (because the RoB is found to contain a total of only about 8 of the 170 billion cells in the human brain), or to the 690 million neurons in the RoB. Moreover, even in the improbable scenario in which all cells in the RoB, composed of massive fiber tracts, were NeuN-negative neurons, they would still amount to only 5% of all brain cells.
More important than the exact number of neurons in the human brain, however, are the implications of how this number compares with that expected for a primate brain of human proportions. We have shown before that a brain with about 100 billion neurons built according to the cellular rules that apply to scaling rodent brains would weigh 45 kg (Herculano-Houzel et al.,2007), well above the largest known whale brain. Humans of 70 kg of body mass built according to these rules would be expected to have a brain of only 145 g, instead of 1,500 g. That is, humans do indeed have a brain that is about ten times larger and holds seven times more neurons than predicted for a nonprimate mammal of its body size.
Remarkably, however, here we find for the first time that the human brain conforms to the scaling rules observed for a given group of mammals: six other primate species (Herculano-Houzel et al.,2007), the only ones so far whose total brain cellular composition is known. We show that humans, despite the large relative size of the cerebral cortex, hold only 19% of all brain neurons in this structure, as do other primates and rodents of different brain sizes, and demonstrate that the mass and cellular composition of the human brain deviate from the values expected for a primate of 75 kg by only 10%. Given that the cellular scaling rules that apply to primate brains are linear, the conformity of the human brain to these rules strongly indicates that the human brain is a linearly scaled-up primate brain in its cellular composition. The isometric scaling of the human brain cellular composition relative to other primates is in line with other observations that have established that the cerebellum (Frahm et al.,1982) and frontal cortex (Semendeferi et al.,2002) of the human brain have the same relative size as in great apes, even though the distribution of mass within the WM and individual cortical areas in humans may differ from that in other primates (Semendeferi et al.,2001; Rilling and Seligman,2002; Schoenemann et al.,2005).
Our notion that the human brain is a linearly scaled-up primate brain in its cellular composition is in clear opposition to the traditional view that the human brain is 7.0 times larger than expected for a mammal and 3.4 times larger than expected for an anthropoid primate of its body mass (Marino,1998). However, such large encephalization is found only when body-brain allometric rules that apply to nonprimates are used, as stated above, or when great apes are included in the calculation of expected brain size for a primate of a given body size. Our finding thus suggests that the rules that apply to scaling brains are more conserved than those that apply to scaling the body and raises the intriguing possibility that, rather than humans having a larger brain than expected, it is the great apes such as orangutans and, more notably, gorillas that have bodies that are much larger than expected for primates of their brain size. Indeed, the inclusion of great apes (Marino,1998) in the primate species (Herculano-Houzel et al.,2007) that we compare to humans would increase the body size expected of our species, with a brain of 1,509 g, from 77 kg to 216 kg, and decrease the expected brain size for a body of 70 kg from 1,247 g to 557 g. One piece of evidence in support of the possibility that gorillas and orangutans, rather that humans, are outlier species in terms of body size is that, whereas in most primate species, humans included, the brain represents about 2% of total body mass (Marino,1998), the brains of gorillas and orangutans, at about 500 g (Semendeferi and Damasio,2000), represent at most 1% of a body of 50 kg, and only 0.5% or less in typical male gorillas of 100 kg or more. The adaptive value of an enlarged body size in these species can be appreciated from the status of social dominance that comes with the large investment of time and energy necessary to develop large bodies in alpha-male gorillas and orangutans (Leigh,1995).
We are currently investigating whether the brains of gorillas and orangutans also conform to the cellular scaling rules found to apply to other primates, including humans. Such conformity would substantiate the intriguing possibility that, rather than humans having too large a brain for their bodies, gorillas have too large a body for their brains, although both species have brains built according to the same rules that apply to other primates. Body size (Jerison,1973), after all, may not be a relevant parameter when it comes to cognition (Roth and Dicke,2005). In light of the recent finding that absolute brain size is the parameter that best correlates with cognitive abilities (Deaner et al.,2007), our cognitive advantage over other primates might be simply a consequence of having the largest brain, built with an isometrically enlarged number of neurons compared with other smaller-brained primates, regardless of body size.
Acknowledgements
We thank all of our colleagues who helped with tissue collection and homogenization.




