Steroid-triggered programmed cell death of a motoneuron is autophagic and involves structural changes in mitochondria
Abstract
Neuronal death occurs during normal development and disease and can be regulated by steroid hormones. In the hawkmoth, Manduca sexta, individual accessory planta retractor (APR) motoneurons undergo a segment-specific pattern of programmed cell death (PCD) at pupation that is triggered directly and cell autonomously by the steroid hormone 20-hydroxyecdysone (20E). APRs from abdominal segment six [APR(6)s] die by 48 hours after pupal ecdysis (PE; entry into the pupal stage), whereas APR(4)s survive until adulthood. Cell culture experiments showed previously that 20E acts directly on APRs to trigger PCD, with intrinsic segmental identity determining which APRs die. The APR(6) death pathway includes caspase activation and loss of mitochondrial function. We used transmission electron microscopy to investigate the ultrastructure of APR somata before and during PCD. APR(4)s showed normal ultrastructure at all stages examined, as did APR(6)s until approximately stage PE. During APR(6) death, there was massive accumulation of autophagic bodies and vacuoles, mitochondria became ultracondensed and aggregated into compact clusters, and ribosomes aggregated in large blocks. Nuclear ultrastructure remained normal, without chromatin condensation, until the nuclear envelope fragmented late in the death process. Light microscopic immunocytochemistry showed that dying APR(6)s were TUNEL-positive, which is diagnostic of fragmented DNA. These observations indicate that the steroid-induced, caspase-dependent, cell-autonomous PCD of APR(6)s is autophagic, not apoptotic, and support an early role for mitochondrial alterations during PCD. This system permits the study of neuronal death in response to its bona fide developmental signal, the rise in a steroid hormone. J. Comp. Neurol. 457:384–403, 2003. © 2003 Wiley-Liss, Inc.
Programmed cell death (PCD) is an active program of cell elimination that occurs during nervous system development, homeostasis, injury, and disease (for review see Burek and Oppenheim, 1996; Deshmukh and Johnson, 1997). In contrast to necrosis, which represents a loss of homeostatic control in the dying cell, PCD is a carefully choreographed cellular suicide. Ultrastructural analysis, using transmission electron microscopy (EM), complements physiological and molecular studies of PCD by providing a high-resolution view of subcellular events and the temporal sequence in which these events occur.
PCD can be classified into three ultrastructural categories: type 1, or apoptosis; type 2, or autophagic cell death (also termed “autophagocytosis”); and type 3, or nonlysosomal PCD (this type is not considered further here; Schweichel and Merker, 1973; Clarke, 1990). EM is at present the definitive method by which to distinguish among the different types of death. Apoptosis is characterized by early condensation of the chromatin into dense masses, often concentrated on the inner surface of the nuclear envelope. The nucleus becomes increasingly condensed (pyknotic). At the same time, the cell shrinks, cytoplasmic contents increase in electron density, the number of free ribosomes increases, and portions of the cell pinch off to form apoptotic bodies that are phagocytosed by surrounding cells. Autophagic bodies do not increase in number, and material from the dying cell is degraded by lysosomes in other cells (heterophagy). In contrast, during autophagic cell death, cellular contents are destroyed by the cell's own lysosomes after being sequestered within autophagic bodies. The number and size of autophagic bodies increase, the cell shrinks, and cytoplasmic contents increase in electron density. Chromatin condensation either is absent or occurs as a relatively late event. Eventually, the nuclear envelope ruptures.
The error of referring to all instances of PCD as “apoptosis” has been amply reviewed by others (Schwartz et al., 1993; Schulte-Hermann et al., 1997; Savitz and Rosenbaum, 1998; Bursch et al., 2000a; Bursch, 2001; Thummel, 2001; Kucharova and Farkas, 2002). This usage has fostered an underappreciation of the important role of autophagic cell death during normal development of the nervous system (Decker, 1974; Stocker et al., 1978; Lamborghini, 1987; Hornung et al., 1989); in neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, and Huntington's disease (for review see Nixon and Cataldo, 1993; Bursch, 2001; Larsen and Sulzer, 2002; see also Cataldo et al., 1996; Anglade et al., 1997; Kegel et al., 2000; Peterson et al., 2001); and in tumorigenesis (Liang et al., 1999). The cytological differences between apoptosis and autophagic cell death provide clues for identifying underlying molecular mechanisms. In Drosophila, some genes (e.g., dronc, which encodes a caspase) participate in both types of PCD, whereas other genes (e.g., E93) are unique to autophagic cell death (Lee et al., 2000; Lee and Baehrecke, 2001; for review see Abrams, 1999; Thummel, 2001). The role of cytoskeletal filaments differs in the two types of PCD (Bursch et al., 2000b), and the lysosomal system plays a dominant role in autophagic cell death but not in apoptosis (for review see Bursch, 2001). Not unexpectedly, intermediate forms of PCD that share apoptotic and autophagic features also occur (for review see Clarke, 1990; Bursch, 2001; see also Nitatori et al., 1995; Bursch et al., 1996; Dai and Gilbert, 1997; Schätzl et al., 1997; Ohsawa et al., 1998; Xue et al., 1999; Terwel and Van de Berg, 2000; von Bültzingslöwen et al., 2001). The nature of the triggering stimulus can determine whether PCD in a neuronal population exhibits apoptotic or autophagic features (Pilar and Landmesser, 1976; for review see Nixon and Cataldo, 1993). Genetic deletion of caspases in mice eliminates apoptotic PCD during spinal cord development, but the normal complement of neurons is nevertheless eliminated by a non-apoptotic form of PCD (Oppenheim et al., 2001). The dichotomous classification of neuronal deaths as apoptotic or necrotic oversimplifies the diversity of neuronal responses to developmental signals, cellular injury, or disease.
The present study investigated ultrastructural events during the PCD of a motoneuron during metamorphosis in the hawkmoth, Manduca sexta. Accessory planta retractor (APR) motoneurons undergo a segment-specific pattern of PCD at pupation (entry into the pupal stage) that is triggered by the prepupal peak of the steroid molting hormone 20-hydroxyecdysone (20E). Previous studies have investigated APRs from abdominal segment 6 [APR(6)s], which die during the first 48 hours of pupal life, and APR(4)s, which survive through the pupal stage and die after adult eclosion (emergence of the moth from the pupal case; Weeks and Ernst-Utzschneider, 1989; Weeks et al., 1992; Zee and Weeks, 2001). By placing APRs in primary cell culture at different developmental stages and treating them with physiological levels of 20E, we determined that prepupal 20E acts directly on APR(6)s to trigger PCD and that intrinsic segmental identity determines which APRs live or die (Streichert et al., 1997; Hoffman and Weeks, 1998). Both APR(4)s and APR(6)s have ecdysteroid receptors during the prepupal period (J. Ewer, G.K., and J.C.W., unpublished data), and the molecular mechanisms by which APRs in different body segments respond differently to 20E are under investigation (Hazelett et al., 2002; and unpublished data). Because the bona fide biological signal for APR(6) death—the prepupal rise in 20E—is known, and APRs respond cell autonomously to this developmental signal, this system is advantageous for investigating cellular mechanisms of PCD. Furthermore, the steroid-induced death of APRs occurs in a well-defined electrophysiological and behavioral context within the insect's life cycle (Streichert and Weeks, 1995; Sandstrom and Weeks, 1996, 1998; Lubischer et al., 1999; for review see Weeks et al., 1997; Weeks, 1999).
A working model of the sequence of events during 20E-induced PCD of APR(6)s (Fig. 1) was derived from experiments in which APR(6)s were cultured at different developmental stages in the presence or absence of 20E and/or pharmacological agents that disrupt specific steps in the pathway (Hoffman and Weeks, 2001). Histological markers were used to assess mitochondrial function [e.g., enzyme activity and inner transmembrane potential (ΔΨm)] and other cellular attributes, such as integrity of the nucleus and plasma membrane. The model of the APR(6) death pathway begins with activation of ecdysteroid receptors, followed by transcription of mRNA and translation of new proteins. Treatment with the protein synthesis inhibitor cycloheximide interrupts PCD at this step (Weeks et al., 1993; Hoffman and Weeks, 1998). Treatment of cultured APR(6)s with the caspase inhibitor benzyloxycarbonyl-aspartate-2,6-dichlorobenzoyloxymethylketone (Z-Asp-CH2DCB) at different developmental stages revealed two steps of caspase activation lying upstream and downstream of mitochondrial events (Hoffman and Weeks, 2001). Activation of initiator (upstream) caspases during the premitochondrial phase of PCD commits mitochondria to a loss of function and sets in motion the postmitochondrial, execution phase of PCD. During the postmitochondrial phase, mitochondrial enzyme activity and ΔΨm are lost, and effector (downstream) caspases cause shrinkage and rounding of the cell body, fragmentation of the nucleus, and ultimately fragmentation of the plasma membrane (Hoffman, 1999; Hoffman and Weeks, 2001; Rutherford et al., 2002).
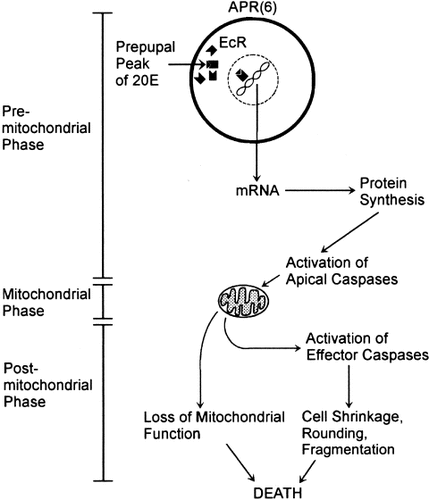
Working model of the 20E-induced programmed cell death pathway in APR(6)s. See text for details. 20E, 20-hydroxyecdysone; EcR, ecdysteroid receptor. Modified from Hoffman and Weeks (2001).
To complement physiological studies of PCD, we examined the EM ultrastructure of APR(4)s (which survive) and APR(6)s (which die) during the larval and early pupal stages. These results show that APR(6) death is autophagic, not apoptotic. Furthermore, during the PCD of APR(6)s, mitochondria undergo striking morphological alterations consistent with an early role in the death pathway. Some of these results have appeared previously in an abstract (Weeks et al., 2000).
MATERIALS AND METHODS
Rearing and staging of experimental animals
M. sexta larvae were reared individually in cups containing artificial diet (modified from Bell and Joachim, 1976) under a 17 hour:7 hour light:dark photoperiod (lights off at 00:00) and a 27°C:25°C thermoperiod. At wandering (see below), larvae were placed in small chambers drilled out of wooden blocks. Both male and female insects were used. Insects were staged as follows (see Fig. 2): Stage L2 larvae were timed to be 48 hours after ecdysis (cuticle shedding) to the final (fifth) larval instar, which occurs on day L0; stage W0 larvae, which initiated wandering behavior during the previous night (gate I; Truman and Riddiford, 1974), were selected at 12:00; and “dorsal bar” prepupae, which were ∼10 hours before pupal ecdysis (PE) and had darkly sclerotized dorsal bars on the metathorax (Truman et al., 1980). The latter insects were designated “PE–10h.” Stage PE (pupal ecdysis) pupae were selected up to 3 hours after completing ecdysis. Subsequently, pupae were staged by the number of hours elapsed since PE (e.g., PE+12h, PE+24h).
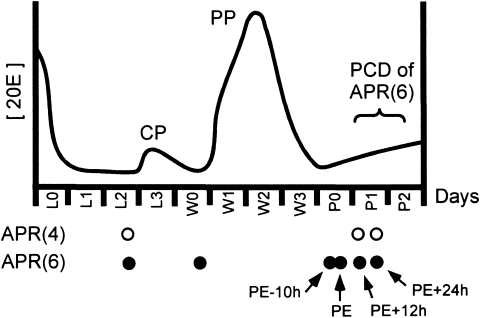
Sampling protocol of APRs for transmission electron microscopy. The time line shows days of development spanning the final larval instar and early pupal stage. L0, day of entry into the fifth (final) larval instar; L1, L2, etc., days after L0; W0, day of wandering behavior (when the larva leaves the food to burrow underground); W1, W2, etc., days after wandering; P0, day of entry into the pupal stage; P1, P2, days after P0. The times at which APR(4)s and APR(6)s were removed from ganglia for EM analysis are indicated by open and solid circles, respectively, below the time line. PE (pupal ecdysis) occurs on day P0, and other developmental stages timed with respect to PE are indicated by arrows. The curve at top shows the relative levels of 20E during this period (from Bollenbacher et al., 1981). The commitment pulse (CP) and prepupal peak (PP) of 20E are indicated. In vivo, all APR(6)s are alive on P0 and dead on P2, in response to the prepupal peak of 20E (Weeks et al., 1992).
Preparation of APRs for transmission EM
Unlike some Manduca motoneurons (Stocker et al., 1978), APR somata cannot be identified accurately by location in sectioned ganglia. Accordingly, EM was performed on APRs that were dissociated from the nervous system using the methods of Hoffman and Weeks (1998, 2001). APR(4)s or APR(6)s were fluorescently labeled in situ by injecting ∼5 μl of a suspension of DiI (10 mg/ml in petroleum jelly; Molecular Probes, Eugene, OR) adjacent to the left and right accessory planta retractor muscle (APRM; the target muscle of APR) in either segment A4 or A6 of midfourth-instar larvae. Each APRM is innervated by two APRs (Sandstrom and Weeks, 1996), so up to four APRs were labeled per ganglion. After DiI injection, insects were allowed to develop to the desired stage; in some cases, they were held at 2°C for up to 24 hours to arrest development prior to dissection.
After anesthesia in CO2 gas, the ganglion of the DiI-injected segment was removed under physiological saline (Weeks and Truman, 1984), transferred to “basal” culture medium (modified L-15; see Hoffman and Weeks, 2001), and desheathed using fine tungsten needles. The anterior ventral portion of the ganglion, containing APR cell bodies, was removed using the needles and transferred to collagenase (1 mg/ml, Sigma type XI; Sigma, St. Louis, MO) and dispase (4 mg/ml, Boehringer Mannheim grade II; Boehringer Mannheim, Indianapolis, IN) in Ca2+-free, Mg2+-free Hanks' balanced salt solution. The time from opening the body to transferring the tissue into enzyme solution was 10–20 minutes. After incubation for 2 minutes, the tissue was transferred to a well containing basal culture medium and mechanically dissociated into single cells using finely pulled capillary glass. The culture dish was then placed under an inverted microscope (Zeiss Axiovert 135) with epifluorescence and phase-contrast optics, to select APRs for EM processing. After dissociation, APRs lacked processes except for a short stump of the primary neurite, which usually survived (see Figs. 2, 3). As in previous studies (Streichert et al., 1997; Hoffman and Weeks, 1998, 2001), only APRs with bright DiI labeling (viewed under rhodamine optics) and intact morphology (viewed under high-power phase-contrast optics) were selected. In this study, all APR(4)s, and all APR(6)s through stage PE, had large, ovoid cell bodies characteristic of normal live neurons (Hoffman and Weeks, 1998; see Fig. 2). Some APR(6)s cultured at PE+12h and PE+24h had rounded, shrunken somata diagnostic of PCD (Hoffman and Weeks, 1998; see Results). The morphology of each APR was recorded.
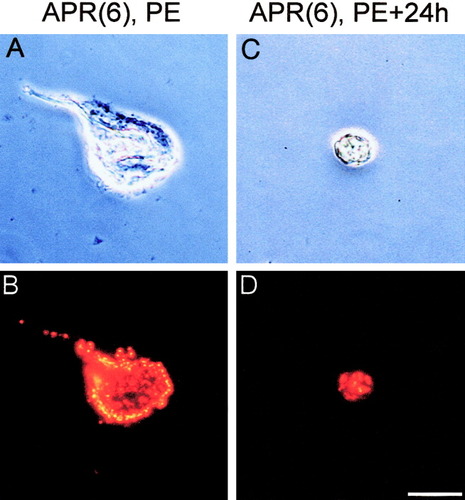
Light microscopic appearance of acutely dissociated APRs. Top two photomicrographs (A,C) are under phase contrast optics, whereas bottom two photomicrographs (B,D) are the same neurons under epifluorescent illumination (rhodamine optics) to visualize DiI labeling. A,B: An APR(6) dissociated at PE, with a large, teardrop-shaped soma and stump of neurite. C,D: An APR(6) dissociated at PE+24h, which is rounded and shrunken. In both APR(6)s, the DiI label appears as bright, punctate spots in the cytoplasm. The most intensely fluorescent spots appear yellow rather than red (see also Streichert et al., 1997; Hoffman and Weeks, 1998). Scale bar = 50 μm.
APRs were transferred to another culture dish for fixation. By following the method of Beadle et al. (1982), APRs were fixed for 1 hour at 4°C in 2% gluteraldehyde (grade I, 25% aqueous; Sigma) in 0.1 M cacodylate buffer (pH 7.2). The interval between removal from the ganglion and fixation was 30–60 minutes, depending on the number of cells to be processed. APRs were then rinsed, and in some cases stored for up to 3 days, in 0.1 M cacodylate buffer. APRs were postfixed for 1 hour in 1% osmium tetroxide, dehydrated in an ethanol series (including staining with 2% uranyl acetate during the 50% ethanol rinse, 20 minutes), embedded in Epon 812, and placed in an oven overnight or until the Epon was hardened. Silver (∼700 Å) or gold (∼1,000 Å) sections were cut with a diamond or glass knife on an LKB microtome, stained in Pb citrate (10 min), and in some cases carbon coated before being viewed and photographed in a CM 12 transmission electron microscope. After sectioning, APRs that appeared damaged or necrotic (e.g., with large, abnormal vacuoles or ruptured plasma membrane; Hoffman and Weeks, 2001) were excluded from analysis. Figure 2 indicates the time points at which APR(4)s and APR(6)s were selected for EM analysis.
Electron micrographs presented in this paper were printed from photographic negatives, scanned at a resolution of 300 dpi, and assembled into figures using Canvas version 7.0 (Deneba Software, Inc., Miami FL) without altering any image features.
TUNEL staining of APRs
The terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method (Gavrieli et al., 1992) was used to visualize fragmented DNA. APR(4)s and APR(6)s were retrogradely labeled with DiI and dissociated from ganglia as described above. In some cases (see Results), unidentified motoneurons (identified by their large somata) were used instead of APRs for controls. All neurons were removed from animals between stages PE and PE+36h. After dissociation and rinsing, groups of APR(4)s, APR(6)s, and/or unidentified motoneurons were placed in separate 10 μl wells. The TUNEL method was performed using an ApopTag kit (Intergen, Purchase, NY). Neurons were rinsed four times in insect saline, fixed for 30 minutes in 4% formaldehyde, rinsed four times in phosphate-buffered saline (PBS; pH 7.2) with 0.01% Triton (PBSX), rinsed twice in PBS, incubated for 10 minutes in kit-provided equilibration buffer, and incubated for 1 hour in terminal deoxynucleotidyl transferase (TdT) reaction solution (to add digoxigenin-labeled nucleotides to DNA fragments). The neurons were then incubated for 20 minutes in stop/wash solution, rinsed four times in PBSX, rinsed twice in PBS, and incubated for 12 hour at 4°C in a mixture of fluorescein-conjugated antidigoxigenin antibody and blocking solution. Next, the neurons were rinsed four times in PBS, transferred onto a glass coverslip, allowed to dry, then mounted in 2% n-propyl gallate (in 90% glycerol, 10% PBS) to prevent fading. For negative controls, TdT was omitted from the reaction mixture. For positive controls, neurons were pretreated with DNAse (100 μg/ml for 10 minutes; Sigma) to cause DNA fragmentation.
The intensity of TUNEL staining was quantified in one experiment in which motoneurons (see Results) were dissociated between PE and PE+36h, pooled, and processed simultaneously under identical conditions (except for controls). Neurons were observed in a Zeiss LSM 310 confocal laser scanning microscope under fluorescein optics using a 40× objective (N.A. 0.75) and a 3× hard magnification in the software, using identical pinhole, brightness, and contrast settings. The depth (along the z-axis) of the nucleus was determined for each neuron, and 10 optical sections were acquired (each being the average of 16 scans) to encompass the entire nuclear volume. A “brightest point” projection was generated for each neuron using Scion Image (Scion Corporation, Frederick, MD). In this method, at each point in the projection plane, a ray passed normally to the plane through the stack of optical sections, and the brightest point encountered along each ray was used to generate the brightest point projection. From the brightest point projections, a threshold pixel brightness was set that separated nuclear TUNEL staining from background cytoplasmic fluorescence [which was low, except for one TUNEL-positive APR(6) with excessive cytoplasmic fluorescence, which was excluded from the study]. The same threshold brightness was used for all neurons in the analysis. The number of pixels that exceeded the brightness threshold was computed for each neuron in Scion Image. Statistical analysis of pixel counts was performed using StatGraphics Plus software (Englewood Cliffs, NJ).
RESULTS
Light microscopic appearance of APRs used for EM
Figure 3 shows the typical light microscopic appearance of APR(6)s immediately after dissociation from the ganglion, before (Fig. 3A,B) and during (Fig. 3C,D) overt morphological signs of PCD. Under epifluorescent illumination (Fig. 3B,D), both APR(6)s exhibited bright DiI labeling, which was intracellular and punctate. The APR(6) cultured at stage PE had the morphology typical of normal, healthy APRs (Streichert et al., 1997; Hoffman and Weeks, 1998, 2001; Zee and Weeks, 2001). The soma was large and ovoid, with a teardrop shape (long and short somatic dimensions of ∼78 μm and ∼63 μm, respectively), and bore a short segment of neurite. In contrast, the APR(6) cultured at stage PE+24h was shrunken and rounded (∼30 μm diameter), with a spherical shape. The decrease in somatic size and acquisition of a spherical shape are quantitative markers of PCD in APR(6)s, both in vivo and in vitro (Hoffman and Weeks, 1998).
Low-magnification ultrastructural appearance of APR(6)s
The same differences in somatic size and shape were apparent when normal and dying APR(6)s were observed by transmission EM. Figure 4A shows a low-magnification electron micrograph of an APR(6) on day W0. This APR(6) had a large, ovoid soma with a teardrop shape (long and short somatic dimension of ∼50 μm and ∼30 μm) and a neurite stump. The somatic dimensions of neurons processed for EM were typically smaller than those of live neurons (cf. Figs. 3 and 4), presumably as a result of fixation-induced shrinkage and the fact that EM sections were not always obtained through the largest profile of the neurons. The general ultrastructural features of the APR(6) shown in Figure 4A were similar to those of many other types of neurons (for review see Peters et al., 1991; Strausfeld and Meinertzhagen, 1998). The centrally placed nucleus contained a matrix of fine material, with some denser aggregates, and exhibited one eccentrically placed nucleolus. The plasma membrane was generally smooth, with fine cytoplasmic fingers in some regions. The cytoplasm exhibited a relatively uniform distribution of organelles with a range of electron densities, from light to dark. As described in more detail below, these ultrastructural features were typical of APR(6)s prior to the onset of PCD and of APR(4)s at all developmental stages examined.
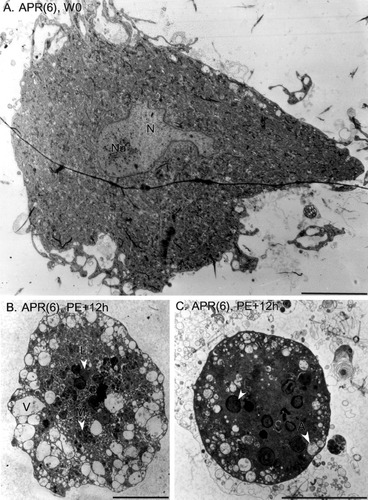
Low-magnification EM appearance of normal and dying APR(6)s. A: An APR(6) on day W0. The soma is large and teardrop-shaped, the nucleus (N) is intact and contains a nucleolus (Nu), and cytoplasmic organelles are distributed uniformly. A stump of neurite extends from the soma at right. These features were characteristic of APR(4)s at all developmental stages and of APR(6)s prior to PCD. The wavy horizontal line crossing the field of view is a flaw in the section. B and C show two APR(6)s cultured at stage PE+12h illustrating the characteristic features of PCD. The somata are shrunken and rounded, the nucleus is missing, the cytoplasm has increased in electron density (evident in C), mitochondria (M) are electron dense and clustered, and there is massive accumulation of autophagic bodies (A), including multilamellar bodies (L) and vacuoles (V). Scale bars = 10 μm.
The two APR(6)s shown in Figure 4B,C, which were dissociated at stage PE+12h while undergoing PCD, had a strikingly different appearance. The cell outlines were rounded and in the case of the APR(6) shown in Figure 4C nearly spherical. The plasma membranes were smooth, without cytoplasmic fingers. The EM sections shown in Figure 4B,C were obtained near the middle of each neuron, to reveal maximal somatic diameters of ∼38 μm and ∼17 μm, respectively. The percentages of APRs with the rounded phenotype (grouped by segment and stage) are given in Table 1. No APR(4)s at any developmental stage, nor any APR(6)s through stage PE, were shrunken and rounded. One-third to one-half of the APR(6)s used for EM analysis at PE+12h and PE+24h were shrunken and rounded. In vivo, approximately 60% of APR(6)s are shrunken and rounded at PE+24h (Hoffman and Weeks, 1998).
| Stage | n | Percentage of APRs exhibiting | ||
|---|---|---|---|---|
| Shrunken, rounded soma | Fragmented nucleus | Condensed chromatin | ||
| APR(4) | ||||
| L2 | 3 | 0 | 0 | 0 |
| PE + 12h | 3 | 0 | 0 | 0 |
| PE + 24h | 5 | 0 | 0 (4)1 | 0 (4)1 |
| APR(6) | ||||
| L2 | 2 | 0 | 0 | 0 |
| W0 | 3 | 0 | 0 | 0 |
| PE−10h | 3 | 0 | 0 | 0 |
| PE | 3 | 0 | 0 | 0 |
| PE + 12h | 4 | 50 | 50 | 0 (2)2 |
| PE + 24h | 6 | 33 | 50 | 0 (3)2 |
- 1 No usable EM sections were obtained through the nucleus for one of five APR(4)s at PE+24h, so observations are based on four neurons.
- 2 Observations on chromatin condensation pertain only to the subset of APR(6)s that had intact nuclei at stages PE+12h (n = 2) and PE+24h (n = 3).
In addition to shrinkage and rounding of the soma, other striking ultrastructural changes were apparent in low-magnification electron micrographs of dying APR(6)s. For example, both APR(6)s shown in Figure 4B,C lacked intact nuclei. Dying APR(6)s also exhibited increased electron density of cytoplasmic contents, massive accumulation of autophagic bodies and vacuoles, and aggregation and increased electron density of mitochondria. These and other ultrastructural features of PCD are described in more detail below.
Nuclear ultrastructure
Figure 5A shows a representative electron micrograph of an APR(4) nucleus. The nuclear envelope showed shallow folds in some regions. Nuclei in APR(4)s typically had elongated oval or crescent shapes, with the outline of the nuclear envelope varying from smooth to convoluted. Pores were apparent in the nuclei of most (8 of 10) APR(4)s. In 8 of 10 APR(4)s, the EM sections passed through a nucleolus (see, e.g., Fig. 5A). Nucleoli were highly electron dense and located eccentrically near (but not contacting) the nuclear envelope. The karyoplasm of APR(4)s was heterogeneous, consisting of a matrix of fine material with small, irregular masses of denser material scattered throughout. Unlike the nucleoli, the small masses of denser material were located in the central region of the nucleus rather than adjacent to the nuclear envelope. The APR(4) shown in Figure 5A was typical in this respect. In 9 of 10 APR(4)s, the electron density of the karyoplasm was less than that of the cytoplasm (see, e.g., Fig. 5A); in one case (at stage L2), the densities were approximately equal. The nuclear envelope was intact in every APR(4) examined (Table 1). Clumping of the chromatin, aggregation of chromatin along the inner surface of the nuclear envelope, or condensation (pyknosis) of the nucleus was never observed in APR(4)s (Table 1). In summary, the nuclei of APR(4)s appeared normal at all developmental stages examined.
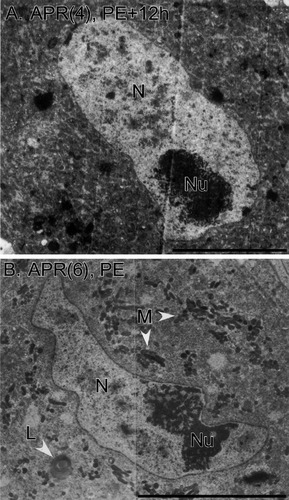
Nuclear ultrastructure. A: An APR(4) at PE+12h. The nucleus (N) contains finely granular karyoplasm with irregular densities. The nucleolus (Nu) is prominent. B: An APR(6) at PE. Ultrastructural features of the nucleus are similar to those of the APR(4). Mitochondria (M) are clustered, and the mitochondrial matrix exhibits increased electron density. A multilamellar body (L) is indicated. Scale bars = 10 μm.
Strikingly, the nuclei of APR(6)s had the same ultrastructural appearance as those of APR(4)s, both before and during PCD, for as long as the nucleus remained intact. Figure 5B shows a representative APR(6) at stage PE, illustrating the same features as seen in APR(4)s. In this example, the nucleus was crescent shaped, with a prominent nucleolus adjacent to (but not contacting) the nuclear envelope. The nuclear outline had shallow convolutions in several regions. The karyoplasm was heterogeneous, with a network of fine, granular material plus small, irregular masses of denser material located in the central region. Despite the normal appearance of the nucleus, signs of degeneration (described in more detail below) were apparent in the cytoplasm of the APR(6) shown in Figure 5B; in particular, the mitochondria were electron dense and aggregated together. Table 1 shows that the nuclear envelope was intact in all APR(6)s through stage PE, whereas half of the APR(6)s examined at PE+12h and PE+24h lacked intact nuclei (see, e.g., Fig. 4B,C). For APR(6)s that lacked intact nuclei, we were unable to identify a nuclear envelope (either intact or in fragments) or material recognizable as nuclear contents. The absence of an intact nucleus was associated with the size and shape of the soma; 5 of the 6 APR(6)s that lacked nuclei at stages PE+12h and PE+24h also had shrunken and rounded somata. In 14 of 16 APR(6)s with intact nuclei, the karyoplasm was less electron dense than the cytoplasm; in 2 cases (1 at stage PE–10h and 1 at stage PE+12), the density was approximately equal. This situation was similar to that observed in APR(4)s (see above). In 9 of 16 APR(6)s with intact nuclei, the EM sections passed through a nucleolus, which was located eccentrically near the nuclear envelope (see, e.g., Fig. 5).
Importantly, clumping of the chromatin, aggregation of chromatin beneath the nuclear envelope, or condensation (pyknosis) of the nucleus—features considered diagnostic of apoptosis—was never observed in APR(6)s, even at the most advanced developmental stages examined (Table 1). Figure 6 provides high-magnification views of the nuclear envelope in APR(6)s at different developmental stages (see also Fig. 12). The double-layered structure of the nuclear envelope was apparent, and nuclear pores were visible [see Fig. 6B; pores were observed in 13 of 16 APR(6)s with intact nuclei]. Typified by the example in Figure 6C, the 50% of APR(6)s that had intact nuclei at stages PE+12h and PE+24h (Table 1) did not exhibit any changes in the karyoplasm or nuclear envelope (see also Fig. 9). These observations at the EM level match previous light microscopic findings using a fluorescent nuclear stain (Hoffman and Weeks, 1998; see Discussion).

Nuclear envelope. The nuclear envelope appears vertically in each panel, with karyoplasm (K) to the left and cytoplasm (C) to the right. A: An APR(6) on day L2. B: An APR(6) at PE, with a prominent nucleolus (Nu). Nuclear pores (P) appear as regularly spaced, darkened narrowings of the double-layered nuclear envelope. C: An APR(6) at PE+24h. In each case, the nuclear envelope is intact, and the karyoplasm is pale and finely granular. Condensation of chromatin is absent (see Table 1). Scale bars = 1 μm.
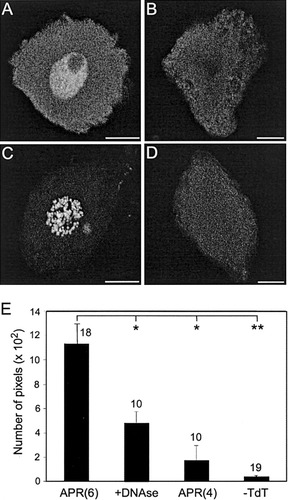
Nuclear TUNEL staining. A–D are each a “brightest point” projection of confocal optical sections that spanned the nucleus. Motoneurons were dissociated between PE and PE+36h, processed by the TUNEL method (using fluorescein-conjugated antibodies), and scanned using identical parameters. A: An APR(6), exhibiting uniform nuclear fluorescence, except for the nucleolus. B: An APR(4), which is TUNEL-negative. C: An unidentified motoneuron pretreated with DNAse, as a positive control. D: An unidentified motoneuron processed without TdT, as a negative control. Nuclear staining is absent. E: Quantification of fluorescence intensity. Each bar shows the mean ± SEM of the number of pixels in the nucleus that exceeded a specific brightness threshold (see Materials and Methods) for the four experimental groups shown in A–D; n is given above each bar. Horizontal lines denote statistical comparisons: *P < 0.05, **P < 0.00001 (Student's t-test). Scale bars = 10 μm.
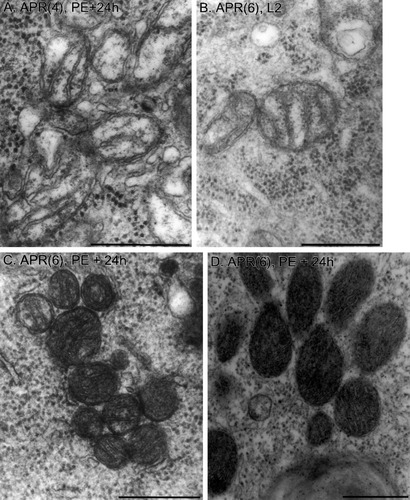
Mitochondria. Examples of normal mitochondria in an APR(4) at PE+24h (A) and an APR(6) on L2 (B) are shown. The cristae are obvious, and the mitochondrial matrix is light and similar in electron density to the cytoplasm. Mitochondria typical of dying APR(6)s are shown in C and D for two different APR(6)s at PE+24h. In C, the mitochondria are clustered, and the mitochondrial matrix has darkened, but cristae are still visible. D shows an example of clustered, ultracondensed mitochondria in which the cristae are barely visible or mitochondrial contents are completely obscured. The electron density of the cytoplasm was similar in all four examples. Scale bars = 0.5 μm.
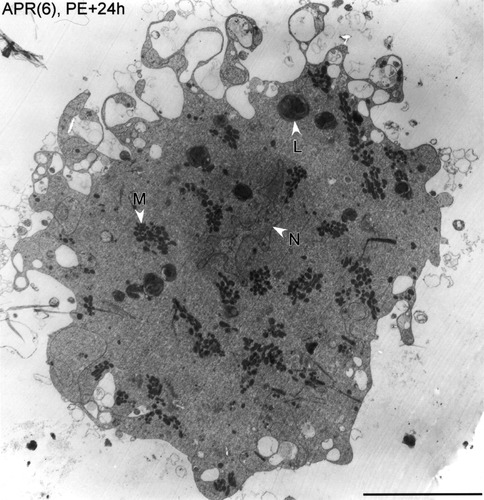
Low-magnification view of mitochondria in a dying APR(6). An APR(6) at PE+24h is shown. Mitochondria (M) are ultracondensed and aggregated into clusters. The nucleus (N) is still intact and large multilamellar bodies (L) are present. Scale bar = 10 μm.
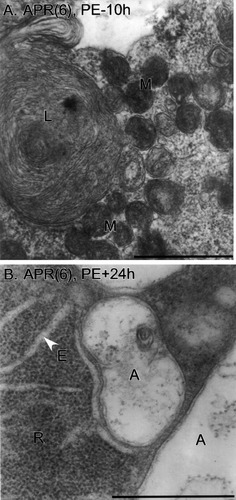
Autophagic bodies. A: An APR(6) at PE–10h is shown, with a large multilamellar body (L) and ultracondensed, clustered mitochondria (M). B: An APR(6) at PE+24h exhibits typical autophagosomes (A) with double limiting membranes. Densely packed blocks of ribosomes (R) and ER cisternae (E) are present. Scale bars = 1 μm.
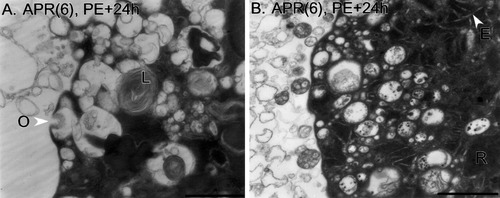
Plasma membrane. A heterogeneous population of autophagic bodies and vacuoles is present at the plasma membrane of two different APR(6)s at PE+24h. The inside of the APRs is to the right. In A, an omega figure (O) is present. Autophagic bodies exhibit a variety of contents, and multilamellar bodies are present (L). The APR(6) in B is surrounded by a halo of membrane-bounded debris similar in appearance to intracellular autophagic bodies. ER cisternae (E) and densely packed blocks of ribosomes (R) are visible. Scale bars = 5 μm in A, 2 μm in B.
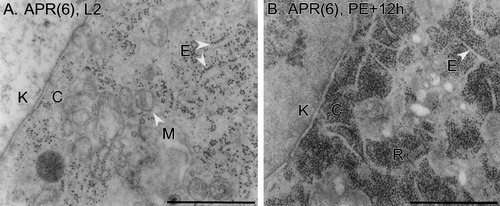
Ribosomes and ER. The nuclear envelope appears diagonally at left in both panels (K, karyoplasm; C, cytoplasm). A: An APR(6) on day L2. Ribosomes are arrayed on rough ER (E) or are free in the cytoplasm. The matrix of mitochondria (M) is electron lucent. B: An APR(6) at PE+12h. RER is still present (E), but large blocks of densely packed free ribosomes (R) are located throughout the cytoplasm. Scale bars = 1 μm.
Increased convolution of the nuclear envelope occurs during some instances of PCD (see, e.g., Hornung et al., 1989). To examine this possibility, observers blind to the identity of the material rank-ordered the tortuosity of the nuclear envelope of APR(4)s and APR(6)s from tracings of the nuclear outlines. There was no relationship between tortuosity and APR segment or stage (data not shown).
In summary, we observed no differences in nuclear ultrastructure between APR(4)s and APR(6)s or among APR(6)s examined at different developmental stages. No changes were observed in the nuclei of APR(6)s prior to nuclear fragmentation.
TUNEL staining of APRs
Chromatin condensation and DNA fragmentation are early, diagnostic events during apoptosis (Clarke, 1990). DNA fragmentation can be detected by the presence of nucleosomal laddering on agarose gels or by labeling the ends of DNA fragments by the TUNEL method (Gavrieli et al., 1992; Kaufman et al., 2000). To complement EM examination of APR(6) nuclei, we performed TUNEL staining.
In initial studies, we visualized TUNEL-positive neurons in chains of abdominal ganglia removed from Manduca pupae at different developmental stages (J. Ewer, G.K., and J.C.W., unpublished data). Beginning at about PE+12h, TUNEL-positive somata were sometimes observed in the appropriate location for APR(6)s and APR(5)s (the latter motoneurons also die at pupation). However, the TUNEL processing eliminated DiI fluorescence, so the APRs could not be identified definitely in situ (not shown). Accordingly, we utilized DiI-labeled APR(4)s and APR(6)s that were dissociated between PE and PE+36h and processed immediately by the TUNEL method. Unidentified motoneurons served as negative controls (TdT omitted from the reaction mixture) or positive controls (pretreated with DNAse; see Materials and Methods). To facilitate handling, APRs from the same segment were pooled during processing [i.e., APR(6)s at all developmental stages were pooled, and APR(4)s at all developmental stages were pooled]. Figure 7 shows representative confocal microscopic images. TUNEL-positive APR(6)s typically exhibited bright, relatively uniform fluorescence throughout the nucleus (excluding the nucleolus; Fig. 7A). Some APR(6)s showed minimal or no TUNEL fluorescence (not shown; see below). The nuclei of APR(4)s (Fig. 7B) and negative controls (Fig. 7D) showed minimal or no fluorescence, whereas positive controls exhibited bright fluorescence (Fig. 7C) that was more punctate than that in dying APR(6)s.
To extend these qualitative observations, we quantified the intensity of TUNEL labeling in groups of motoneurons dissociated between PE and PE+36h that were processed simultaneously under identical conditions. Sample sizes are given in Figure 7E. For each neuron, the entire volume of the nucleus was scanned and collapsed into a “brightest point” projection, and the number of pixels that exceeded a brightness threshold was counted (see Materials and Methods). The same threshold brightness was used for all neurons. As shown in Figure 7E, the nuclei of APR(6)s exhibited the greatest mean fluorescence, followed by the positive controls, the APR(4)s, and the negative controls. Mean fluorescence in the nuclei of APR(6)s was significantly greater than in the other three groups (Fig. 7E). Thus, APR(6)s became TUNEL-positive during PCD, indicating that DNA fragmentation occurred. The relationship between chromatin morphology visualized by EM and TUNEL staining is considered in the Discussion.
The APR(6)s processed for quantitative TUNEL analysis (Fig 7E) were combined from stages PE to PE+36h. Because the shape and size of the APR(6) soma reflect the cell's location along the death pathway (Fig. 3; Hoffman and Weeks, 1998), comparison of TUNEL staining and somatic morphology in individual APR(6)s provided information on when DNA fragmentation had occurred. Confocal microscopic images of the somata of the 18 APR(6)s from Figure 7E were scored by observers blind to the identity of the material as 1) ovoid or teardrop-shaped or 2) rounded and shrunken. APR(6)s were classified, based on the pixel counts given in Figure 7E, as TUNEL-positive if the number of fluorescent pixels was greater than 2 SD above the mean number of fluorescent pixels of the APR(4)s in Figure 7E [2 SD above the mean APR(4) value was 751 pixels]. Twelve of the eighteen APR(6)s were TUNEL-positive by this criterion. Among these, 75% (9 of 12) were ovoid or teardrop-shaped. These data indicate that DNA fragmentation, as detected by the TUNEL method, begins before APR(6)s acquire a rounded and shrunken morphology and, hence, relatively early in the PCD pathway (see Discussion)
Mitochondrial ultrastructure
Figure 8A,B shows representative high-magnification electron micrographs of normal mitochondria in an APR(4), and in an APR(6) prior to PCD, respectively. The mitochondria were oval or elongate and bounded by an outer membrane. The highly folded inner membrane formed longitudinal or transverse cristae, often associated with intracristal granules. The mitochondrial matrix was pale and similar in electron density to the surrounding cytoplasm. Mitochondria were scattered homogeneously throughout the cytoplasm; they sometimes abutted each other (see, e.g., Fig. 8A,B) but did not occur in compact aggregates. These features were characteristic of mitochondria in APR(4)s at all developmental stages examined (n = 8) and of APR(6)s through stage W0 (n = 5).
In contrast, mitochondria in APR(6)s undergoing PCD exhibited two marked abnormalities (Figs. 8C,D, 9). First, the electron density of the mitochondrial matrix increased dramatically, becoming many times darker than the surrounding cytoplasm. In some cases, internal structures such as cristae were still visible (Fig. 8C), whereas, in more extreme examples, the internal details were obscured, and mitochondria appeared as featureless gray or black blobs (Fig. 8D). The size of mitochondrial profiles decreased, suggesting a decrease in mitochondrial volume. This “ultracondensed” mitochondrial conformation is observed in other examples of PCD (Jia et al., 1997b; Mancini et al., 1997; Zhuang et al., 1998; see Discussion). The second abnormality was the aggregation of mitochondria in dying APR(6)s into tightly packed clusters. The profiles of mitochondria in clusters were generally smaller and more rounded than in normal APRs, although some elongated mitochondria were still apparent (see, e.g., Fig. 9). Compared with mitochondrial ultracondensation, aggregation is a less commonly observed accompaniment to PCD (see Discussion).
Figure 9 shows a low-magnification electron micrograph of an APR(6) at PE+24h illustrating typical mitochondrial abnormalities during PCD. The ultracondensed mitochondria were aggregated into dense clusters amid large expanses of mitochondrion-free cytoplasm. It is notable that, in this APR(6), the nuclear envelope was still intact and the soma had not yet become rounded. Thus, mitochondrial abnormalities preceded nuclear fragmentation and the loss of cytoskeletal integrity in the soma. Comparison of APR(6)s at different developmental stages was consistent with this sequence of events. As noted above, mitochondria in all APR(4)s, and in APR(6)s through stage W0, appeared normal. The first consistent indication of mitochondrial changes occurred at about stage PE; for example, the APR(6) at stage PE–10h shown in Figure 10A had dark, clustered mitochondria, as did the APR(6) at stage PE shown in Figure 5B. At stages PE–10h and PE, only a subset of APR(6)s had unambiguous mitochondrial abnormalities [1 of 3 APR(6)s at PE–10h, 2 of 3 at stage PE], but all had large, normal somata and intact nuclei (Table 1). Mitochondrial ultracondensation and aggregation appeared together in individual APR(6)s, with no suggestion that one phenomenon preceded the other. At stages PE+12h and PE+24h, most (7 of 10) APR(6)s exhibited mitochondrial abnormalities. Within the group of APR(6)s at stages PE+12h and PE+24h that were rounded and shrunken and lacked intact nuclei (n = 4), all had severely aggregated and ultracondensed mitochondria (see, e.g., Fig. 4B). Although the electron density of the cytoplasm of APR(6)s increased during the terminal stages of PCD, notably after the motoneurons became rounded and shrunken (see, e.g., Fig. 4C), this was not the case during the earlier developmental stages, when the electron density of the mitochondrial matrix first increased. This is apparent when comparing the electron density of the cytoplasm of APRs shown in Figure 8 and in other figures (Figs. 4-6, 9, 10, 12). Thus, the increase in electron density of the mitochondrial matrix during ultracondensation was specific to this organelle and not a nonspecific change in all cytoplasmic contents, nor was it a result of sectioning anomalies. Recently, we have confirmed mitochondrial aggregation during PCD by staining mitochondria with vital fluorescent dyes in APR(6)s (Rutherford et al., 2002; see Discussion).
Mitochondrial ultracondensation and aggregation were thus consistent features of dying APR(6)s and preceded nuclear fragmentation or rounding and shrinkage of the soma. These results are consistent with the postulated early role of mitochondrial events in the PCD pathway (Fig. 1; see Discussion).
Autophagic bodies
During autophagic cell death, segments of endoplasmic reticulum (ER) envelop cytoplasmic components and pinch off to form autophagosomes, which are bounded by a double membrane. Autophagosomes fuse with primary lysosomes to form autolysosomes (also termed “autophagic vacuoles”), which have a single limiting membrane. The contents of autolysosomes are broken down by acid phosphatase and other enzymes contributed by the lysosomes. After hydrolysis of their contents, autolysosomes are termed “residual bodies” (for review see Dunn, 1994). In the present study, we refer to autophagosomes, autolysosomes, and residual bodies collectively as “autophagic bodies” unless the number of limiting membranes was clearly apparent (see, e.g., Fig. 10B). Some autophagic bodies contained tightly packed, concentric membrane whorls (Fig. 10A; termed “multilamellar bodies”; Hariri et al., 2000), whereas the contents of most were heterogeneous (Figs. 4B,C, 10B, 11). The term “vacuole” is sometimes used to refer to autophagic bodies (Dunn, 1990); here, we use it to refer to an empty, electron-lucent, membrane-bounded organelle (see Fig. 4B).
At all developmental stages examined, APR(4)s (n = 8) had occasional small vacuoles, autophagic bodies, and multilamellar bodies, but these structures were present in low abundance (see Fig. 4A). APR(6)s showed a similarly low abundance of vacuoles, autophagic bodies, and multilamellar bodies through stage W0 (n = 5). APR(6)s at stages PE–10h and PE (n = 6) were generally similar to those at earlier stages, although the number of autophagic organelles may have increased slightly. However, by stages PE+12h and PE+24h (n = 10), APR(6)s exhibited a massive increase in the abundance and size of autophagic bodies, multilamellar bodies, and vacuoles (Figs. 4B,C). Figure 10A shows a representative multilamellar body (also see Figs. 5B, 9, 11A), and Figure 10B illustrates two autophagosomes with double membranes. The contents of autophagic bodies were difficult to identify with certainty, but debris resembling rough ER (RER) and mitochondria was observed (Figs. 10B, 11). Lysosomes, identified as electron-dense, round bodies (Peters et al., 1991), were sometimes observed in APR(4)s and APR(6)s, but their abundance did not appear to change (not shown).
During PCD, vacuoles and autophagic bodies were concentrated near the plasma membrane of APR(6)s (Figs. 4B,C, 11). In some cases, omega figures, suggestive of exo- or endocytosis, were observed (Fig. 11A). These were rarely observed in APR(4)s or in APR(6)s that were not yet shrunken and rounded. The highest abundance of omega figures was seen in APR(6)s at the most advanced stages of PCD. Clathrin coating of omega figures or vesicles near the plasma membrane (see Fig. 11), suggestive of endocytosis, was not observed. Regarding possible exocytosis, some dying APR(6)s were surrounded by a “halo” of debris that appeared to consist of autophagic bodies and their contents. For example, a membrane whorl lies adjacent to the APR(6) shown in Figure 4C, and material similar to that seen within autophagic bodies adjoins the APR(6) shown in Figure 11B. APRs were fixed 30–60 minutes after being placed in culture, potentially allowing time for expelled material to accumulate around the cell.
Ribosomes and ER
The distribution of ribosomes changed during PCD. APR(4)s at all developmental stages, and APR(6)s prior to PCD, exhibited RER as well as free ribosomes and polyribosomes that were not associated with ER cisternae. Agranular or smooth ER was less abundant than RER. An example of normal RER and ribosomes is shown in Figure 12A; rows of ribosomes were arrayed along the external surfaces of ER cisternae, whereas other ribosomes were free in the cytoplasm. During PCD, RER was still apparent, but large blocks of densely packed free ribosomes appeared within the cytoplasm (Fig. 12B; see also Figs. 4C, 10B, 11B). The release of ribosomes from ER and ribosomal aggregation is observed during the PCD of other neurons as well (Pilar and Landmesser, 1976; Yaginuma et al., 2001). The dense aggregations of ribosomes (Nissl bodies; Peters et al., 1991), in conjunction with the mitochondrial ultracondensation (see above), contributed to the increased electron density of cytoplasm characteristic of shrunken and rounded APR(6)s. Dilation of ER cisternae, reported in some instances of autophagic PCD (Pilar and Landmesser, 1976; Stocker et al., 1978; Hornung et al., 1989), was observed in some APR(6)s (see, e.g., Fig. 10B). The transcriptional competency of APR(6)s during PCD is unknown, but, by stage PE, the protein synthesis required to execute PCD has been completed (Hoffman and Weeks, 2001). Thus, ribosomal function may be expendable after this stage.
Other organelles
Golgi complexes were common in the middle zone of APR somata, but no changes in their shape or abundance were obvious during PCD. Multivesicular bodies were observed at low abundance and also appeared to have no relationship to PCD. Microtubules and neurofilaments were not examined in detail, so their relationship to PCD (particularly somatic shrinkage and rounding) is unknown.
DISCUSSION
Ultrastructural analysis provides insight into cellular events during PCD and underlying biochemical and molecular mechanisms. We used electron and light microscopy to examine APR(6) motoneurons in Manduca, which undergo PCD in response to the steroid hormone 20E. Previous work suggested a model of the PCD pathway in APR(6)s (Fig. 1; Hoffman and Weeks, 2001), and many details of the electrophysiological and behavioral roles of APRs are known (for review see Weeks et al., 1997; Weeks, 1999). Hence, the biological context in which APRs are eliminated during metamorphosis is particularly well understood (see below).
APR(6)s exhibit massive autophagic activity during PCD
Two ultrastructural features that differ in apoptotic and autophagic cell death are the early occurrence of chromatin condensation and progressive pyknosis of the nucleus in the former and massive up-regulation of autophagic organelles in the latter (Clarke, 1990; see the introductory paragraphs above). Nuclear events in APR(6)s are discussed below. In APR(4)s at all developmental stages examined, and in APR(6)s prior to the onset of PCD, autophagic bodies were rare (Fig. 4A). In contrast, during PCD, the cytoplasm of APR(6)s became filled with a variety of autophagic bodies, including autophagosomes, autolysosomes, multilamellar bodies containing densely packed whorls of membrane, and vacuoles (Figs. 4,9-11). In rounded and shrunken APR(6)s, which are late in the death process, autophagic organelles occupied most of the cell volume (Figs. 4B,C, 11). Thus, APR(6)s abundantly fulfill the key criterion for autophagic cell death. The large number of autophagic bodies in dying APR(6)s presumably reflects ongoing self-destruction of the neuron, as cytoplasmic components are first sequestered into autophagosomes by projections of ER membrane, followed by fusion with lysosomes and enzymatic hydrolysis of the contents of the autolysosome (Dunn, 1990, 1994). Thus, a fundamental difference between autophagic cell death and apoptosis is that most of the dying cell's contents are digested “in house” in the former case and by neighboring cells in the latter case (heterophagy).
In principle, because APR(6)s were removed from the CNS prior to EM processing, it is possible that autophagic cell death was activated because the APR(6)s were removed from the influence of other cells that would normally perform heterophagy. However, this is highly unlikely for two reasons. First, only 30–60 minutes elapsed between the times when APRs were dissociated and fixed, which is unlikely to provide sufficient time to develop the extremely autophagic phenotypes that were observed. Second, an autophagic phenotype similar to that observed in APR(6)s is exhibited by a different class of Manduca motoneurons that dies after adult emergence, as shown in an in vivo EM study (see below; Stocker et al., 1978). We conclude that the massive accumulation of autophagic bodies in dying APR(6)s is the normal phenotype for 20E-triggered PCD.
A recent review of the cell death field states that “there is growing recognition that autophagic death is not a caspase death …” (Lockshin et al., 2000), so it is important to emphasize here that the 20E-triggered death of APR(6)s is both autophagic (this study) and caspase dependent (Hoffman and Weeks, 2001). Thus, the involvement of caspases is not unique to apoptotic PCD.
APR(6)s become TUNEL-positive but do not exhibit chromatin condensation during PCD
Condensation of chromatin in dying cells is a defining feature of the apoptotic form of PCD (Clarke, 1990). In all APR(4)s and APR(6)s examined, at all developmental stages, chromatin condensation was absent (Table 1), and the karyoplasm had the same characteristic features: a matrix of fine material with small, irregular masses of denser material and a prominent nucleolus (Figs. 4A, 5, 6, 9, 12). These EM observations corroborate a previous study in which APR(6)s placed in culture at stage PE were stained with the nuclear dye Hoechst 3342 (Hoffman and Weeks, 1998). At stage PE, before any morphological signs of PCD, Hoechst 3342 staining in APR(6) nuclei was diffuse and uniform. After 24 hours in culture, APR(6)s that had become rounded and shrunken showed bright spots of Hoechst-stained material scattered throughout the cytoplasm, suggesting that the nuclear envelope had fragmented and released nuclear contents. Condensation of chromatin within the nucleus was never observed with Hoechst 3342 staining (Hoffman and Weeks, 1998). Thus, both EM and light microscopic observations suggest that chromatin does not condense in APR(6)s during PCD, with the chromatin maintaining a normal ultrastructural appearance until the nuclear envelope fragments. A similar conclusion was reached in the EM study of other Manduca motoneurons undergoing PCD in vivo (see below; Stocker et al., 1978). Chromatin condensation accompanies some instances of autophagic cell death, but only late in the death program (for review see Clarke, 1990), e.g., the autophagic cell death of human breast cancer cells treated with antiestrogens (Bursch et al., 1996).
The finding that chromatin condensation is not observed in dying APR(6)s but that their nuclei become TUNEL-positive (Fig. 7) has several possible explanations. First, DNA fragmentation and chromatin condensation can occur separately; using isolated cell nuclei, Sun et al. (1994) showed that DNA fragmentation produced by endonuclease activity does not produce chromatin condensation and that, conversely, treatment with the potassium ionophore valinomycin produces chromatin condensation but no DNA fragmentation. Other treatments produced both DNA fragmentation and chromatin condensation. These results show that DNA fragmentation and chromatin condensation need not co-occur in APR(6)s. Second, even if DNA fragmentation and chromatin condensation are normally interrelated, DNA fragmentation sufficient to render a cell TUNEL-positive may not produce a condensation phenotype. DNA is degraded in a stepwise fashion into progressively shorter fragments (Walker et al., 1993), and chromatin condensation may occur late in the cleavage process, or not at all. During the ischemia-induced PCD of CA1 pyramidal cells, positive TUNEL staining precedes chromatin condensation observed at the EM level (Nitatori et al., 1995) and, during 3-acetylpyridine-induced PCD in neurons of the rat inferior olive and cerebellum, individual neurons that are TUNEL-positive lack condensed chromatin (Wüllner et al., 1997). Roth (2001) argues that positive TUNEL staining can reflect sublethal levels of DNA damage in neurons that are not dying and hence will not develop condensed chromatin. These studies are consistent with our observation that APR(6)s become TUNEL-positive but do not exhibit chromatin condensation. A third possibility is that TUNEL-positive APR(6)s do develop a condensed chromatin phenotype but that it is a transient event that was missed in the EM or Hoechst 3342 studies. This possibility seems unlikely but cannot be excluded. Even if this were the case, this situation would differ fundamentally from apoptosis, in which chromatin condensation is a prominent, early event. Nonconcordance between the assessment of DNA fragmentation by the TUNEL method and light microscopy or EM examination of chromatin structure has been reported in other systems as well (van Lookeren Campagne et al., 1995; Collins et al., 1997; Tatton et al., 1998; Turmaine et al., 2000; Barenberg et al., 2001). Resolution of this issue in APR(6)s would require further experiments, ideally involving processing the same neurons for TUNEL staining and EM.
These considerations aside, nuclear events in APR(6)s support three general conclusions. First, the absence of chromatin condensation demonstrates that the 20E-triggered death of APR(6)s fails a key ultrastructural criterion for apoptosis. Instead, this and other ultrastructural features (see above) identify the death of APR(6)s as autophagic, or type 2 (Clarke, 1990). Dying cells can exhibit features of both apoptotic and autophagic cell death (see the introductory paragraphs), but APR(6)s exhibit an indisputably autophagic phenotype, with no features characteristic of apoptosis. Second, the finding that APR(6)s become TUNEL-positive during autophagic PCD is consistent with other reports that this method is not diagnostic for the apoptotic form of PCD (Lucassen et al., 1997; Schulte-Hermann et al., 1997; Savitz and Rosenbaum, 1998; Roth, 2001). APR(6) represents a clear example of a cell undergoing autophagic cell death that becomes TUNEL-positive; it is incorrect to assume that positive TUNEL staining indicates that a cell is dying by apoptosis. At present, EM is the definitive method for making this determination (although molecular markers will become increasingly useful). Third, the finding that a substantial fraction of TUNEL-positive APR(6)s had ovoid or teardrop-shaped somata (see Results) suggests that DNA cleavage begins during the premitochondrial and/or mitochondrial phases of PCD (Fig. 1). The protein synthesis required for APR(6)s to execute PCD is complete by stage PE (Hoffman and Weeks, 2001), so, in principle, nuclear function may be expendable after this time.
During PCD, mitochondria in APR(6)s undergo ultracondensation and aggregation
Mitochondria can assume a variety of conformations related to osmotic or respiratory state (Hackenbrock, 1968; Laiho and Trump, 1975; for review see Frey and Mannella, 2000). The “orthodox” state is characterized by a large volume of pale matrix, regular cristae, and close apposition between inner and outer membranes, whereas the “condensed” state has a reduced matrix volume, increased matrix density, dilated cristae, and increased space between inner and outer membranes. Condensed mitochondria are believed to have a decreased respiratory rate. The term “ultracondensed” is applied to mitochondria with dense matrix and decreased mitochondrial volume, with minimal or no alterations in membrane or intercristal spacing (for discussion see Zhuang et al., 1998). The release of cytochrome c and other death-promoting factors from mitochondria can involve mitochondrial swelling and rupture of the outer membrane, or mitochondria may condense and release signaling molecules by other means (Jia et al., 1997b; Zhuang et al., 1998; Kluck et al., 1999; for review see Frey and Mannella, 2000).
Mitochondria in APR(4)s at all stages examined, or in APR(6)s prior to PCD, have the orthodox configuration (Fig. 8A,B). In contrast, mitochondria in APR(6)s undergoing PCD (Figs. 8, 10A) have the ultracondensed conformation shown by others to occur during PCD in some cell types (Jia et al., 1997b; Mancini et al., 1997; Zhuang et al., 1998; Cai et al., 2000). The metabolic state of ultracondensed mitochondria is uncertain. The ultracondensation of mitochondria in APR(6)s occurs relatively early in the death process, before cellular rounding and shrinkage or nuclear fragmentation (Fig. 9); this timing is consistent with ultracondensation being associated with the mitochondrial phase of the PCD pathway (Fig. 1). Mitochondria in APR(6)s lose ΔΨm during PCD, and treatment with cyclosporin A (which inhibits opening of the mitochondrial permeability transition pore) blocks rounding and shrinkage but not the loss of mitochondrial function in APR(6)s (Rutherford et al., 2002; and in preparation). Dinsdale et al. (1999) showed in monocytes undergoing PCD that ultracondensation is caspase dependent, that ultracondensed mitochondria had low ΔΨm, and that cytochrome c release precedes ultracondensation. In colorectal adenocarcinoma cells, ultracondensation appears to precede the loss of ΔΨm (Ikebukuro et al., 2000). Additional experiments will be required to determine the sequence of events in APR(6)s.
The other striking mitochondrial abnormality in APR(6)s was the formation of tightly clustered aggregates (Figs. 8, 9). Ultracondensation and aggregation occurred together (see Results), suggesting that the two phenomena may be linked. Recently, we confirmed mitochondrial aggregation during PCD by confocal microscopic imaging of unfixed APR(6)s stained with the fluorescent dye MitoTracker Green (Rutherford et al., 2002); this method can be utilized with other fluorescent markers (for ΔΨm, caspase activation, etc.) to ascertain the relationship between mitochondrial abnormalities and other events. In two-dimensional micrographs, mitochondria appear as isolated organelles but may in fact belong to a complex tubular reticulum; three-dimensional reconstruction or imaging (for review see Perkins and Frey, 2000) could reveal whether mitochondria in APR(6)s are reticular in the normal or aggregated state. The functional significance of mitochondrial aggregation in APR(6)s is unknown. De Vos et al. (1998) report that PCD induced by tumor necrosis factor involves perinuclear mitochondrial clustering, which appears to result from impaired activity of the molecular motor kinesin. In yeast cells, inactivation of the Hsp70 molecular chaperone system causes mitochondrial aggregation, which requires normal actin function (Kawai et al., 2001). Mitochondrial clustering has been reported during other instances of PCD (Li et al., 1998; Garland et al., 2000) and in cardiomyopathic disorders (Burbach, 1987).
During PCD induced by the tyrosine kinase inhibitor herbimycin A, mitochondria in human colon carcinoma cells exhibit both condensation and aggregation and appear strikingly similar to those in dying APR(6)s (see Mancini et al., 1997; Fig. 11A); in both cases, the outer and inner membranes remain closely associated, the cristal spacing remains normal, mitochondria become smaller, and the matrix becomes highly electron dense. The authors note that this morphology has sometimes been termed “mitochondrial pyknosis.” In the colon carcinoma cells, mitochondrial condensation is followed by swelling and lysis (Mancini et al., 1997), which does not occur in APR(6)s.
When a death-inducing stimulus is presented in the presence of caspase inhibitors to block the execution phase of PCD, followed by a return to survival-promoting conditions, some cells exhibit selective autophagic destruction of all mitochondria yet survive for a period of time in an anaerobic state (for review see Tolkovsky et al., 2002). In some cases mitochondrial destruction is preceded by perinuclear clustering. Tolkovsky et al. suggest that mitochondrial damage during the release of death-signaling molecules triggers the autophagic engulfment and destruction of these organelles.
Endo- or exocytosis
One challenge faced by dying neurons is to dispose of plasma membrane as the cell shrinks. During PCD, the sarcolemma of Manduca intersegmental muscles becomes wrinkled and folded (Schwartz et al., 1993), whereas shrunken and rounded APR(6)s have smooth plasma membranes (Fig. 4B), indicating that membrane infolding does not contribute to shrinkage. The area of plasma membrane lost during APR(6) death can be estimated from the dimensions of DiI-labeled APR(6)s in vivo (data from Hoffman and Weeks, 1998). At PE, the area of the somatic plasma membrane of APR(6)s is ∼9,230 μm2 (mean long by short dimensions of 62 × 39 μm), whereas, at PE+24h, somatic membrane area is ∼2,945 μm2 (mean long by short dimensions of 34 × 24 μm; plasma membrane area was computed by treating somata as rotated ellipses). Thus, somatic membrane area decreases by 68%, representing a loss of 6,285 μm2 of membrane in less than 24 hours. During apoptosis, plasma membrane and cytoplasmic material are lost via “apoptotic bodies” that bud off from the dying cell (Clarke, 1990). The possibility that exocytosis contributes to the reduction in plasma membrane area and cell volume in APR(6)s is suggested by occasional omega figures and the material that surrounded some cells (Figs. 4C, 11). Autophagic bodies are expelled by exocytosis during the PCD of cultured sympathetic neurons following the withdrawal of nerve growth factor (NGF; Xue et al., 1999) and from T-lymphoblastic leukemic cells during PCD triggered by tumor necrosis factor-α (Jia et al., 1997a; cf. their Fig. 1B with our Fig. 11).
Alternatively, or in addition, plasma membrane could be eliminated by endocytosis, perhaps contributing to the large, multilamellar bodies in dying APR(6)s (Figs. 4B,C, 9, 10A,11). Hornung et al. (1989) demonstrated abundant endocytosis (indicated by uptake of horseradish peroxidase) by isthmo-optic neurons in chick embryos during autophagic cell death and suggest that this process may serve to destroy plasma membrane; endocytosed materials can rapidly enter autophagic bodies and combine with materials autophagocytosed from within the cytoplasm (Gordon et al., 1992). Uchiyama (2001) proposed that both inward and outward “blebbing” of the plasma membrane contributes to cell shrinkage during PCD. The occurrence of exo- and/or endocytosis during the PCD of APR(6)s could be resolved by the use of various labeling techniques (see, e.g., Hornung et al. 1989; Consoulas and Levine 1998).
Comparison with PCD in other Manduca neurons
The results reported here corroborate those of Stocker et al. (1978), who examined the ultrastructure of Manduca motoneurons that innervate intersegmental (ISM) abdominal muscles and degenerate after adult eclosion (emergence of the moth from the pupal case). Unlike APRs, the somata of ISM motoneurons can be recognized by their location in sectioned ganglia, so they were examined by EM in situ. The ultrastructure of ISM motoneurons prior to the onset of PCD is similar to that reported here for APR(4)s and APR(6)s (Stocker et al., 1978). Initial events (∼12–24 hours post-eclosion) in ISM motoneurons include a change in mitochondrial shape from elongated to ovoid, darkening of the mitochondrial matrix, increase in the number of free ribosomes, and increase in the number and size of autophagic bodies [Stocker et al. (1978) used the terms “isolation bodies” and “lamellar bodies” to describe what we term “autophagic bodies” and “multilamellar bodies,” respectively]. Later (∼32–48 hours post-eclosion), the nuclear envelope ruptures without prior aggregation of chromatin or nuclear shrinkage. There is a further increase in autophagic bodies (especially multilamellar bodies), and mitochondria become “polygonal” and aggregated. By 72 hours post-eclosion, soma diameter has not yet decreased, and the cytoplasm is filled with autophagic profiles. By 96 hours, the somata are shrunken and highly electron dense [similarly to the APR(6) shown in Fig. 4C]. Features reported for ISM motoneurons that were not prominent in dying APR(6)s were a swelling of RER cisternae, a decrease in the number of Golgi complexes, and a late phase of degeneration when the number of ribosomes decreases and some mitochondrial remnants and autophagic bodies swell (the latter phenomenon is attributed to osmotic dysfunction). Comparison of electron micrographs of APR(6)s (this study) and ISM motoneurons (Stocker et al., 1978) suggests that large, electron-lucent, autophagic bodies and vacuoles (Figs. 4B,C, 11) were more abundant in APR(6)s; whether this is a neuron-specific difference or is related to APR(6)s being in culture is unknown. However, cultured APR(4)s did not exhibit large vacuoles and autophagic bodies, so this phenotype was associated with the death process. Because APRs were examined in vitro, we did not obtain information on glial events during PCD. Stocker et al. (1978) showed that glia surrounding ISM motoneurons take up and digest degenerating remnants of ISM motoneurons. Thus, although the PCD of ISM motoneurons is autophagic, final remnants of the motoneurons are eliminated by heterophagy.
Interestingly, APR(4)s—which survive the larval-pupal transformation—undergo PCD following adult eclosion at the same time as ISM motoneurons. Just as for APR(6)s, the PCD of APR(4)s is a direct, cell-autonomous response to 20E; as demonstrated both in vivo and in vitro, the death-inducing cue for APR(6)s is the prepupal rise in 20E, whereas for APR(4)s the signal is the decline in 20E before eclosion (Zee and Weeks, 2001). The light microscopic phenotype of APR(4) death is indistinguishable from that of APR(6)s. No aspects of the model presented in Figure 1 have yet been tested for APR(4)s, but we predict that, with the exception of the sign inversion of the 20E signal, the same PCD pathway is utilized by APRs at both developmental stages. We similarly expect that the same ultrastructural events occur in dying APR(4)s as in APR(6)s, a prediction that awaits experimental testing.
As in Drosophila (Lee and Baehrecke, 2001), the degeneration of the labial (salivary) glands in Manduca occurs by autophagic cell death. Chromatin condensation occurs relatively late in the death process and is accompanied by DNA fragmentation detected by both the TUNEL method and nucleosomal laddering (Jochová et al., 1997). Interestingly, a low level of DNA fragmentation is detected prior to the ultrastructural onset of labial gland degeneration, which is similar to our observations of APR(6)s.
Relationship between ultrastructural changes and the PCD pathway in APR(6)s
The finding that the 20E-triggered PCD pathway in APR(6)s (Fig. 1) produces autophagic cell death permits us to assign ultrastructural correlates provisionally to some steps in the model and generates specific predictions of the model that can be tested experimentally. For example, the striking mitochondrial abnormalities (ultracondensation, aggregation) observed in dying APR(6)s may correspond to the mitochondrial phase of the death pathway. The finding that positive TUNEL labeling occurs in many ovoid or teardrop-shaped APR(6)s suggests that endonucleases begin DNA degradation during the premitochondrial or mitochondrial phases, perhaps because of initiator caspase activation. The postmitochondrial phase may include the exocytosis of autophagic bodies to help produce the dramatic loss of plasma membrane area and cell volume during somatic rounding and shrinkage. Finally, the massive mobilization of autophagocytotic activity suggests that 20E-induced transcription and translation may up-regulate enzymes in the lysosomal system (for review see Nixon and Cataldo, 1993). All of these hypotheses are amenable to further testing.
Advantages of this system and relationship to other studies of PCD
The cellular and molecular mechanisms involved in PCD are under investigation in a variety of neural and nonneural cell types, with each system providing specific advantages or points of entry for analysis. The steroid-induced death of APR(6) motoneurons in Manduca offers several unique advantages as an experimental system. First, the bona fide biological signal that triggers PCD—the prepupal rise in 20E—is known, and APR death in response to this signal can be studied both in vivo and in vitro. Many studies of PCD, particularly those utilizing cultured cells, use pharmacological agents (e.g., treatment with the kinase inhibitor staurosporine) or relatively undefined stimuli (e.g., removal of serum from the medium) to trigger death. In these cases, the extent to which the death program evoked by the experimental stimulus relates to naturally occurring PCD in vivo can be problematic (see, e.g., Deshmukh and Johnson, 2000). In the case of APR(6)s, the biochemical (Fig. 1) and ultrastructural (this study) events during PCD occur in response to the natural signal, 20E. Similarly, the PCD of vertebrate sympathetic neurons triggered by a biologically relevant signal—the withdrawal of NGF—can be studied both in vivo and in vitro (for review see Deshmukh and Johnson, 1997). A second advantage of APRs is that the effect of 20E is direct and cell autonomous (Streichert et al., 1997). In many systems, neuronal survival is regulated by indirect antero- or retrograde signals (for review see Mennerick and Zorumski, 2001) such that the neurons of interest are not themselves targets of steroid hormone action. For example, the PCD of rat spinal motoneurons during embryonic development is controlled by androgen action on the motoneurons' target muscles (for review see Breedlove, 1992). This begs the question of how steroids affect their direct targets, an issue that can be readily addressed in APRs. A third advantage is that the segmental pattern and stage specificity of APR death provide powerful controls for experiments; at pupation, APR(4)s survive whereas APR(6)s die in response to a rise in 20E, and, at adult emergence, the APR(4)s die in response to a fall in 20E (Zee and Weeks, 2001). Thus, homologous motoneurons that exhibit dramatically different hormonal responses can be compared by electrophysiological (Streichert and Weeks, 1995), endocrinological (Weeks et al., 1992), pharmacological (Hoffman and Weeks, 1998), and anatomical (this study) methods. This feature can also be exploited to identify differences in gene expression (Hazelett et al., 2002). Finally, the behavioral roles and synaptic connections of APRs are extensively characterized (Streichert and Weeks, 1995; Sandstrom and Weeks, 1996; for review see Weeks et al., 1997 and Weeks, 1999), with the segmental pattern of APR death at pupation directly related to neuromuscular requirements in the pupal stage (Sandstrom and Weeks, 1998; Lubischer et al., 1999). The functional context of PCD is thus known. Interestingly, the segmental pattern of APR death at pupation differs in another moth species, Bombyx mori (the flightless, domesticated silkworm; Zee and Weeks, 2002), providing an opportunity to investigate the regulation of neuronal death in a comparative, evolutionary context.
This study demonstrates that the steroid-induced death of APR(6)s is autophagic, not apoptotic. The use of “programmed cell death” and “apoptosis” as synonyms has muddied the field of cell death research, but increasing attention is being paid to the importance of differentiating between apoptotic and autophagic cell deaths, a distinction that is particularly compelling given that autophagic cell death is implicated in numerous neurodegenerative disorders (see the introductory paragraphs; for review see Bursch, 2001). APRs provide an accessible system for investigating the cellular and molecular underpinnings of autophagic cell death. They also reveal two important points about PCD in general. First, the present study shows that TUNEL staining is not diagnostic for apoptosis (see other examples above). Therefore, the use of this method to identity “apoptotic” neurons, in the absence of supporting evidence provided by another method, is invalid. We speculate that some instances of neuronal PCD that have been designated “apoptotic” based on TUNEL labeling may in fact occur by autophagic or other forms of cell death (Clarke, 1990). In addition, APRs illustrate that a PCD pathway involving caspases and mitochondrial signaling (Fig. 1) can produce autophagic, rather than apoptotic, cell death. Thus, the ultrastructural phenotype expressed by a dying neuron cannot be predicted a priori by the involvement of caspases and mitochondria. The differences in gene expression and intracellular signaling that cause cells to execute one or the other form of death, or intermediate forms, remain to be determined. This issue also has clinical relevance. For example, neuronal death triggered by intracellular accumulation of the mutant form of huntingtin, the protein that causes Huntington's disease, has been termed “apoptotic” by Saudou et al. (1998) based on Hoechst dye staining and TUNEL labeling, whereas Kegel et al. (2000) designated the death “autophagic” based on massive up-regulation of endosomal-lysosomal activity and accumulation of autophagic bodies. The recognition that neurodegeneration comes in multiple forms, including intermediate phenotypes, is important for targeting therapeutic interventions to the specific mechanisms involved. Further analysis of autophagic neuronal death in model systems should facilitate achievement of this goal.
Acknowledgements
We thank Dr. John Ewer for pilot experiments using the TUNEL method, Dr. Johnathan Melville for advice on confocal microscopy and analysis, Dr. William M. Roberts for computation of membrane areas and blind scoring, Mr. Mark Rutherford for sharing unpublished data, Ms. Tregony Bucknell-Pogue for help with figure preparation, Ms. Tsitsi M. Magaya for help with manuscript preparation, and Dr. Jeanne Selker and Mr. Eric Schabtach for assistance with electron microscopy. The Marine Biological Laboratory (Woods Hole, MA) provided facilities for data analysis. This research was supported by NIH grant R01 NS23208 and a John Simon Guggenheim fellowship to J.C.W., predoctoral traineeships (NIH grant T32 HD07348) to K.L.H. and M.C.Z., and funding from the University of Oregon Summer Program for Undergraduate Research and the McNair Scholar's Program to E.M.R.




