Odor-evoked activity is spatially distributed in piriform cortex
Abstract
Much data on the olfactory bulb (OB) indicates that structural characteristics of odorant molecules are encoded as ordered, spatially consolidated sets of active cells. New results with “genetic tracing” (Zou et al. [2001] Nature 414:173–179) suggest that spatial order is also present in projections from the OB to the olfactory cortex. For the piriform cortex (PC), results with this technique indicate that afferents conveying input derived from single olfactory receptors (ORs) are distributed to well-defined patches in the anterior PC (APC) but that these patches are much larger than in the OB. We have used c-fos induction to examine how input patterning for single ORs is translated into patterns of odor-evoked cellular activity in the PC. The laminar distribution of labeled cells and dual-immunostaining for γ-aminobutyric acid (GABA)ergic markers indicated that activity was detected largely in pyramidal cells. In odor-stimulated rats, labeled cells were present throughout the posterior PC (PPC) but were concentrated in prominent rostrocaudal bands in APC. Analysis of responses to different odorants and concentrations revealed that locations and shapes of bands conveyed no apparent information regarding odor quality, rather, they appeared to correspond to subregions of the APC distinguished by cytoarchitecture and connectivity. Small-scale variations in labeling density were observed within APC bands and throughout the PPC that could reflect the presence of a complex topographical order, but discrete patches at consistent locations as observed by genetic tracing were absent. This finding suggests that as a result of afferent overlap and intracortical processing, odor-quality information is represented by spatially distributed sets of cells. A distributed organization may be optimal for discriminating biologically relevant odorants that activate large numbers of ORs. J. Comp. Neurol. 457:361–373, 2003. © 2003 Wiley-Liss, Inc.
It has been estimated that there are ∼1,000 different olfactory receptors (ORs) in the rat and mouse, each one of which may be expressed by a separate population of olfactory sensory neurons (Buck and Axel, 1991). Rather than specifically binding whole molecules, ORs recognize substructural features; consequently, a given compound can activate many different sensory neurons (Sicard and Holley, 1984; Malnic et al., 1999; Araneda et al., 2000; Floriano et al., 2000; Ma and Shepherd, 2000; Singer, 2000; Wachowiak and Cohen, 2002). This combinatorial coding yields robust responses to an exceedingly large number of odors, both familiar and novel. However, as a result of a dependence of binding on concentration, and the multicomponent nature of most biologically relevant odors, combinatorial coding imposes a heavy computational burden on the central nervous system.
Studies with a variety of techniques have revealed that sensory neurons expressing each OR converge onto a small number of glomeruli in the olfactory bulb, thereby creating a highly organized arrangement of olfactory information (Sharp et al., 1975; Imamura et al., 1992; Johnson et al., 1998; Rubin and Katz, 1999; Uchida et al., 2000; Wachowiak and Cohen, 2001). Based on findings from other systems, it might be expected that this order would be preserved in the piriform cortex, the traditional primary olfactory cortex. A recent “genetic tracing” study (Zou et al., 2001) has provided evidence that supports such an order (also see Ojima et al., 1984; Buonviso et al., 1991). In the study of Zou and coworkers, barley lectin was introduced into the olfactory sensory neurons that express specific ORs and traced transneuronally through the olfactory bulb to the piriform cortex. The results revealed comparatively little spatial order in the posterior piriform cortex (PPC) but well-defined patches of labeled neurons in the anterior piriform cortex (APC). However, the patches in the APC were much larger than olfactory bulb glomeruli; only ∼20 could be accommodated without overlap. It was concluded that these patches reflect patterns of input connectivity from the olfactory bulb. An important question is how these afferent patterns are reflected in spatial patterning of odor-evoked cellular activity, i.e., postsynaptic patterns. Are the cells activated by a given odorant in the piriform cortex also concentrated in patches, and, if so, are there differences in numbers, locations, and morphologies of the patches activated by different odorants?
Many factors would be expected to participate in shaping postsynaptic patterns. Because most odorants activate a large number of ORs (Sicard and Holley; 1984; Malnic et al., 1999; Araneda et al., 2000; Ma and Shepherd, 2000), the comparatively large afferent patches activated by pathways from individual ORs could blend together and be obscured in responses to odorants. However, inhibitory and excitatory systems within the piriform cortex, and between the piriform cortex and other cortical areas (Haberly, 1998), provide mechanisms that could substantially alter the form of postsynaptic patterns relative to afferent patterns. Broad patterns of afferent fiber activation could be shaped by lateral inhibition or converging associational connections into well-defined spatial patterns of cellular activation. Conversely, the highly distributed excitatory connections between pyramidal cells could activate neurons that receive no afferent input, thereby broadening postsynaptic patterns relative to afferent patterns.
To gain insight into spatial patterning of odor-evoked cellular activity in the piriform cortex, we examined induction of c-fos, an immediate-early gene (IEG) that has been used to visualize spatial components of coding in the olfactory bulb (e.g., Guthrie et al., 1993) and other cortical areas (e.g., Melzer and Steiner, 1997; Arckens et al., 2000). This technique has been previously applied to the piriform cortex to study seizure activity (Morgan et al., 1987; Maggio et al., 1993; White and Price, 1993; Zimmer et al., 1998) and changes in the numbers of labeled cells associated with olfactory learning (Datiche et al., 2001) and the diurnal cycle (Funk and Amir, 2000), but the spatial distribution of odor-evoked activity has not been analyzed. Attempts to visualize spatial patterns of odor-evoked activity in the piriform cortex by 2-deoxyglucose uptake and optical imaging have been unsuccessful for reasons that include a lack of cellular-level resolution, the relative inaccessibility of the piriform cortex surface, and the superficial myelinated layer in APC.
MATERIALS AND METHODS
Odor exposure
Experimental procedures followed NIH guidelines for the use of animals, with approval by the University of Wisconsin IACUC. Approximately 18 hours prior to odor exposure, littermate pairs of adult (200–250 g) male Long-Evans hooded rats (n = 47 littermate pairs) were placed in identical chambers of an olfactometer. Chambers were polypropylene cages with a floor constructed of stainless steel grid (2.5-cm spacing) elevated over a layer of activated charcoal to minimize odors from animal wastes. To minimize background odors further, purified air was pulled through the chambers at a high rate (200 liters/min). The air stream was purified with high-capacity HEPA/activated charcoal filters connected to the chambers by large-diameter glass tubes.
Odorant stimuli (Sigma, St. Louis, MO; PheroTech, Vancouver, BC) were generated by bubbling dehumidified, purified air through high-purity odorants contained in tall aspirator bottles. Concentration was varied by dilution in nominally odor-free mineral oil (Sigma) and by mixing odorized and purified air streams through a system of tubing, valves, and flow meters constructed from Teflon and glass. Odor exposure was performed 2 hours after the onset of the animals' 12-hour daylight period to minimize effects of circadian rhythm on odor-induced Fos labeling (Funk and Amir, 2000). Animals were exposed as littermate pairs in parallel channels of the olfactometer, with one animal receiving odorant and the other serving as a control (exposed to clean air only, or clean air bubbled through mineral oil for experiments in which odorants were diluted). Stimulation was intermittent to minimize response habituation (Wilson, 1998, 2000): 30-second periods of odorant delivery were separated by 90 seconds of clean air over a period of 1 hour.
Immunocytochemistry
Immediately following odor stimulation, animals were anesthetized with sodium pentobarbital (75 mg/kg) and transcardially perfused with 4% formaldehyde, freshly depolymerized from paraformaldehyde. Brains were stored overnight in cryoprotection solution (20% sucrose, 5% glycerol in 0.1 M phosphate buffer), and sections were cut at 60 μm in the coronal plane as defined in the atlas of Paxinos and Watson (1986) using a freezing microtome. Particular care was taken to orient brains consistently during sectioning to decrease the variability encountered when results were compared across animals.
Visualization of c-fos induction used a polyclonal antibody to Fos protein (Ab-5, 1:30,000 dilution; Calbiochem, San Diego, CA). Tissues from experimental and control animals were processed together. Sections were washed three times in 0.9% NaCl with 0.01 M phosphate buffer at pH 7.4 (PBS) + 2% bovine serum albumin + 0.3% Triton, followed by PBS + 20% normal goat serum. Incubation in primary antiserum was overnight at room temperature. After four washes in PBS, sections were incubated for 3 hours in biotinylated goat-anti-rabbit IgG, washed four times in PBS, incubated for 1 hour in ABC reagent (standard kit; Vector, Burlingame, CA), washed five times in PBS, and reacted with 0.04% 3, 3′ diaminobenzidine (DAB) and 0.01% H2O2 for 4 minutes at 25°C (monitored with a thermocouple probe for consistency). No intensification procedures were applied because of the tendency of these methods to obscure quantitative differences in labeling intensity. Sections were dried onto gelatin-coated slides, dehydrated, and coverslipped with Eukitt (Calibrated Instruments, Ardesly, NY). γ-Aminobutyric acid (GABA)ergic cells were stained with a monoclonal antibody to GAD-67 (AB108; 1:3,000; Chemicon), parvalbumin (P-3088; 1:10,000; Sigma), calbindin (C-9848; 1:25,000; Sigma), or cholecystokinin (C-2581; 1:10,000; Sigma). Double staining was carried out with secondary antisera conjugated to AlexaFluor (Molecular Probes, Eugene, OR) and Cy3 (Sigma). Double-labeled sections were examined as wet mounts using VectaShield (Vector).
Data analysis
Sections were imaged under standard conditions using a SPOT-2 camera (Diagnostic Instruments) and microscope equipped with planapochromatic objectives (Nikon). A correction for nonuniform illumination was made during image acquisition using the SPOT-2 software. Data analysis was automated using MetaMorph software (Universal Imaging) to avoid experimenter bias. Labeled cells were detected with a combination of an optical density threshold (described below) and a size window that excluded profiles with a diameter of less than 5 μm. To compensate for differences in staining density introduced by tissue processing, the optical density threshold was defined as a percentage of the difference between the optical density of the darkest stained cell and the noncellular background in layer Ia (65% unless otherwise specified). This threshold was determined independently for each set of sections from each animal. Cells identified by this procedure are termed Fos-positive. Subdivisions and layers of piriform cortex were visualized using darkfield illumination (sections were not counterstained). The illustrated images were optimized by adjusting tone scale and applying unsharp masking using Photoshop 6.0 (Adobe).
-
Procedure 1, designed to confirm the presence of cell-rich bands, compared the number of Fos-labeled cells in dorsal and ventral sectors of APC using a paired t-test. A dorsal sector was defined as the region of the cortex between the lateral margin of the LOT and the lateral border of the APC (Fig. 1B). A ventral sector was defined as the region extending medially from the dorsal sector for a distance equal to the width of the dorsal sector. All borders were defined by lines oriented perpendicular to the cortical surface that extended through all layers.
-
Procedure 2 was used to confirm visual observations regarding differences in the dorsoventral location of the band of labeled cells in the dorsal C-shaped region of APC. An arc fitted to layer II in the dorsal sector (as defined above) was bisected into superior and inferior segments by a line through the center of the curvature. Numbers of Fos-labeled cells in the superior and inferior segments were compared in 15 sections from five animals using a t-test for independent groups.
-
Procedure 3 was used to determine whether variations in the density of cellular labeling along the rostrocaudal axis of APC were significant. Counts of labeled cells in the dorsal sector were made in a series of sections extending over the full rostrocaudal extent of the APC (from the rostral border with the anterior olfactory nucleus to the caudal end of the LOT). Counts were made for every other 60-μm-thick section in each of 12 odor-exposed animals (34–36 sections per animal). Cell counts were compared using a two-way (odor × rostrocaudal level) analysis of variance.
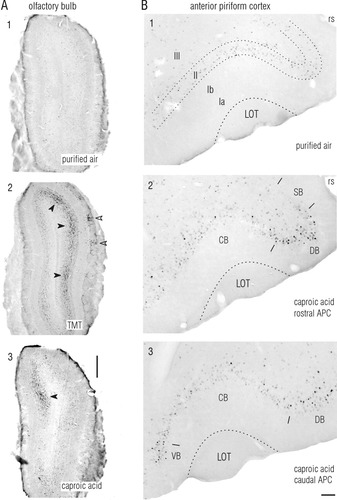
Odor-evoked c-fos induction was concentrated in patches in the olfactory bulb and in longitudinal bands in anterior piriform cortex (APC). c-fos induction was visualized by immunocytochemical staining for Fos protein. A: Responses in the olfactory bulb to purified air control (A1), TMT at 1:10,000 dilution (A2), and caproic acid at 1:100,000 (A3). Confirming previous reports, Fos-positive neurons in the olfactory bulb were concentrated in patches that extended from the glomerular through the granular layer (open and solid arrowheads, respectively), and these patches were at different locations in response to different odorants. B: Responses in APC to purified air control stimulus (B1) and to 1:100,000 caproic acid at rostral (B2) and caudal (B3) levels in the same animal. Darkly labeled cells in the dorsal curved portion of layer II were concentrated in a longitudinally oriented dorsal band (DB). A narrower ventral band (VB) consisting of darkly labeled cells at the ventral border of APC (arrow in B3) was confined to approximately the caudal one-half of its extent. Dorsal and ventral cell-rich bands were separated by a wide cell-poor central band (CB). Labeling was also sparse in a sulcal band (SB) that extended into the rhinal sulcus (rs) from the dorsal boundary of the dorsal band. Sections were cut in the coronal plane at 60 μm thickness. Roman numerals denote layers in APC; LOT, lateral olfactory tract. Dorsal is up and lateral is right in A and B, and in all subsequent figures. TMT, 2,5-dihydro-2,4,5-trimethylthiazoline. Scale bar = 500 μm in A; 100 μm in B.
Odorant selection
- 1
Four-carbon (butyric) and 6-carbon (caproic) aliphatic acids that have similar odors (sweat-like) and patterns of olfactory bulb activation (Johnson et al., 1999).
- 2
An aliphatic alcohol, nonanol, that activates glomeruli positioned laterally to those activated by the aliphatic acids in the rostral olfactory bulb (Uchida et al., 2000). Nonanol has a subtle spice-like odor that is distinctly different from the aliphatic acids.
- 3
Limonene and isoamyl acetate share a “fruity” quality (orange- and banana-like, respectively) but are very different structurally. Both activate large regions in the medial and lateral parts of the olfactory bulb (Johnson et al., 1998; Linster et al., 2001), patterns that are very different from those elicited by the aliphatic acids and alcohol.
- 4
The sixth compound, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), which is found in fox feces, was chosen because it elicits unconditioned autonomic, endocrine, and behavioral responses in the rat (Morrow et al., 2000; Wallace and Rosen, 2000). TMT has a pungent odor. Its pattern of olfactory bulb activation has not been reported.
Because high odorant concentrations usually broaden spatial patterns in the olfactory bulb (i.e., activate more glomeruli) without altering odor quality (Rubin and Katz, 1999), we reasoned that any spatial patterning in the olfactory cortex would be more apparent at lower stimulus concentrations. Most experiments were therefore performed with dilutions that were perceived as low to moderate in intensity by the human nose (1:2,000–1:20,000).
RESULTS
General features of odor-evoked Fos expression
In odor-exposed animals, Fos staining was prominent in the olfactory bulb, piriform cortex, and other cortical areas that receive direct (e.g., amygdaloid and entorhinal areas) and disynaptic input (e.g., insular and orbitofrontal areas) from the olfactory bulb. Although many cells with light Fos label were present in the olfactory bulb and piriform cortex of control animals (Figs. 1, 2), substantial numbers of heavily labeled cells were only observed after odor exposure. Automated counts of Fos-positive cells with the detection threshold set at 65% of maximum (corrected for background staining; see Materials and Methods) revealed an average of 23.5 ± 3.16 Fos-positive cells per section in the APC versus 7.3 ± 0.74 cells per section in the littermate control animals (t(40) = 4.98, P < 0.001). Few Fos-positive cells were detected in cortical areas that lack mono- or disynaptic olfactory input.
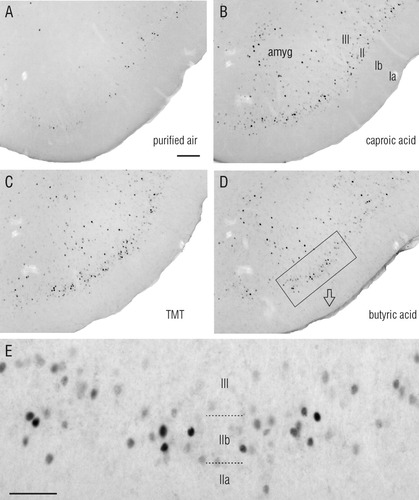
Odor-evoked Fos expression in the posterior piriform cortex (PPC) was in spatially distributed neurons. Coronal sections through the central region of PPC illustrate responses to purified air in a control animal (A), 1:100,000 caproic acid (B), 1:10,000 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), a constituent of fox feces (C), and 1:20,000 butyric acid (D). E: Enlargement of indicated region in D. Note the concentration of labeled cells in layer IIb (compact pyramidal cell layer) and the relative absence in layer IIa (semilunar cell layer). Amyg, amygdala. Scale bar = 100 μm in A–D; 50 μm in E.
In the olfactory bulb, Fos-positive cells were concentrated in patches that were narrow in the glomerular layer and wider in the granule cell layer (Fig. 1A), matching previous results in which c-fos induction was detected by in situ hybridization (Guthrie et al., 1993). For the five test odorants for which data are available (all except TMT), patches of darkly labeled cells were observed at locations that corresponded to previous findings obtained by 2-deoxyglucose (2-DG) uptake (Johnson et al., 1998, 1999; Johnson and Leon, 2000), optical imaging (Rubin and Katz, 1999; Uchida et al., 2000), in situ hybridization to c-fos (Guthrie et al., 1993), and single-unit recording (Imamura et al., 1992; Yokoi et al., 1995).
Distributions of Fos-positive cells in the piriform cortex of odor-exposed animals were very different from the spatial patterns observed in the olfactory bulb (Figs. 1, 2). Darkly labeled cells were widely dispersed in the piriform cortex, and although spatial patterns could be readily discerned in the APC (Fig. 1B), they were much larger and less well-defined than those in the olfactory bulb. The distribution of Fos-positive cells over depth corresponded to the distribution of pyramidal cell somata (highest density in layer IIb and the superficial part of layer III) in both the APC (Fig. 1B) and PPC (Figs. 2, 3). There were comparatively few labeled cells in layers where neurons are predominantly nonpyramidal: layers I and deep-III, where GABAergic cells are concentrated, and layer IIa, which consists largely of semilunar cells (Haberly, 1998).

Computer-generated plots for PPC showing Fos-positive neurons with different degrees of label in response to low and high odorant concentrations. Symbols indicate cells with different degrees of label; dotted lines indicate layer II (see Fig. 2 for orientation); solid lines are the cortical surface. Filled circles indicate darkly labeled cells (65% threshold setting; see Materials and Methods); open circles, an intermediate level (40% threshold); dots, light label (25% threshold). A: Labeled cells at three levels separated by 120-μm intervals in the central region of PPC in response to a moderately low concentration of butyric acid (1:20,000). Note that the images in Figure 2D and E are from the same section plotted in A1; arrowheads in A indicate the region that was imaged. B: Corresponding plot from the littermate control (purified air) for the animal illustrated in A. C: Response to a much higher concentration of butyric acid (1:200). Note the diffuse distribution of labeled cells throughout the dorsal to ventral extent of PPC, the interspersion of cells with different degrees of label, and the concentration of labeled cells in layers IIb and superficial III where pyramidal cells are the dominant population. Also note that labeled cells are not homogeneously distributed, rather, there are regions where the concentration of labeled cells is comparatively high (clusters) and low (voids) in both layers IIb and III. Examples of clusters and voids in layer IIb are indicated by solid and open arrows, respectively, in C1. Scale bar = 200 μm.
Odor-evoked activity in posterior piriform cortex
Spatial distributions of Fos-positive cells were analyzed by using a computer microscope system that detected cells based on an objectively defined threshold as described earlier. Results are described first for the PPC, where patterning was simpler than in the APC. Labeled cells in the PPC of odor-exposed animals were widely distributed, with no well-defined spatial patterning (Figs. 2, 3). Local variations were observed in the distribution of labeled cells in the form of small “clusters and voids” (Fig. 3); however, no consistency was detected in the locations of these variations in different animals that received identical stimuli. Furthermore, no differences were detected in comparisons of patterns evoked by different odorants, as illustrated for three of the six test odorants in Figures 2 and 3. Visual observations at high power and quantitative analysis with multiple threshold settings revealed a continuous variation in the density of cellular labeling (as opposed to all-or-none staining), and an interspersion of labeled cells among a substantially larger number of cells with little or no label (Figs. 2, 3). Changes in stimulus dilution altered the number of labeled cells, but the overall distributed character was observed from the lowest to highest dilutions (Fig. 3).
Odor-evoked activity in the anterior piriform cortex
Fos-positive cells were present throughout the APC in odor-exposed animals, but, in marked contrast to the PPC, prominent spatial patterns were consistently observed (Figs. 4, 5). These odor-associated spatial patterns consisted of longitudinal (rostrocaudally extended) bands of Fos-positive cells that contrasted with the more restricted patches observed in the olfactory bulb (Fig. 1A). Two cell-rich and two cell-poor bands were observed. The cell-rich bands were a narrow strip at the ventral limit of the APC (the ventral band; Figs. 1B3, 4B, 5A2, 6D) and a broader zone in the dorsal curved region (the dorsal band; Figs. 1B2, 4B, 5A1, 6D). The ventral band was confined to approximately the caudal half of the APC, whereas the dorsal band was more prominent rostrally (see summary plot in Fig. 6E). A wide cell-sparse central band encompassed the region between the cell-rich bands (Figs. 1B, 6), and a narrower sulcal band with very few labeled cells was observed in the dorsalmost part of the APC located in the ventral bank of the rhinal sulcus (Figs. 1B2, 4B, 5A1, 6E). Quantitative analysis confirmed that the dorsal band contained a significantly higher density of Fos-positive cells than the adjoining central band: an average of 10.7 ± 1.96 cells/section was observed in a sector that encompassed the dorsal band, versus 2.5 ± 0.53 in an adjoining ventral sector of identical size (P < 0.001 by paired t-test, n = 25 sections from five animals; see procedure 1 in Materials and Methods). High-power examination of the dorsal and ventral cell-rich bands revealed that, as in the PPC, large numbers of cells with no detectable Fos staining were interspersed with labeled cells in all experiments, and a small scale patterning was present within bands as described below.
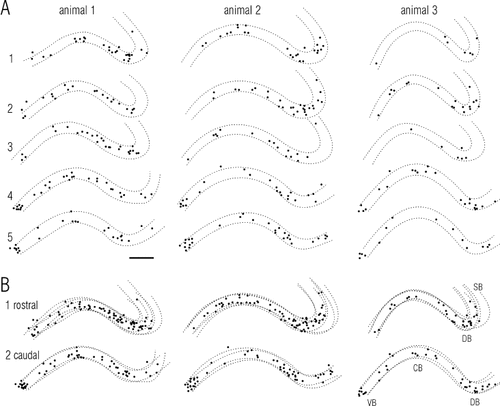
Extent of animal-to-animal variation in patterns of odor-evoked Fos labeling in APC. Filled circles are cells with labeling that exceeded a threshold level (65% setting) as described for Figure 3; dotted lines indicate layer II (see Fig. 1B for orientation). Responses to a low concentration (1:10,000 dilution) of caproic acid are shown for three animals (columns). Individual sections are shown at the top (A); summary plots generated by stacking individual sections are shown at the bottom (B). Sections in A extend from ∼3.2 mm (at top) to ∼1.2 mm anterior to Bregma (coordinates of Paxinos and Watson, 1986); B1 is a composite of A1–A3; B2 is A4–A5. The cell-rich dorsal band (DB) is well defined at rostral levels in all three animals, and the narrow cell-rich ventral band (VB) is present at the ventral boundary at caudal levels. Differences between animals include a smaller number of labeled cells in animal 3 relative to animals 1 and 2, and the locations of clusters and voids within the dorsal band. SB, sulcal band. Scale bar = 200 μm.
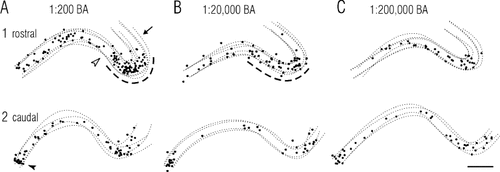
Effects of stimulus concentration on banding patterns in rostral and caudal regions of APC. Results are shown as summary plots of Fos-positive cells for rostral and caudal regions of APC that were generated with a computer-microscope system as described for Figures 3 and 4. Stimuli were butyric acid (BA) at dilutions of 1:200 (A), 1:20,000 (B), and 1:200,000 (C). Subtle but consistent changes in banding pattern with concentration included less well-defined patterning at low concentrations (C) and a more sharply defined dorsal band at high concentrations. The better definition of the dorsal band resulted from the emergence of an adjoining cell-poor zone (open arrowhead in A) that shifted its location (compare dashed lines in A and B). Arrow indicates the cell-poor sulcal band at the dorsal boundary of APC. Closed arrowhead indicates the cell-rich ventral band. Scale bar = 200 μm.

Patterns of Fos-labeled cells in APC in response to odorants with diverse structures and qualities. A: 1:200 limonene. B: 1:100,000 nonanol. C: 1:20,000 isoamyl acetate. D: 1:10,000 TMT. Plots are stacks of individual sections for rostral (top row) and caudal (bottom row) levels in APC (see Fig. 4B). Note the dorsal cell-rich band (DB) at rostral levels and the ventral cell-rich band (VB) at caudal levels in the responses to all four odorants. Also note that approximate matches can be found for each pattern within the sets of responses to different concentrations of butyric and caproic acids illustrated in Figures 4 and 5, suggesting that the form of banding patterns reflects both quantity and quality in ambiguous fashion. E: Summary diagram showing consistent features of odorant-evoked Fos labeling. Left, location of piriform cortex in ventrolateral view of rat brain (rotated upward 45°); dashed line indicates regions of olfactory cortex that extend into the rhinal sulcus (rs). Right, enlargement of the anterior piriform cortex (APC) and the posterior piriform cortex (PPC), with the distribution of odorant-induced Fos labeling indicated by stippling; vertical dashed lines indicate the approximate levels of rostral and caudal plots in A–D and previous figures. Locations of the dorsal and ventral cell-rich bands (DB, VB), and central and sulcal cell-sparse bands (CB, SB) are indicated in APC. AON, anterior olfactory nucleus; OT, olfactory tubercle; TMT, 2,5-dihydro-2,4,5-trimethylthiazoline; hatching denotes the LOT. Scale bars = 200 μm in A–D; 1 mm in E.
Stimulus-related differences in banding pattern.
Visual observations indicated that stimulus-evoked banding patterns in the APC were similar across animals for a given odorant at a given concentration, but these could differ in response to different odorants or to different concentrations of the same odorant. Experiments were carried out to examine how these patterns are shaped by odor quality as opposed to intensity. Studies in the olfactory bulb (Johnson and Leon, 1999; Uchida et al., 2000) have shown that the set of glomeruli activated by aliphatic acids on its dorsal aspect shifts systematically in location with chain length and that this change is largely independent of concentration. We therefore asked whether a comparable concentration-independent change in banding patterns evoked by aliphatic acids can also be detected in the APC. If odor quality is encoded in terms of band location or form, then at least one feature related to odorant structure must be relatively insensitive to stimulus intensity. Observations were made in the olfactory bulb and APC of 17 littermate pairs in response to dilution series of 4-carbon (butyric) and 6-carbon (caproic) aliphatic acids.
Observations in the olfactory bulb revealed that butyric and caproic acids activated comparatively small groups of glomeruli (as indicated by Fos-positive periglomerular cells; e.g., Fig. 1A). As reported in previous studies using other methods, the locus of the group that was activated by butyric acid on the dorsomedial aspect was dorsal to that activated by caproic acid at all concentrations of both odorants (not shown).
Patterns of Fos-positive cells in the APC for the butyric acid dilution series (1:200–1:2 × 106 of saturation) are illustrated in Figure 5. At the highest concentration, both cell-rich and cell-poor bands were apparent (Fig. 5A). The ventral cell-rich band was indistinguishable in location and shape in response to all concentrations, and labeling density in the cell-sparse sulcal band remained very low at all concentrations. However, changes were observed in both the dorsal cell-rich, and the central cell-sparse bands. At increasingly higher concentrations, the number of Fos-positive cells in the dorsal band increased (Fig. 5), and the density of cells in the adjoining portion of the central cell-sparse band decreased (open arrowhead in Fig. 5A1). This resulted in an apparent shift in location of the dorsal band (compare dashed lines in Fig. 5). Statistical analysis confirmed that this shift was significant, with a higher proportion of Fos-positive cells in a “superior” segment of the dorsal APC following exposure to a high concentration of odor (38 ± 3.4%) than following a low concentration (23.7 ± 2.8%; t(13) = 3.10, P < 0.01; see procedure 2 in Materials and Methods).
Responses to a dilution series of caproic acid revealed a progression in the form of dorsal and central bands that was indistinguishable from that observed for butyric acid, as well as an absence of any consistent change in ventral or sulcal bands. Although there appeared to be subtle differences in the location of the dorsal band in response to identical dilutions of butyric and caproic acids, no difference was detected for comparisons in which the concentration of caproic acid was higher than that of butyric acid. We conclude that, in contrast to the olfactory bulb, in which a concentration-independent difference in spatial patterns is observed for butyric and caproic acids, differences in the forms of banding patterns in the APC are ambiguous with regard to the identity and concentration of these aliphatic acids.
Banding patterns from odorants that evoke disparate spatial patterns in the olfactory bulb.
Because butyric and caproic acids are structurally similar and activate sets of glomeruli at nearby locations in the olfactory bulb, we examined responses in the APC to more structurally diverse odorants. We reasoned that any spatial ordering of cellular activity would be more apparent in a comparison of responses to odorants that activate different parts of the olfactory bulb. Responses were analyzed in 21 littermate pairs to nonanol, which evokes a patch of activity in the dorsolateral olfactory bulb at approximately the same rostrocaudal level as the dorsomedial patches evoked by butyric and caproic acids (Uchida et al., 2000), and to limonene and isoamyl acetate, which activate large overlapping regions in the lateral bulb at a more caudal level than the prominent butyric and caproic acid patches. If banding patterns in the APC represent a mapping of inputs from the olfactory bulb, then readily apparent differences would be expected for these odorants in view of their widely divergent patterns of olfactory bulb activation. However, if the same features of APC banding patterns are altered by stimulus intensity and quality, as suggested by the dilution-series experiment for aliphatic acids, then responses to the additional odorants would not be unique.
As illustrated in Figure 6, the form of banding patterns evoked by each of these structurally diverse odorants approximately matched patterns within the set of responses to different concentrations of butyric acid. Any possible differences were comparable to variations between animals in responses to identical stimuli (cf. Fig. 4). Dorsal and ventral cell-rich bands were apparent in all responses, and the dorsal band was again more prominent rostrally, with the ventral band restricted to the caudal part of the APC. These results strengthen the conclusion from the dilution-series experiment that the overall forms and locations of banding patterns are ambiguous with respect to odor quality and quantity.
Do banding patterns in the APC reflect nonolfactory associations?
Physiological studies have provided evidence that neurons in the APC encode information regarding the behavioral significance of odors in addition to their identity (Schoenbaum and Eichenbaum, 1995). To examine this possibility, we asked whether the morphology of banding patterns evoked by the predator-associated odorant, TMT, which evokes unconditioned autonomic and behavioral responses in the rat (Morrow et al., 2000; Wallace and Rosen, 2000), differs from responses to the five presumed behaviorally neutral test odorants (n = 9 littermate pairs for TMT). Four of the nine rats exposed to TMT displayed apparent escape-attempt behavior that was not observed in control animals or animals exposed to other odorants. As illustrated in Figure 6, no unique features were observed in the banding patterns evoked by TMT. Furthermore, no differences were detected between animals that displayed behavioral activation and those that did not. Because the odor of TMT (pungent) and its spatial pattern of olfactory bulb activation differed from the other test odorants (widespread and particularly strong caudally), this experiment also provided additional evidence that banding patterns in the APC have little or no relationship to odor quality.
Small-scale variations in labeling density.
In addition to banding patterns, local variations in the density of cellular labeling were also observed that created fine-scale clusters and voids within labeled regions of the APC (Fig. 4). The most prominent of these features were on the order of 200 μm in extent. This contrasts with patches of transneuronally transported label derived from afferent fibers in the mouse that were ∼250–800 μm in length in the APC (Zou et al., 2001), which would extrapolate to ∼450–1,500 μm for the larger rat cortex. Quantitative analysis was carried out in the dorsal band, where local variations were most prominent, to assess the degree of consistency across animals and to determine whether stimulus-related differences in these fine-scale features could be detected. We examined the rostrocaudal distribution of Fos-positive cells in the dorsal cell-rich band in 12 animals exposed to four different odors. Cells were counted in each stained section for the entire length of the APC, from the AON border to the caudal end of the LOT (see procedure 3 in Materials and Methods). As illustrated in Figure 7, the small-scale variations observed in individual animals were apparent in the counts of Fos-labeled cells. However, we found no evidence for a correspondence in this fine-scale patterning between animals exposed to a particular odor (Fig. 7A), or evidence that patterns differed significantly for different odors (Fig. 7B; ANOVA F76,269 = 1.17; P > 0.1; see procedure 3 in Materials and Methods).
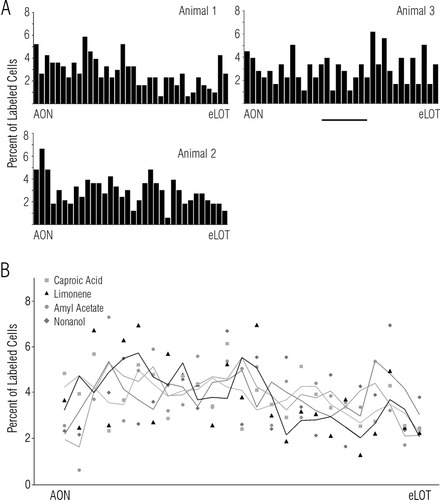
Quantitative analysis of odorant-evoked Fos label in APC. A: Rostrocaudal distribution of Fos-labeled cells in the dorsal part of APC following exposure to caproic acid for the three animals shown in Figure 4. Bars represent the number of cells labeled in single sections (60 μm, every second section shown), normalized as a percent of all cells counted; see text for details. Note the lack of correspondence across animals in the locations of local maxima (clusters) and minima (voids). B: Pooled data for four odorants. Symbols represent the mean percent of labeled cells at each of 25 rostrocaudal levels in APC for three animals exposed to the odorants identified at upper left. Lines connect smoothed values computed from the illustrated points for the four odorants. Smoothing was carried out with a sliding window using a gaussian average over 5 points for levels 3–23, and progressively fewer points at each end. AON, anterior olfactory nucleus; eLOT, end of the LOT. Scale bar = 1 mm in A.
Does c-fos induction in GABAergic cells obscure a spatially discrete code?
Studies in the olfactory bulb have demonstrated c-fos induction in GABAergic cells in the granule cell layer over a region that extends for several hundred microns beyond active glomeruli (Guthrie et al., 1993). In light of this finding, it is conceivable that odor-evoked activation of principal cells is discretely patterned in the piriform cortex but that the patterns are obscured by Fos labeling in a larger cloud of GABAergic cells. To examine this possibility, we double-labeled tissue sections with the Fos antiserum and with antisera to markers of GABAergic function. Because the optimal fixative for Fos detection is incompatible with GABA immunostaining, we used an antiserum to GAD-67 that stains cell bodies in the piriform cortex in a pattern that matches those observed with GABA antisera (Ekstrand et al., 2001a, b) and antisera to parvalbumin, calbindin, and cholecystokinin, which colocalize with GABA in the piriform cortex (Kubota and Jones, 1992; Cho and Takagi, 1993; Ekstrand et al., 2001a). In marked contrast to the olfactory bulb, where Fos-positive granule cells were double-labeled with the GAD-67 antiserum (inset, Fig. 8), consistent with their known GABAergic nature (Ribak et al., 1977), less than 2% (2/142 cells from eight animals) of Fos-positive cells in the piriform cortex were also labeled with any of the GABAergic markers (Fig. 8). Based on this finding, and on the laminar distribution of Fos-positive cells described earlier (comparatively few in layers I, IIa, or deep-III), we conclude that the observed odor-associated c-fos induction was predominantly in pyramidal cells in the piriform cortex.
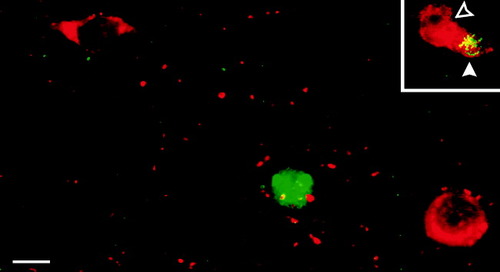
Very few Fos-positive cells were immunoreactive for GAD-67 in the piriform cortex. The illustrated section from APC of an odor-exposed animal was double-labeled using antisera against Fos (green fluorescence) and GAD-67 (red). GAD-67 immunoreactivity was concentrated in the cytoplasm and Fos reactivity in the nucleus; small GAD-67-positive spots are boutons. Less than 2% of neurons were double-labeled in either APC or PPC, suggesting that few GABAergic cells display substantial activity-induced Fos expression. The inset illustrates a double-labeled cell in the granule cell layer of the olfactory bulb (yellow, solid arrowhead) and adjoining cells labeled for GAD-67 only (red, open arrowhead). Scale bar = 10 μm for main panel and inset.
DISCUSSION
The results revealed marked differences in patterns of odor-evoked c-fos induction between the olfactory bulb and piriform cortex, and between the APC and PPC. The observed patterning was complex in nature, with both well-defined bands and widely distributed cells with Fos label. To investigate relationships between these patterns and the spatial representation of odorant information, stimuli were selected on the basis of molecular structure, perceived quality, and behavioral significance. Interpretations are discussed in light of the technical limitations of the c-fos method.
Technical limitations
IEG labeling provides the only functional imaging tool that can achieve cellular-level resolution over the full extent of large neuronal systems. However, the interpretation of results obtained with IEG-based methods is complex and must be made with caution. Factors that must be taken into account include the multifactorial relationship between neuronal activity and IEG induction, differences in the extent to which activity induces IEGs in different neuronal populations, and the lack of a capacity to visualize fast temporal aspects of sensory activity.
Complex relationship between activity and induction.
Like most other imaging methods, including endogenous optical, functional magnetic resonance imaging, and Ca2+-sensitive dyes, the induction of IEGs can be initiated by processes that are not directly related to stimulus-evoked postsynaptic activity. This is of particular concern for neuromodulatory inputs from cholinergic and monoaminergic cell groups that are not well localized spatially. Nevertheless, despite the potential of these processes to obscure the visualization of patterned activity, there is considerable empirical evidence suggesting that c-fos induction can provide insight into the structure of sensory representations in cortical systems. Such evidence is available for the olfactory bulb (Guthrie et al., 1993) and sensory areas of the neocortex (e.g., Melzer et al., 1997; Arckens et al., 2000), all of which receive inputs from the same neuromodulatory systems as the piriform cortex (Shipley et al., 1995; Haberly 1998, 2001). Findings from the current study that support the validity of the c-fos approach include the presence of discrete odor-related patches in the olfactory bulb at locations that match those observed by single-unit recording and 2-DG uptake (which is quantitatively related to activity; Kennedy et al., 1995), and the restriction of robust c-fos induction by odorants to areas that receive strong olfactory input.
We also examined the effects of manipulations that would be expected to increase or decrease the extent of sensory-evoked neuromodulator actions. If the observed spatial features are derived predominantly from neuromodulatory rather than from direct sensory input, they should be substantially altered by these manipulations. The finding that neither stimulation with TMT, an odorant that elicits behavioral and autonomic activation, nor anesthesia with halothane or urethane (Illig and Haberly, unpublished data) had apparent effects on spatial patterns of odor-evoked c-fos induction therefore supports the dominance of direct sensory-evoked activation. Because these manipulations would also be expected to alter the extent of “higher order” activity, our results also discount a substantial contribution of nonolfactory inputs from the prefrontal cortex or other areas to the observed patterning.
There is also independent evidence from studies of 2-DG uptake that supports our findings (Sharp et al., 1977; Astic and Cattarelli, 1982; Cattarelli et al., 1988). Although the lack of cellular-level resolution in the 2-DG studies precludes a detailed comparison, these studies demonstrated a spatially distributed pattern of activation in the piriform cortex that contrasted with well-defined patches in the olfactory bulb. It is also worth noting that a recent study in the CA1 region of the hippocampus, where connectivity is spatially distributed as in the piriform cortex, demonstrated a spatially distributed pattern of induction of an IEG (Arc) by a behavioral task (Guzowski et al., 1999). Through application of a new double-labeling method, it was shown that properties of the cellular-level patterning revealed by gene induction matched those originally demonstrated by single-unit recording.
Neuronal population dependence.
A finding of potentially general significance is that the extent of c-fos induction differed in three neuronal populations that could be distinguished. There was robust c-fos induction in pyramidal cells, but minimal induction in semilunar cells in layer IIa and in several classes of GABAergic cells that were defined immunocytochemically. Although there has been no analysis of odorant-evoked responses in semilunar cells, electrophysiological studies have shown that probable GABAergic fast-spiking cells in the piriform cortex respond to odorant stimuli (McCollum et al., 1991; Illig, unpublished data), suggesting that the absence of label in these cells reflects an insensitivity of c-fos induction to activity. This finding is of particular interest in view of the finding that c-fos induction in the olfactory bulb is predominantly in GABAergic cells (Guthrie et al., 1993).
For study of the spatial representation of olfactory information, the observed dominance of pyramidal cell labeling was fortuitous, because these cells are the primary population of principal cells in the piriform cortex, i.e., the focus of integrative processes and the primary source of output projections. Although GABAergic cells play central roles in integrative processes, evidence for the piriform cortex and other areas of the cerebral cortex indicates that their actions are largely exerted through principal cells (Haberly, 2001). Consequently, it can be argued that spatial patterns of GABAergic cell activation are of secondary importance for discerning the manner in which odorant information is represented.
Temporal encoding.
It is important to recognize that olfactory responses are patterned both spatially and temporally and that IEG induction provides information on the spatial component alone. Although there have been no demonstrations that temporal components of olfactory responses contribute to discrimination in vertebrates, the evidence for such a role in insects whose olfactory systems have many features that are homologous to vertebrates (Stopfer et al., 1994) underscores the need for caution in the interpretation of results that reflect spatial information alone.
Functional implications
Banding patterns in APC.
An unexpected feature was the concentration of labeled cells in prominent bands in the APC. Although subtle but significant differences in the locations of bands were observed in response to different stimuli, all could be reproduced by varying concentration alone, suggesting that they play a minimal role in the representation of odor quality.
An intriguing feature of these bands is the presence of parallels in form to subregions of the APC defined by connectivity and cytoarchitecture. Studies with axonal tracers have shown that projections from regions that correspond to the dorsal cell-rich and central cell-sparse Fos bands in the APC differ in spatial and laminar patterns of termination (Behan et al., 1995; Haberly, 2001), and a region that corresponds to the ventral cell-rich Fos band has a distinctive cytoarchitecture (Ekstrand et al., 2001b; Fig. 1) and receives a unique projection from the amygdala (Luskin and Price, 1983). Because differences in connectivity and cytoarchitecture can be assumed to be related to function (Kass and Collins, 2001), it can be proposed that the observed odor-independent banding patterns reflect functional differences. If banding patterns stem from differences in the proportion of active cells (sparsity of coding), they could reflect differences in learning capacity (Hertz et al., 1991; Rolls and Treves, 1998); if they stem from differences in the strength of activity-to-gene coupling rather than differences in physiologically defined activity, they could reflect differences in slow processes that require gene induction.
A prominent characteristic of odor-evoked bands in the APC was the reciprocal relationship in the density of label in adjoining regions: in experiments in which density was high in dorsal and ventral cell-rich bands, adjacent regions within the central cell-sparse band exhibited particularly low concentrations of labeled cells (Fig. 5). The presence of such flanking regions, together with inhibitory basket cells in the APC with sufficiently broad axonal fields (Ekstrand and Haberly, unpublished observations), suggests that lateral inhibition between adjoining subregions may contribute to the differences in form of banding patterns in response to different odorants and concentrations.
Spatial patterns of olfactory evoked activity.
Because the available evidence regarding the interpretation of c-fos-derived findings is indirect, any conclusions from our data regarding the nature of spatial encoding will require independent verification. However, a rather strong argument can be made that our data rule out the representation of odor information in the form of discrete, topographically ordered patches in the piriform cortex, as observed in the olfactory bulb and in primary areas of neocortex. As detailed above, supporting data include the following: 1) the demonstrated capacity of c-fos induction to reveal discrete patterning in systems where it is known to be present; 2) independent evidence from studies with 2-DG uptake that support a distributed nature of olfactory responses; and 3) our evidence that the shapes and locations of APC bands, the only well-defined spatial patterns, play little or no role in the encoding of odor quality. At the very least, our results appear to rule out an enhancement of the degree of order in the spatial representation of olfactory information through lateral inhibition or a spatially organized convergence of afferent or intrinsic fiber systems.
Based on the findings of Zou et al. (2001) with their new genetic tracing method, one might expect a well-defined patchy distribution of odor-evoked cellular activity in the piriform cortex. However, the findings obtained by genetic tracing reflect patterns derived from single OR clones, whereas the current results were obtained with stimuli that activate multiple ORs. This cannot be avoided at present because no odorants have been identified that activate single ORs or small identified sets of ORs. Therefore, even if postsynaptic activity in the piriform cortex were to be determined exclusively by afferent input, it follows that spatial patterns of cellular activity would be more complex and spatially distributed than the patterns observed by genetic tracing. The dense, widely divergent associational connections between pyramidal neurons in the piriform cortex (Johnson et al., 2000; Haberly, 1998) also could render odor responses more spatially distributed than could be predicted from afferent connectivity alone. It is important to point out, however, that a spatially distributed character does not rule out the presence of a systematic odotopic representation consisting, for example, of many small patches at disparate locations that are related to odor quality.
At the other extreme, overlapping inputs and divergent intrinsic connectivity could generate an ensemble code by which each cell responds to diverse odorants and neighboring cells are no more likely to respond to common stimuli than distant cells, as recently demonstrated in the hippocampus (Redish et al., 2001). Unfortunately, the scale of the clusters and voids we observed within labeled regions was too small to determine with the current method (a single marker that necessitated across-animal comparison) whether they reflect a complex topographical order as opposed to an unstructured ensemble code.
CONCLUSIONS
Our findings suggest that single odorants activate principal cells that are widely distributed in both anterior and posterior parts of the piriform cortex. Although our results do not rule out the presence of a complex, spatially distributed topographical order in the representation of odorant information, they appear to be incompatible with a discrete spatial segregation of principal cells with similar response properties, as observed in the olfactory bulb and primary areas for other systems. We conclude that the coarse patchy organization of afferent input to the piriform cortex suggested by patterns of transneuronal transport derived from single ORs (Zou et al., 2001) becomes obscured at the postsynaptic level as a result of overlap in inputs and the action of associational fibers that interconnect pyramidal cells.
In the olfactory bulb and sensory areas of the neocortex, it appears that the juxtaposition of patches of cells with systematically varying response properties serves to facilitate the extraction of specific stimulus features (e.g., Yokoi et al., 1995; Weliky et al., 1996). The spatially distributed representation of information in the piriform cortex suggests that rather than participating in the extraction of specific features, this system plays a role in recognizing complex combinations of odorant features by which little advantage would be afforded by a segregation of the cells that encode individual features. Studies with artificial networks have revealed that the distributed representation of information can be an efficient strategy for storing and discriminating large numbers of complex patterns (Hertz et al., 1991; Haberly, 2001).
Acknowledgements
The authors thank Susan M. K. Osting for her excellent technical assistance, David Jukam for his work with TMT-exposed animals, and Kevin Neville for valuable discussion.




