Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice
Abstract
S100B, the EF-hand Ca++-binding protein with gliotrophic and neurotrophic properties implicated in the pathogenesis of Alzheimer's disease, is coined as a glial marker, despite its documented presence in rodent brain neurons. We have generated a transgenic mouse whose EGFP reporter, controlled by the −1669/+3106 sequence of the murine S100B gene, allows the direct microscopic observation of most S100B-expressing cells in the central nervous system (CNS). From embryonic day 13 onward, EGFP expression was targeted to selected neuroepithelial, glial, and neuronal cells, indicating that cell-specific expression of S100B is regulated at the transcriptional level during development. In adult mice, the highest level of EGFP expression was found in ependymocytes; astrocytes; and spinal, medullar, pontine, and deep cerebellar S100B neurons. Our results, thus, agree with earlier reports suggesting that S100B is not a CNS glial-specific marker. In addition, we detected EGFP and S100B in forebrain neurons previously thought not to express S100B in the mouse, including neurons of primary motor and somatosensory neocortical areas, the ventral pallidum and prerubral field. Another interesting finding was the selected EGFP targeting to neonatal S100B oligodendrocytes and adult NG2 progenitors as opposed to mature S100B oligodendrocytes. This finding suggests that, except for oligodendrocytes at the last stage of myelin maturation, the −1669/+3106 sequence of the S100B gene is a useful reagent for driving expression of transgenes in most S100B-expressing cells of mouse brain. J. Comp. Neurol. 457:404–419, 2003. © 2003 Wiley-Liss, Inc.
S100B, the small EF-hand Ca++, Zn++, and Cu++ binding protein of the S100 family discovered more than 30 years ago (Moore, 1965), is highly conserved among vertebrates (Allore et al., 1990; Maeda et al., 1991; Jiang et al., 1993). Because S100B is highly expressed in brain and has been implicated in the pathogenesis of neurodegenerative diseases, most S100B functional studies have been performed in glial or mixed glial-neuronal cultures (Sheng et al., 1996; Griffin et al., 1998). Intracellularly, S100B is implicated in the modulation of enzyme activity, energy metabolism, secretion, and cell shape. S100B is also exported from glial cells but lacks a secretion signal sequence and resembles in this respect several other extracellularly active proteins like FGF and amphoterin (Cooper et al., 1990; Muesch et al., 1990). Hence, whether extracellular S100B is internalized or binds to a specific surface receptor is unknown. The secreted S100B homodimer has trophic, as well as pro-apoptotic properties toward neurons and glia maintained in culture (Zimmer et al., 1995; Schäfer and Heizmann, 1996; Heizmann and Cox, 1998; Donato, 1999). However, evidence that S100B subserves these functions in vivo is scarce. S100B null mice exhibit no overt abnormalities except for a higher sensitivity of cerebellar astrocytes to treatment with KCl or caffeine (Xiong et al., 2000; Nishiyama et al., 2002). Moreover, two transgenic mice have been created that overexpress the human (Friend et al., 1992) or the mouse (Reeves et al., 1994) S100B gene. The first one tolerates 100-fold higher than normal levels of S100B without any noticeable phenotype. On the other hand, astrocytosis and axonal sprouting occurs in the hippocampus of the other transgenic mouse, at only twice the normal S100B level.
The evidence for the existence of a robust expression of S100B in brain neurons is convincing for the mouse (Friend et al., 1992) and rat (Rickmann and Wolff, 1995a; Yang et al., 1995, 1996) yet, S100B is coined as a glial-specific marker of the CNS. Moreover, the consensus regarding the late ontogenic expression of S100B in the brain is at odds with several studies reporting the presence of S100B at early stages of prenatal development in several species, including humans (Landry et al., 1989, 1990; Sarnat, 1992; McKanna, 1993). Knowing the exact cellular makeup and spatiotemporal pattern of S100B expression in the CNS of the mouse, which is the mainstay of transgenic technology, is essential for understanding its physiological and pathophysiological roles. To achieve these goals, we have generated a mouse (designated pBG) whose EGFP reporter, controlled by S100B genomic sequences, allows the direct microscopic observation of most S100B-expressing cells in the organism. We have confronted the developmental pattern of EGFP expression to that of S100B in the CNS. The results show that the murine (−1169/+3116) S100B genomic sequence targets EGFP to S100B ependymocytes, astrocytes, oligodendroglial precursors, and specific neurons according to a spatiotemporal pattern that is less restricted than previously thought.
MATERIALS AND METHODS
Animals
Transgenic pBG mice and S100B knock-out mice (Xiong et al., 2000) were housed under standard laboratory conditions in a 12-hour light/dark cycle with access to food and water ad libitum. Experiments were performed according to the principles of laboratory animal care following the guidelines approved by INSERM. Adult animals were deeply anesthetized with sodium pentobarbital and perfused through the ascending aorta with phosphate buffered saline (PBS, pH 7.4) followed by 300 ml of fixative composed of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Mice in the pre- and postnatal stages (from embryonic day [E] 13 to postnatal day [P] 10) were anesthetized by hypothermia and killed by decapitation.
Construction of the transgene
A 4.8-kb S100B promoter-containing sequence (−1669/+3106) was derived from Swiss mouse genomic DNA by polymerase chain reaction (PCR) with the following forward and reverse primers, respectively, 5′-agtcagaattcCACAGGAACCAGAGACTCCTCACCCAAGAC-3′ and 5′-ggctaccggtAGCTGCAGAGGAAGGATACGTTTTCATGTT-3′ (Fig. 1). The PCR was carried out by using the Expand Long Template PCR System (Boehringer Mannheim, Mannheim, Germany) for 30 cycles (94°C for 10 seconds, 66°C for 30 seconds, and 68°C for 3 minutes 30 seconds). The 4.8-kb S100B genomic fragment was subcloned in the TA-cloning pGEM-T Easy Vector (Promega, Madison, WI), retrieved by EcoRI digestion and finally ligated into the pEGFP-1 promoterless expression vector (Clontech Laboratories, Palo Alto, CA) and entirely sequenced on both strands by the method of Sanger. The pBG transgene was excised by double BglII/AflII digestion (New England Biolabs, Beverly, MA) electrophoresed on agarose and extracted-purified with a WIZARD kit (Promega).
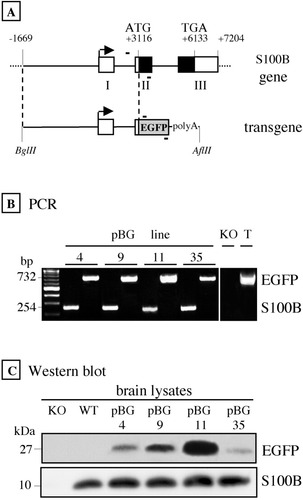
The making of pBG transgenic mice. A: Structure of the S100B gene and pBG construct. Boxed regions represent exons I–III of the murine S100B gene, the solid part corresponding to the protein-coding region. The transgene comprises the −1669 to +3116 S100B gene sequence fused to the EGFP cDNA. B: Polymerase chain reaction (PCR) amplification of EGFP and S100B gene DNA fragments from transgenic tail DNA. The PCR primers (-) are shown in A. The same 732-bp EGFP fragment was amplified from a control plasmid containing the transgene (T) and genomic DNA obtained from F1 mice of lines 4, 9, 11, and 35. As expected, a 234-bp S100B-specific fragment was amplified from transgenic but not S100B null mice (KO). C: Western blot analysis of the brain EGFP content in pBG lines 4, 9, 11, and 35. Overexpression of EGFP (upper blot) has no influence on the endogenous S100B level of pBG mouse brain that is very similar to that of wild-type (WT) mice (lower blot).
Generation of stable pBG transgenic lines of mice
Fertilized (C57BL/6N × DBA2/N) pronuclear-stage embryos were microinjected at the S.E.A.T transgenic facilities of the CNRS (Villejuif, France). Four founders were back-crossed to C57BL/6N mice, and F1 progenies were screened for DNA integration of the transgene in two ways. First, the fluorescence of tail cartilage (adults) or whole skeleton (embryos and newborns) was evaluated under a stereomicroscope (Leica MZ FL-III). Second, tail DNA was PCR amplified, generating one 732-bp EGFP fragment (forward, 5′-GCCACCATGGTGAGCAAGG-3′; reverse, 5′-CAGGAAGTGAGAGAGCTCGT-3′) and one 254-bp S100B-exon-2 fragment (forward, 5′-CTCTCCCTGGTAGGCCTCT-3′; reverse 5′-CAGGAAGTGAGAGAGCTCGT-3′; Fig. 1). PCR reactions were carried out by using DyNAzyme EXT DNA polymerase (Finzymme, Espoo, Finland) for 30 cycles (94°C/1 minute, 65°C/1 minute, 72°C/1 minute 30 seconds) preceded by an initial denaturation step at 94°C for 3 minutes, and followed by a final step at 72°C for 4 minutes. PCR products were electrophoresed on 2% (wt/vol) agarose gel. When the heterozygous progeny of founder 11 was mated to produce homozygous mice, it appeared that two insertion sites segregated independently. All pBG mice are fertile and showed no apparent abnormality in development or behavior upon visual inspection at up to 12 months of age.
Western blot analysis
Total lysates were prepared from frozen brain powder homogenized in 10 mM Tris (pH 7.4), 2 mM ethylenediaminetetraacetic acid, 0.01% phenylmethyl sulfonyl fluoride (Sigma, St. Louis, MO) and 20 μg/ml leupeptin (Euromedex, Souffelweyersheim, France) and spun at 10,000 × g for 5 minutes. The Bradford protocol (Bio-Rad, Hercules, CA) was used for protein quantification. Proteins were electrophoresed under reducing conditions on 11% (sodium dodecyl sulfate-Tris-Tricine) polyacrylamide gels and transferred onto HYBOND C nitrocellulose membranes (Amersham, Les Ulis, France) in a buffer containing 95 mM glycine, 12.5 mM Tris pH 8.3, 10% ethanol, and 5 mM CaCl2. Membranes were first incubated overnight at 4°C with a rabbit anti-human S100B (1:200, Dako, High Wycombe, UK) or a monoclonal anti-GFP antibody (1:1,000, Boehringer Mannheim). Blots were then incubated with peroxidase-linked anti-rabbit (1:6,000, Pierce, Rockford, IL) or anti-mouse (1:4,000, Chemicon, Temecula, CA) IgG conjugates for 1 hour at room temperature. One millimolar CaCl2 was included in all solutions, and immunoblots were revealed by using the BM chemiluminescence blotting substrate (Boehringer Mannheim).
Developmental study
Adult mice were allowed to mate overnight, females were inspected for the presence of vaginal plugs the next morning (E0.5), and pups were born after 19 days (P0). Whole heads or dissected brains were post-fixed for several hours in 4% paraformaldehyde, 0.1 M phosphate buffer, pH 7.4 at 4°C. Tissues were embedded in 7% agarose, and coronal and sagittal Vibratome 50-μm sections were collected and stored in PBS pH 7.5, containing azide at 4°C. To confirm embryonic stages and identify brain structures, we used the Atlas of Mouse Development (Kaufman, 1995) and the Mouse Brain in Stereotaxic Coordinates atlas (Franklin and Paxinos, 2001).
Immunofluorescence procedure
After several rinses in PBS, Vibratome sections were incubated for 48 hours at 4°C with the primary antibodies: rabbit polyclonal antibodies specific for S100B (1:1,000, Dako), glial fibrillary acidic protein (GFAP; 1:1,000, Dako), NG2 (1:500, Chemicon), and monoclonal antibodies against NeuN (1:800, Chemicon) and CNPase (2′,3′-cyclic nucleotide phosphodiesterase; 1:500, Sigma). After three washes in PBS, sections were incubated for 1 hour at RT with the secondary antibodies: anti-rabbit or anti-mouse-IgG CY3-F(ab′)2 conjugates (1:1,000, Jackson ImmunoResearch, West Grove, PA). Primary and secondary antibodies were diluted in PBS containing 0.1% Triton X-100 and 2% BSA. After rinsing in PBS, sections were mounted in Mowiol (Calbiochem, La Jolla, CA) containing 2.5% DABCO (1,4-diazabicyclo-[2.2.2] octane). The specificity of S100B immunolabeling was confirmed (1) on sections of brains and spinal cords of S100B null mice, and (2) with a primary antibody prereacted with recombinant S100B or S100A1.
Macroscopic and microscopic fluorescent images
Macroscopic images were acquired by using a Leica MZ FL-III stereomicroscope equipped with a mercury lamp and a Leica DC300F digital camera. Conventional epifluorescence microscopic images were obtained by using a Zeiss Axiophot 2 microscope equipped with a BP 450-490 band pass filter and a beam splitter FT510 for EGFP and a BP 546/12 band pass filter and a beam splitter FT580 for CY3. Pictures were captured with a Photometrics Coolsnap camera (4,096 gray levels). Nonstacked or stacked confocal laser scanning microscopic images representing optical sections with a depth of field of 3–5 μM or 10–20 μM, respectively, were obtained by using the Bio-Rad 1024 CLSM system equipped with an argon/krypton mixed gas laser. Two laser lines emitting at 488 nm and 568 nm were used for exciting EGFP and CY3-conjugated secondary antibodies, respectively. The background noise of each confocal image was reduced by averaging four image inputs, and green and red images were collected sequentially. Data acquisition and processing was controlled by the Laser Sharp 1024 software and processing system.
RESULTS
Generation of pBG transgenic mice
As shown in Figure 1A, the transgene spans 4.8 kb of a murine S100B gene sequence located 5′ to the translation initiation site and includes 1.7 kb of promoter sequence. This choice was justified by the fact that the natural rostral-to-caudal positive gradient of S100B mRNA is maintained in the brain of transgenic mice overexpressing the murine S100B gene and bearing the same 4.8-kb fragment (Reeves et al., 1994). PCR analysis performed on genomic DNA from F1 mice showed that the transgene had been stably integrated in the genomes of four germline-transmitting founders (Fig. 1B). Western blot analysis revealed the existence of large differences in the EGFP content of brain lysates among the four transgenic lines (Fig. 1C, upper). To ensure that EGFP overexpression does not lead to abnormal expression of the endogenous S100B protein, we compared the S100B level of transgenic mouse brain with that of wild-type mouse. As shown in Figure 1C (lower), the levels of endogenous S100B were found to be very similar in all brain lysates, whether obtained from transgenic or wild-type mice.
Spatiotemporal pattern of EGFP expression in developing pBG mouse brain
To identify EGFP and S100B-positive cells and structures in the embryonic CNS, we used morphologic and topographical criteria (Kaufman, 1995) coupled to classic and confocal laser scanning immunofluorescence microscopy. A series of preliminary experiments were designed (1) to detect potential artefacts attributable to the site of integration of the transgene in the genome, and (2) to select the best suited lines for direct microscopic observation. We found that lines 4, 9, and 11 displayed identical EGFP expression patterns, indicating a lack of effect of the transgene integration site. However, due to the high level of EGFP expression of lines 9 and 11, most of the subsequent work was done in parallel with mice of both lines until we were sure that line 11, displaying the highest level of EGFP expression, presented no abnormality in breeding or during development. The specificity of our immunolabeling method was routinely controlled by including the following positive and negative controls: (1) brain sections of wild-type and S100B null mice, (2) absorption of the S100B antibody with recombinant S100A1 (no effect), or (3) S100B (extinction of the signal).
Non-neural cells.
At E13, the craniofacial, costal, vertebral, limb, and tail cartilages were readily visualized in whole transgenic embryos by virtue of their EGFP content (Fig. 2A). In addition to being strongly expressed in the nasal capsule and septum, EGFP stained olfactory nerve branches from their sites of emergence in the vomeronasal organ and nasal epithelium to the nerve layer of the olfactory bulb (Fig. 2B). The strong fluorescence of an olfactory nerve branch traversing the cribriform plate of an E17 embryo is illustrated in Figure 2C. Based on their shape and location, it is likely that the densely packed EGFP cells of the olfactory nerve are the so-called ensheathing cells. In contrast to astrocytes derived from the embryonic neuroepithelium, the cells of the olfactory bulb nerve layer as well as ensheathing cells of the olfactory nerve are thought to arise from progenitors that migrate out of the olfactory epithelium at early stages of development (Chuah and Au, 1991). Together with chondrocytes, these cells are known to express S100B during embryonic and adult life in several mammalian species including human, rat, and mouse, (Stefansson et al., 1982; Landry et al., 1990; Moiseiwitsch and Lauder, 1997; Astic et al., 1998). We therefore interpreted these results as the first indication that the pBG transgene was expressed in a cell-specific manner during embryonic development.
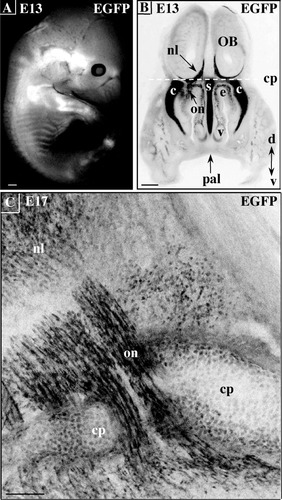
Embryonic S100B promoter activity in non-neural cells. A: Close-up lateral view of the EGFP expression in the cartilaginous skeleton of the embryonic day (E) 13 pBG fetus by using a fluorescence stereomicroscope. B: Negative stereomicroscopic view of an E13 frontal head section featuring the EGFP-positive nerve layer (nl) of the olfactory bulb (OB), cartilaginous nasal capsule (c) and septum (s), and olfactory nerve (on). The plane of the cribriform plate of the ethmoid bone (cp) is represented by a stippled line. e, olfactory epithelium; v, vomeronasal organ; pal, maxillar palatal shelf; d, dorsal; v, ventral. C: Negative composite picture generated from stacked confocal images of an E17 parasagittal head section. EGFP is expressed in the cartilage primordium of the cribriform plate (cp), the ensheathing cells of the olfactory nerve (on), and the cells of the olfactory bulb nerve layer (nl). Scale bars = 500 μm in A,B, 100 μm in C.
Radial Midline Raphe Glial Structure.
Beginning at E10 in the mouse, the embryonic radial glia forms different patterns in different regions of the CNS in relation to the direction followed by the axon bundles (Dupouey et al., 1985; Edwards et al., 1990). The radial processes emitted by the cells located in the ventricular zone, with endfeet terminating at the pial surface, become fasciculated, and this fasciculation is prominent for the embryonic midline raphe glial structure (MRGS), extending from the ventral cervical spinal cord and medulla to the floor of V4 (Edwards et al., 1990). In the pBG mouse, the MRGS was strongly EGFP- and S100B-positive from E16 until P3 (Fig. 3A). As nuclear and cortical structures developed, the trajectories of the fascicles underwent distortions in their initially linear configuration, resulting in the wavy appearance of the midline illustrated in Figure 3A. From P3 to P8, radial glia progressively disappeared, leaving a place for astrocytes (data not shown). From our results, we can conclude that (1) EGFP expression faithfully reflects S100B expression in the mouse MRGS, and (2) the temporal pattern of EGFP/S100B expression in the mouse MRGS is very similar to that previously reported for the rat MRGS (Van Hartesveldt et al., 1986).
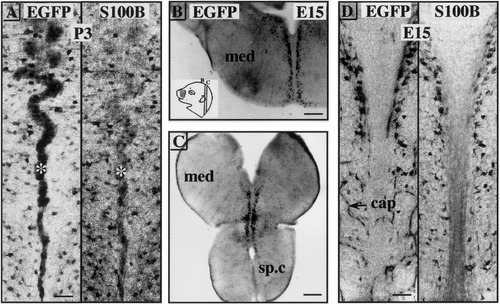
EGFP and S100B gene expression in the midline raphe glial structure (MRGS) and other glial-like cells. A,D: Negative stacked confocal images. cap, autofluorescent capillary. B,C: Negative fluorescent stereomicroscopic images. A: Coronal section through the medullar MRGS at postnatal day (P) 3. EGFP stains both the midline (asterisk) and free cells flanking it, whereas S100B stains only the cells. B,C: Coronal sections through the embryonic day (E) 15 medulla (med) and dorsal half of the cervical spinal cord (sp.c). The EGFP cells are disposed in two columns flanking the midline (inset in B). Scale bars = 50 μm in A,D, 150 μm in B,C.
Macroglia.
In parasagittal sections of E13 heads, we noted the presence of strongly EGFP-positive cells disposed along the ventral portion of the brain and, like the MRGS, extending from the ventral cervical spinal cord and medulla to the floor of V4 (data not shown). Between E13 and E16, their number and level of EGFP expression increased so that we could easily map them on coronal sections. One medial cluster was located at the junction between the midbrain and the pons (data not shown), and two columns of cells flanked the midline in the medulla (Fig. 3B) and cervical spinal cord (Fig. 3C). As shown in Figure 3D, EGFP expression was matched by S100B in all cells. The shape, location, and timing of emergence of the two columns of strongly EGFP-positive cells bordering the midline in the ventral hindbrain correspond to those described for glial precursors of the E12 mouse brainstem that express myelin gene products such as DM-20 (Timsit et al., 1995) and CNPase and MBP (Peyron et al., 1997).
Contrasting with the branched appearance and bright EGFP-based fluorescence of these cells (Fig. 4A), parenchymal glial-like cells with elongated, unbranched cell bodies (Fig. 4B) occasionally seen contacting capillary blood vessels were less easily detected. Between E17 and P3, there was a tremendous increase in the number of perivascular and parenchymal EGFP/S100B cells with mature glial shapes in gray matter (Fig. 4C) as well as white matter tracts like the fimbria (Fig. 4D).
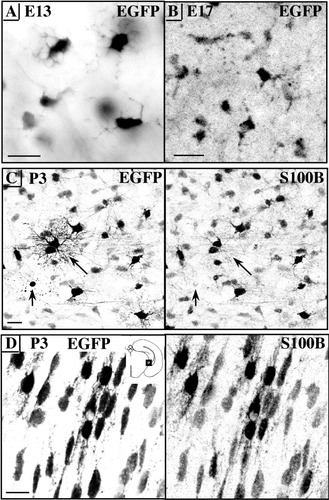
Early onset of macroglial EGFP/S100B expression. A: Negative conventional epifluorescence microscopic image of the first strongly EGFP glial cells flanking the midline raphe glial structure at embryonic day (E) 13 in the ventral medulla. B–D: Negative stacked confocal images. B: At E17, free parenchymal EGFP cells have an undifferentiated appearance. C: At postnatal day (P) 3, EGFP colocalizes with S100B in glial cells. Note the fine arborization of two astrocytes (long arrows) and the apoptotic body (short arrow) more readily visualized through their EGFP content than through S100B staining. D: EGFP/S100B colocalization in the P3 white matter cells of the fimbria. Scale bars = 20 μm in A–C, 10 μm in D.
Classic ependyma and subcommissural organ.
Starting at E13, EGFP stained sharply demarcated zones of the neuroepithelium like the floor of V3 in the hypothalamus (data not shown). EGFP expression then progressively spread to other regions of the neuroepithelium. Figure 5A illustrates the highly overlapping, mosaic-like pattern of EGFP and S100B expression observed in classic ciliated ependymocytes of the lateral ventricles at P3. It is important to note that, although EGFP is not supposed to colocalize with S100B at the subcellular level, it faithfully reproduced the intercellular variations in S100B expression, as illustrated here by the superimposable mosaic patterns of EGFP and S100B in the ependyma. Contrasting with the classic ependymocytes lining V3 and the hypendymal cells overlying them, the subcommissural organ (SCO) ependymocytes were weakly S100B/EGFP positive at P3 (Fig. 5B) and this pattern persisted into adulthood.
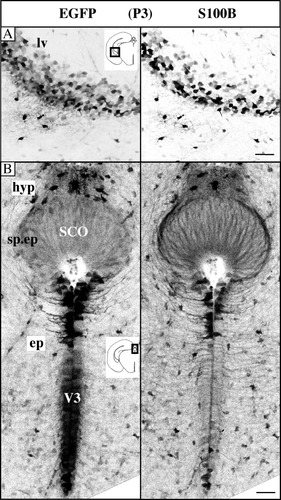
Ependymal EGFP/S100B coexpression at postnatal day (P) 3. A,B: Negative stacked confocal images of a P3 midbrain coronal section. A: EGFP/S100B colocalization in classic ependymocytes of the lateral ventricle (lv). The mosaic pattern reflects intercellular variations in expression. B: EGFP/S100B colocalization in the hypendymocytes (hyp) and specialized ependymocytes of the subcommissural organ (SCO) and V3 classic ependymocytes (ep). Note the over-represented EGFP signal compared with the S100B signal in the V3 region. Scale bars = 50 μm in A, 40 μm in B.
Neurons.
EGFP and S100B started accumulating in CNS neurons during the perinatal period. Due to their low level and diffuse pattern of expression, the precise mapping of neuron nuclei was not possible until a few days after birth. This is illustrated at P3 with the examples of the ambiguus (Fig. 6A) and the mesencephalic trigeminal (Fig. 6B) nuclei. Neuronal EGFP/S100B expression then continued to increase to reach a maximum in the fourth postnatal week.
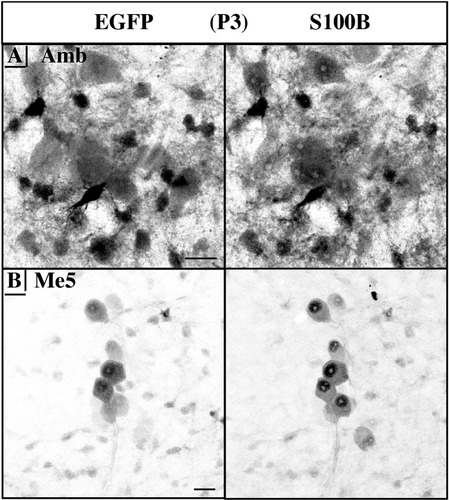
Neuronal EGFP/S100B colocalization at postnatal day (P) 3. A,B: Negative stacked confocal images. A: Ambiguus nucleus (Amb). B: Mesencephalic trigeminal nucleus (Me5). Note the difference in subcellular localization with a stronger nuclear S100B expression and a homogenously diffuse EGFP staining of all subcellular compartments. Scale bars = 20 μm in A,B.
Neuroepithelial EGFP expression and formation of the cerebellum.
At E13, EGFP stained the lateral and isthmal recesses of the V4 neuroepithelium lining the ventral cerebellar anlage (Fig. 7A,B) and at E15, the primary radial glia that provides the substrate for the migration of Purkinje neurons was clearly EGFP positive (Fig. 7C). Elongated cells and radial processes were visible through the entire cerebellar anlage (arrows in Fig. 7C). Between P0 and P1, bipolar EGFP cells accumulated in the presumptive Purkinje cell layer that follows the contours of the cerebellar folia (Fig. 7D). At this stage already, the level of EGFP/S100B colocalization was high (data not shown). Two days later, EGFP cells appeared less densely packed in the Purkinje layer, the EGFP signal being somewhat higher than the S100B signal (Fig. 7E). Within 9–10 days after birth the localization of EGFP in individual S100B Bergmann cells was clearly recognizable and remained present through adulthood (data not shown).
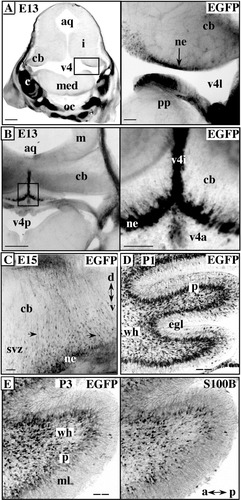
EGFP/S100B expression during histogenesis of the cerebellum (embryonic day [E] 13 to postnatal day [P] 3). A,C: Negative stereomicroscopic views of coronal head sections. A,B: EGFP at E13. A, left: Aqueduct (aq), isthmus (i), rostral part of the cerebellum anlage (cb) at the junction between pons and medulla (med). EGFP is strongly expressed in cochleal (c) and basioccipital (oc) cartilage. A, right: EGFP-positive neuroepithelium (ne) of the ventral cb and posterior pons (pp) in the lateral recess of V4 (v4l). B, left: Midbrain (m), aqueduct (aq), caudal cerebellum (cb), posterior V4 (V4p) and rostral medulla. B, right: Strongly EGFP-positive neuroepithelial cells (ne) located in the isthmal recess of V4 (v4i). C: Close-up view of the E15 cerebellar anlage revealing the concentration of EGFP in the ventral subventricular zone (svz). Radial glial cells extend their EGFP processes (arrows) through the entire cerebellar anlage (cb). D,E: Negative stacked confocal images of cerebellum sagittal sections. D: The presumptive Purkinje cell layer (p) is packed with EGFP-positive cells at P1. wh, white matter; egl, external granule layer containing the premigratory granule neurons. E: EGFP/S100B colocalization in all layers of the cerebellum at P3. Note the thinning of the Purkinje cell layer (P) compared with P1. ml, molecular layer. Scale bars = 500 μm in A left,B left, 100 μm in B right, 50 μm in A right,C, 40 μm in D,E.
EGFP targeting of S100B-expressing glia and neurons in the brain and spinal cord of adult pBG mice
General pattern.
When the brain of an adult transgenic mouse was sagittally sectioned and observed under a fluorescence stereomicroscope, the strongest signal was detected in the brain cavities, cerebellar white matter, and cortical white matter tracts (Fig. 8A). The prominent EGFP expression of the ependyma lining the ventricles, aqueduct, and central canal was confirmed upon examination of brain and spinal cord coronal sections at low magnification (Fig. 8B). From these results, we concluded that EGFP expression faithfully reproduces at least one recognized feature of S100B expression in the adult mouse brain, namely a high expression in the ependyma (De Vitry et al., 1980; Ueda, 1994). In addition to a diffuse EGFP fluorescence that displayed a rostral-to-caudal positive gradient in intensity, lower power microscopic examination revealed the presence of EGFP in motor trigeminal, facial, and deep cerebellar neurons, and the large motoneurons of the spinal cord (Fig. 8B, asterisks). These known features of S100B expression in mouse brain (Cicero et al., 1972; Friend et al., 1992; Reeves et al., 1994) again indicated that the transgene was expressed in a controlled manner.
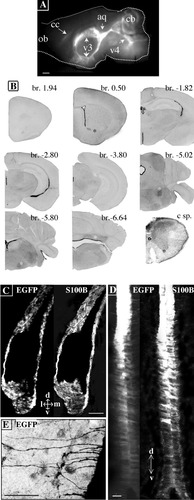
S100B promoter activity is highest in the adult ependyma. A: Fluorescence stereomicroscopic view of a mid-sagittally sectioned brain revealing the strong EGFP fluorescence of the ependyma lining the ventricles. v3, v4, third and fourth ventricles; aq, aqueduct; cb, cerebellum; cc, corpus callosum. B: Negative fluorescence stereomicroscopic views of the coronally sectioned adult pBG brain and cervical spinal cord (c sp.). The position of each coronal section along the anteroposterior axis is indicated by its Bregma point (br.). EGFP expression is strongest in the ependyma lining the ventricles. Other identified EGFP structures are marked by asterisks: the anterior commissure (br. 0.5), motor trigeminal nucleus (br. −5.02), facial (br. −5.8), deep cerebellar nuclei (br. 6.64), and motoneurons of the cervical spinal ventral horn (c sp). C–E: stacked confocal images of the ependyma in brain coronal sections. EGFP/S100B colocalization in classic ependymocytes of the lateral ventricle (C) and in the V3 ependyma (D). Note the dorsoventral gradient of EGFP/S100B expression in V3. E: Negative image of a capillary blood vessel contacted by several long basal processes emitted by V3 ependymocytes. Scale bars = 500 μm in A, 20 μm in C–E.
Classic and modified ependymocytes.
Confocal fluorescence microscopic analysis revealed the high level of EGFP/S100B overlap that prevails in the ependyma and is illustrated for the lateral and third ventricles (Fig. 8C,D). In the latter case, EGFP and S100B steadily decreased from the middle part to the floor of V3. The enwrapping of a blood vessel by the EGFP+ basal processes emitted by several ependymocytes is illustrated in Figure 8E. In agreement with the available data on mouse brain (Chouaf et al., 1989), neither EGFP nor S100B was detected in the choroid plexus epithelium and their respective levels were at the limit of detection in specialized ependymocytes of the subcommissural organ (SCO) located at the junction of the aqueduct and V3 (data not shown).
Astrocytes.
In agreement with the known S100B expression by most types of brain astrocytes (Ludwin et al., 1976; Cocchia, 1981; Didier et al., 1986; Migheli et al., 1999), a high level of EGFP expression was detected in S100B-positive (Fig. 9A) and GFAP-positive (Fig. 9B) astrocytes present in gray and white matter or closely associated with the vasculature (Fig. 9C) and in the cerebellar Bergmann glia (data not shown). Staining for both S100B and EGFP was associated with the nucleus and cytoplasm, cell processes, and endfeet forming the glia limitans (data not shown).
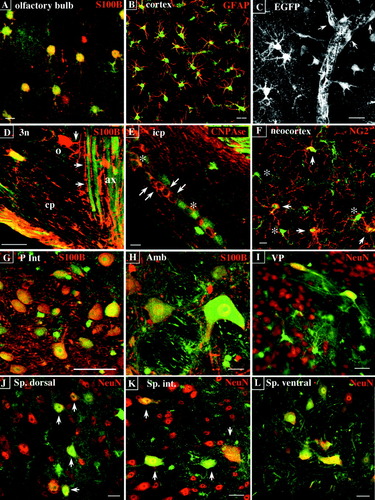
EGFP targeting to S100B macroglia and neurons in the adult pBG mouse. A–H,J–L: Stacked confocal images. I: Conventional epifluorescence image. A–C: Astrocytes. EGFP (green) colocalises with S100B (red) in astrocytes of the external plexiform layer of the olfactory bulb (A) and with GFAP (red) in neocortical astrocytes (B). C: Parenchymal and vascular (arrows) EGFP astrocytes and brain capillary completely ensheathed by EGFP astrocytic processes. D,E: Mature oligodendrocytes. D: EGFP is not expressed in a mature strongly S100B-positive oligodendrocyte (o) and its processes (arrows) ensheathing EGFP axons of the occulomotor nerve (3n). cp, cerebellar peduncle. E: EGFP is not (arrows) or is weakly (asterisks) expressed in CNPase oligodendrocytes (red) of the inferior cerebellar peduncle (icp). The EGFP-positive/CNPase-negative cells intercalated between them may be astrocytes or oligodendroglial precursors. F: EGFP colocalizes with NG2 labelling (red) in neocortical oligodendroglial progenitors. G–I: Brain neurons. G,H: Two examples of neuronal EGFP/S100B colocalization in the posterior part of the interposed cerebellar nucleus (P Int, G) and the ambiguus nucleus (Amb, H). I: EGFP/NeuN colocalization in ventral pallidum neurons (VP) and their long neurites. J–L: EGFP/NeuN colocalization in spinal cord neurons. J: Sensory neurons. K: Interneurons. L: Motoneurons of the spinal cord. Scale bars = 10 μm in A,E,F, 20 μm in B–D,H–K; 30 μm in L; 50 μm in G.
Special case of oligodendrocytes.
Contrasting with the high level of EGFP/S100B colocalization found in white matter tracts of new born mice (Fig. 4D), most oligodendrocytes present in white matter tracts of adult mice expressed high levels of S100B but only few were EGFP positive. A typical example is illustrated in Figure 9D with a mature EGFP-negative/S100B-positive oligodendrocyte and its processes ensheathing EGFP axons of the occulomotor nerve (3n). To distinguish mature cells from oligodendroglial precursors and astrocytes, we used CNPase, a marker of postmitotic myelin-producing cells. We found a reciprocal expression of EGFP and CNPase, strongly CNPase cells being EGFP negative (Fig. 9E, arrows) or weakly EGFP positive (Fig. 9E, asterisks). In contrast, EGFP colocalized with the NG2 integral membrane chondroitin sulfate proteoglycan, that is not expressed in postmitotic cells but persists in adult oligodendroglial progenitors (Levine et al., 1993). As shown in Figure 9F, the NG2 antibody delineated small round or elongated cell bodies and multiple fine branching processes typical of the adult oligodendroglial progenitors scattered in gray and white matter (Reynolds and Hardy, 1997).
Neurons.
Like the rat CNS S100B neurons (Rickmann and Wolff, 1995a), the mouse EGFP neurons are functionally diverse (motor and sensory neurons, interneurons), and display different sizes and shapes. In general, EGFP and S100B colocalized in the cytoplasm and nucleus (Fig. 9G–I) but less often in axons and not at all in the nucleolus where EGFP prevailed. This feature allowed the visualization of entire tracts like the occulomotor nerves (Fig. 10A), the decussation of the superior cerebellar peduncle (Fig. 10B) and the facial nerve, medial longitudinal fasciculus, inferior cerebellar peduncle and sp5 tracts (data not shown). As reported by Friend et al. in 1992, the EGFP/S100B expression was strong in the majority of spinal motoneurons (Fig. 9J–L), medullar, pontine (Fig. 10C–F), and deep cerebellar neuron nuclei but strongly labeled neurons were also found in more anterior regions of the brain like the ventral pallidum and prerubral field (Fig. 10G,H). EGFP was undetectable but, as in the rat (Rickmann and Wolff, 1995a), S100B was present in mouse hippocampal pyramidal neurons (data not shown). Similarly to what was observed in hamster (Tabuchi et al., 1976) and rat (Rickmann and Wolff, 1995a) cerebellum, a weaker EGFP/S100B expression was found in some but not all mouse Purkinje cells (data not shown) and EGFP/S100B expression was variable in cortical neurons known to express S100B in the rat (Rickmann and Wolff, 1995a) like the cortical pyramidal neurons of lamina III and V (Fig. 10I). The EGFP/S100B neurons we could identify according to morphologic, topographical, and molecular (NeuN) criteria, are listed in Table 1, and their topographical distribution along the anteroposterior axis is illustrated schematically in Figure 11.

Strongly EGFP-expressing neurons in the forebrain, midbrain, and hindbrain of adult pBG mice. A–H: Stacked confocal images of EGFP neurons. A,B: EGFP decorates axon bundles of the occulomotor nerve (3n, A) and the axons forming the decussation of the superior cerebellar peduncle (xscp, B). EGFP expression in hindbrain neurons (C,D:), midbrain neurons (E,F:), and forebrain neurons (G,H:), displaying various shapes and expressing high levels of EGFP (compare with astrocytic EGFP signals). I: Conventional epifluorescence image of S100B expression in pyramidal neurons (arrows) of the primary motor cortex (M1). Note the higher nuclear S100B concentration. Gi, gigantocellular reticular nucleus; Me5, mesencephalic trigeminal nucleus; PnO, pontine reticular nucleus, oral part; PR, prerubral field; Tz, nucleus of the trapezoid body; VP, ventral pallidum. Scale bars = 100 μm in B,F, 40 μm in A,C–E, 20 μm in G,I, 10 μm in H.
| Brain | |
| Cortex | |
| Lateral orbital cortex | L0 |
| Secondary motor cortex | M2 |
| Primary motor cortex | M1 |
| Primary somatosensory cortex | S1 |
| Agranular insular cortex | AI |
| Primary somatosensory cortex | S1HL |
| Secondary visual cortex | V2L |
| Lateral enthorinal cortex | Lent |
| Primary visual cortex | V1 |
| Secondary visual cortex | V2ML |
| Subiculum | S |
| Medial enthorinal cortex | Ment |
| Claustrum | Cl |
| Basal ganglia | |
| Ventral pallidum | VP |
| Caudate putamen | CPu |
| Lateral globus pallidus | LGP |
| Medial globus pallidus | MGP |
| Hippocampal formation | |
| Pyramidal cell layer | Py* |
| Oriens layer | Or |
| Dentate Gyrus | |
| Granular layer | GrDG |
| Polymorph layer | PoDG |
| Amigdala | |
| Medial amygdaloid nu, pv part | MePV |
| Amygdaloid hip area, pm part | AHiPM |
| Thalamus | |
| Paracentral thalamic nu | PC |
| Reticular thalamic nu | Rt |
| Ventral postolateral thalamic nu | VPL |
| Laterodorsal thalamic nu, vl part | LDVL |
| Ventrolateral thalamic nu | VL |
| Ventral pm thalamic nu | VPM |
| Posterior thalamic nuclear group | Po |
| Retroethmoid nu | REth |
| Lateral post thalamic nu, lr part | LPLR |
| Suprageniculate thalamic nu | SG |
| Zona incerta | ZI |
| Sub-thalamic nu | STh |
| Substantia nigra, reticular | SNR |
| Prerubral field | PR |
| Red nu | |
| Parvocellular | RPC |
| Magnocellular | RMC |
| Pararubral nu | PaR |
| Superior collicus | |
| Superficial gray layer | SuG |
| Intermediate gray layer | InG |
| Central gray | CG |
| Oculomotor nu | 3 |
| Inferior collicus | |
| Nu of the brachium of the IC | BIC |
| External cortex | ECIC |
| Central nu | CIC |
| Lateral lemniscus | |
| Ventral nu | VLL |
| Intermediate nu | ILL |
| Dorsal nu | DLL |
| Mesencephalic reticular nu | |
| Epimicrocellular | EMi |
| Cuneiform nu | CnF |
| Sagulum nu | Sag |
| Pontine reticular nuclei | |
| Pontine reticular nu, oral part | PnO |
| Paralemniscal nu | PL |
| Ventral tegmental area | VLTg |
| Pontine reticular nu, ventral part | PnV |
| Pontine reticular nu, caudal part | PnC |
| Subcoerulus nu, ventral part | SubCV |
| Parabrachial nuclei | |
| Lateral | LPB |
| Medial | MPB |
| Medullary reticular nuclei | |
| Parvicellular reticular nu, alpha part | PCRtA |
| Gigantocellular reticular nu, alpha part | GiA |
| Gigantocellular reticular nu | Gi |
| Dorsal paragigantocellular nu | DPGi |
| Parvicellular reticular nu | PCRt |
| Ambiguus nu | Amb |
| Superior olivary complex | |
| Rostral periolivary region | RPO |
| Lateral ventral periolivary region | LVPO |
| Mv ventral periolivary region | MVPO |
| Dorsal periolivary region | DPO |
| Lateral superior olive | LSO |
| Superior paraolivary nu | SPO |
| Medial superior olive | MSO |
| Nu trapezoid body | Tz |
| Medial geniculate nu, dorsal part | MGD |
| Cranial nuclei | |
| Mesencephalic trigeminal nu | Me5 |
| Motor trigeminal nu | |
| Peritrigeminal zone | P5 |
| Motor trigeminal nu | Mo5 |
| Supratrigeminal nu | Su5 |
| Accessory trigeminal nu | Acs5 |
| Intertrigeminal nu | I5 |
| Cochlear nu | |
| Ventral, anterior part | VCA |
| Ventral, posterior part | VCP |
| Dorsal | DC |
| Principal sensory | |
| Dorsomedial part | Pr5DM |
| Ventrolateral part | Pr5VL |
| Abducens nu | 6 |
| Facial nu | 7 |
| Accessory facial nu | Acs7 |
| Vestibular nu | |
| Superior | SuVe |
| Medial, parvicellular part | MVePC |
| Medial, mediocaudal part | MVeMC |
| Lateral | LVe |
| Spinal | SpVe |
| Dorsal motor nu of vagus | 10 |
| Spinal trigeminal nu, interpolar part | Sp5I |
| Hypoglossal nu | 12 |
| Raphe nuclei | |
| Interpeduncular nu | IP |
| Dorsal raphe | DR |
| Locus coeruleus | LC |
| Cuneate nu | Cu |
| Gracile nu | Gr |
| Cerebellum | |
| Cerebellar lobules | |
| Purkinje neurons | p** |
| Deep cerebellar nuclei | |
| Interposed cerebellar nu | Int |
| Lateral cerebellar nu | Lat |
| Medial cerebellar nu | Med |
| Vestibulo cerebellar nu | VeCb |
| Spinal Cord | |
| Ventral horn | VH |
| Intermediate zone | IZ |
| Dorsal horn | DH |
- 1 The anatomical nomenclature is derived from The Mouse Brain in Stereotaxic Coordinates by Franklin and Paxinos (2001). The single asterisk indicates undetectable EGFP, and the double asterisk indicates weak EGFP expression. Gigantocellular reticular nu, alpha partMVeMC
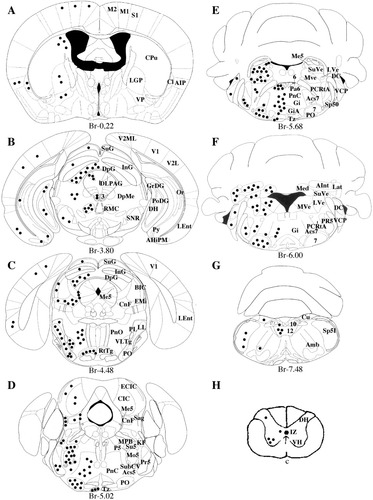
Schematic distribution of S100B neurons in mouse brain and cervical spinal cord. Distribution of EGFP/S100B neurons (black dots) from the anterior to the posterior part of the brain (A–G) and the cervical spinal cord (H). Coronal planes of section according to Franklin and Paxinos (2001). For abbreviations, see Table 1.
DISCUSSION
We have engineered a new transgenic mouse expressing EGFP under the control of S100B gene regulatory sequences and compared the developmental pattern of EGFP expression with that of S100B in the CNS. The results show that the murine (−1169/+3116) S100B genomic sequence targets EGFP in S100B ependymocytes, astrocytes, oligodendroglial precursors, and specific neurons according to a spatiotemporal pattern that is less restricted than previously thought. The high degree of colocalization of EGFP and S100B suggests that selective expression of S100B by specific cell types in the CNS is determined at the level of transcription.
S100B, a late embryonic glial marker
Radial glia.
For the most part, neurogenesis precedes gliogenesis during mammalian brain development; however, one transient specialized cell population, the radial glia, violates this temporal order and proliferates after neural tube closure, between E10 and E17 in the mouse. Macroglial maturation then follows a caudorostral temporal gradient, reaching a peak just before birth (Das, 1979). The earliest EGFP radial structure we could identify was present in the E15 cerebellar anlage. The arrangement of the ventral epithelial EGFP cells and processes (Fig. 11B) provides support for the assumption that they belong to the primordial radial glial scaffold that guides the migration of deep cerebellar and Purkinje neurons to their final destination (Yuasa et al., 1996). Based on our results, the mouse temporal pattern of EGFP/S100B cerebellar expression, thus, appears to be very similar to the rat pattern (Landry et al., 1989).
Oligodendrocytes.
One interesting finding of the present study is the high level of EGFP expression we found in discrete populations of oligodendroglial-like cells at various stages of development. The first EGFP-positive oligodendroglial-like cells appeared at a late embryonic stage (E13). Their shape and disposition (two columns on each side of the midline in the ventral medulla), correspond to those of the presumed oligodendroglial precursors previously observed at E12 in the mouse brainstem and expressing several myelin gene products such as DM-20, CNPase, and MBP (Timsit et al., 1995; Peyron et al., 1997). During the early postnatal period of oligodendrocyte maturation (P3), we noted that EGFP and S100B expressions were closely matched within individual cells of white matter tracts like the fimbria and the corpus callosum, interindividual variations between neighboring cells may be reflecting the asynchronous differentiation and myelination that is characteristic of the postnatal period. In the adult brain, EGFP targeted a population of cells expressing the NG2 chondroitin sulfate marker and displaying the morphologic characteristics of slowly dividing oligodendrocyte progenitors. Like the O-2A cell present in the optic nerve (Raff et al., 1983), the adult NG2 progenitor can differentiate in vitro into type 2 astrocytes or into oligodendrocytes (Wolswijk and Noble, 1989). It is abundant in both gray and white matter and comprises 5–8% of all cells in the adult brain. However, although a high proportion of dividing cortical cells express NG2, the proportion of NG2 precursor cells that belong to the oligodendrocyte lineage is not known (Dawson et al., 2000) and the role of these cells in the adult brain is still debated (Levine et al., 2001). The realization that, although EGFP and S100B levels increase with age, EGFP expression is totally absent from mature oligodendrocytes came as a surprise. Because the same developmental pattern of EGFP expression was observed in oligodendrocytes of lines 4, 9, and 11, we concluded that the pBG construct that functions rather well in oligodendroglial precursors may lack a key regulatory element that is crucial for S100B expression during the last stages of myelin formation. Hence, transcription of the S100B gene may not only be cell type-specific but may require different regulatory elements, depending on the cell type and stage of differentiation.
Although the presence of S100B oligodendrocytes in the CNS of mice overexpressing S100B was not mentioned (Friend et al., 1992; Reeves et al., 1994), and only a minor population of S100B oligodendrocytes was found in the adult rat brain (Rickmann and Wolff, 1995b), our finding of high S100B expression in mature interfascicular oligodendrocytes is corroborated by other reports in the adult mouse (Korr et al., 1994) and rat (Ludwin et al., 1976). In the injured adult vertebrate CNS, oligodendrocytes are thought to prevent axonal regeneration partly by means of the expression of inhibitor molecules like Nogo-66 that bind to specific receptors on the neuronal plasma membrane (Chen et al., 2000; Fournier et al., 2001). On the other hand, the secreted form of S100B is known to promote neurite extension and survival of CNS neurons in vitro (Winningham-Major et al., 1989; Scotto et al., 1998). Hence, the high level of S100B expression we observed during white matter maturation and the S100B-controlled expression of EGFP in adult NG2 oligodendroglial progenitors may have important implications for future studies of demyelinating diseases and axonal regeneration after mechanical injury, excitotoxicity, or viral infection.
Developmental expression of S100B is delayed in neurons compared with glia, but strongly expressing S100B neurons are present in all brain regions of adult mice
In a previous rat study, S100B mRNA expression was first detected at P1 in the mesencephalic trigeminal nucleus, at P3 in the motor trigeminal nucleus, and P7 in the pontine and medullar reticular nuclei (Yang et al., 1996), that is, a few days later than in the pBG mouse (present study). One plausible explanation for this discrepancy could be the higher sensitivity afforded by EGFP/S100B detection compared with the in situ hybridization method used in the rat study. Although our results contradict previous work referring to S100B being excluded from cerebellar, hippocampal, and cortical neurons in the CNS of S100B transgenic mice (Friend et al., 1992; Reeves et al., 1994), they are supported by the comprehensive study on the rat CNS published by Rickman and Wolff (1995a). Moreover, a recent report showing the presence of low levels of S100B mRNA in mouse hippocampal and neocortical pyramidal neurons (Bendotti et al., 2002) lends support to our results and suggest that the traditional view of S100B as a glial-specific marker of the adult rodent brain is no longer tenable.
What can the distribution of S100B in mouse brain tell us about its function(s)?
Several intracellular and extracellular roles have been postulated for S100B in the CNS, based on its gliotrophic and neurotrophic properties (Winningham-Major et al., 1989; Scotto et al., 1998) and interactions with other proteins in vitro (Heizmann and Cox, 1998; Matsumura et al., 1998; Donato, 2001). Up until today, however, and except for the higher sensitivity of cerebellar astrocytes to treatment with KCl or caffeine (Xiong et al., 2000), there is no obvious phenotype associated with targeted inactivation of the S100B gene. Neither axonal regeneration nor adult neurogenesis is affected in S100B null mice (Xiong et al., 2000). In addition, S100B exhibits strong neurite extension activity in cultured serotonergic neurons, and yet, serotonergic neuron development is normal in mice lacking S100B (Nishiyama et al., 2002). Finally, we observed similar rates of motoneuron death and reactive gliosis after section of the facial nerve in newborn and adult S100B null and S100B+/+ pBG mice (V. Vives, M. Dubois Dauphin, A. Marks, and C. Legraverend, unpublished observations). The exact physiological role of S100B, thus, is unknown.
S100B and dynamics of the cytoskeleton.
Like plectin, a major linker and scaffolding protein (Errante et al., 1994; Wiche, 1998), S100B is expressed in neural cells that are rich in intermediate filaments, like vimentin or GFAP in glia (Pixley et al., 1984), and peripherin in brainstem and spinal cord neurons (Parysek and Goldman, 1988; Brody et al., 1989). Double-immunofluorescence analysis revealed that S-100 immunoreactivity is mainly colocalized with GFAP in astrocytes, and microtubular structures in oligodendrocytes (Richter-Landsberg and Heinrich, 1995). S100B has been shown to inhibit the phosphorylation of GAP-43 (Lin et al., 1994), microtubule-associated protein τ (Baudier et al., 1987; Baudier and Cole, 1988), GFAP, and vimentin (Ziegler et al., 1998). It is thought to inhibit microtubule and type III intermediate filament assembly by means of sequestration (Bianchi et al., 1994; Sorci et al., 1998). Hence, one of the main functions of S100B in the CNS could be to regulate the organization of the cytoskeleton.
S100B and the regulation of metal homeostasis and detoxification in the adult mammalian brain.
There is a striking similarity between the distribution pattern of EGFP/S100B expression (present study), that of the heavy metal-binding, cysteine-rich metallothioneins (Choudhuri et al., 1995), and the pattern of accumulation of several xenobiotic metals in rodent brain, including bismuth (Ross et al., 1996) and mercury (Moller-Madsen, 1990). The zones of overlap between EGFP/S100B expression (this study) and bismuth accumulation (Ross et al., 1996) include the ependyma; perivascular and fiber tract astrocytes; neurons in layer V of the motor cortex and the entorhinal cortex; large cranial motor neurons innervating somatic muscle; lateral vestibular, red, pontine/medullary reticular, and sensory nuclei of the subcortical auditory regions (cochlear nuclei, superior olive, and nuclei of the lateral lemniscus); the most conspicuous intraneuronal accumulation of metals being found in the large S100B brainstem motor neurons. What is usually overlooked is that divalent ions other than Ca++ bind S100B in vitro with higher affinity, as is the case of Zn++, which increases the affinity of calcium for S100B and decreases the antagonistic effect of potassium on calcium binding (Baudier et al., 1983, 1986). It is also the case of Cu++, whose sequestration by S100B was shown to prevent Cu++-induced hemolysis of mouse erythrocytes (Nishikawa et al., 1997). Hence, S100B could contribute to the scavenging of toxic substances at the brain-CSF interface (Del Bigio, 1995).
Perspectives
In addition to the neural cell types described above, we have observed EGFP/S100B colocalization in several other cell types known to express S100B: melanocytes, Schwann cells, pituitary folliculostellate cells, dorsal root ganglion cells, sustentacular cells of the adrenal medulla and chondrocytes (V. Vives and C. Legraverend, unpublished observations). Therefore, we believe the pBG mouse will be useful in establishing precise topographical maps of the S100B distribution in most organs. Isolation of selected populations of S100B-expressing cells by virtue of their EGFP expression will afford the possibility to perform transplantation-based cell fate studies, reproducible electrophysiological recordings of EGFP neurons, and functional analysis of genes regulating the differentiation of S100B glial or neuronal precursors. In addition, the −1669/+3106 sequence of the S100B gene could be used for driving expression of other transgenes in most S100B-expressing cells of mouse brain.
Acknowledgements
We thank the Centre Régional d'Imagerie Cellulaire for use of the confocal microscope. We also thank Pr. A. Marks (Banting and Best Institute, Toronto, Canada) for the donation of S100B null mice, G. Salazar for excellent technical assistance, and P. Chavis and V. Perez for their critical comments on the article.




