Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding
Abstract
The homeobox transcription factors Emx1 and Emx2 are expressed in overlapping patterns that include cortical progenitors in the dorsal telencephalic neuroepithelium. We have addressed cooperation of Emx1 and Emx2 in cortical development by comparing phenotypes in Emx1; Emx2 double mutant mice with wild-type and Emx1 and Emx2 single mutants. Emx double mutant cortex is greatly reduced compared with wild types and Emx single mutants; the hippocampus and dentate gyrus are absent, and growth and lamination of the olfactory bulbs are defective. Cell proliferation and death are relatively normal early in cortical neurogenesis, suggesting that hypoplasia of the double mutant cortex is primarily due to earlier patterning defects. Expression of cortical markers persists in the reduced double mutant neocortex, but the laminar patterns exhibited are less sharp than normal, consistent with deficient cytoarchitecture, probably due in part to reduced numbers of preplate and Reelin-positive Cajal-Retzius neurons. Subplate neurons also exhibit abnormal differentiation in double mutants. Cortical efferent axons fail to exit the double mutant cortex, and TCAs pass through the striatum and approach the cortex but do not enter it. This TCA pathfinding defect appears to be non-cell autonomous and supports the hypothesis that cortical efferents are required scaffolds to guide TCAs into cortex. In double mutants, some TCAs fail to turn into ventral telencephalon and take an aberrant ventral trajectory; this pathfinding defect correlates with an Emx2 expression domain in ventral telencephalon. The more severe phenotypes in Emx double mutants suggest that Emx1 and Emx2 cooperate to regulate multiple features of cortical development. J. Comp. Neurol. 457:345–360, 2003. © 2003 Wiley-Liss, Inc.
Proper development of the cerebral cortex is a complex process: cortical neurons must proliferate within internal and external germinal zones, migrate to their proper positions, and differentiate according to a genetic program that determines their correct cell type, layer-specific and area-specific properties, and input and output projections, which reflect their functional specializations (Chenn et al., 1997; O'Leary and Nakagawa, 2002). The homeobox transcription factors, Emx1 and Emx2, are candidates for playing key roles, either independently or cooperatively, in regulating aspects of cortical development (Cecchi and Boncinelli, 2000).
Emx1 and Emx2 expression in the central nervous system is restricted to the forebrain, and almost exclusively to the dorsal telencephalon, where they exhibit nested patterns of expression (Simeone et al., 1992a, b). Emx2 expression begins in the rostrolateral neural plate around E8.5 (Simeone et al., 1992a, b; Shimamura et al., 1995), whereas Emx1 expression begins after neurulation and the onset of telencephalic evagination (∼E9.5). Emx1 expression is restricted to cortical regions, excluding the ventral pallium. Emx2 expression is highest in the cortex, but its expression extends into the ventral telencephalon and also includes a small domain in the diencephalon (Simeone et al., 1992a, b; Guilisano et al., 1996). Within the cortex, Emx1 and Emx2 are expressed in high caudomedial to low rostrolateral gradients (Guilisano et al., 1996; Mallamaci et al., 1998). Their expression includes the medial pallium (hippocampal primordium), dorsal pallium (neocortical primordium), and lateral pallium (olfactory cortex primordium) (Smith-Fernandez et al., 1998; Puelles et al., 2000). Within the medial pallium, Emx2 expression extends closer to the dorsal midline than Emx1 (Tole et al., 2000a, b). Thus, there are some pallial regions where Emx2 is expressed, and Emx1 expression is not detected.
In addition to their expression in progenitor cells in the dorsal telencephalon, Emx1 and Emx2 are also expressed in postmitotic neurons. Emx1 is expressed in the majority of differentiating and mature cortical neurons (Guilisano et al., 1996; Briata et al., 1996) and is restricted to glutamatergic cortical neurons (Iwasoto et al., 2000; Chan et al., 2001), consistent with their generation in the cortical neuroepithelium, whereas γ-aminobutyric acid (GABA)ergic interneurons are generated within the ganglionic eminence, a Emx1-negative domain (Parnavelas, 2000; Corbin et al., 2001; Marin and Rubenstein, 2001; Gorski et al., 2002). In contrast, Emx2 neuronal expression within the cortex is limited to a subset of marginal zone neurons, the Cajal-Retzius cells, between E12.5 and E15.5 (Mallamaci et al., 1998, 2000a).
Analysis of loss-of-function mouse mutants for either Emx1 or Emx2 informs us about some of their functions in forebrain development (reviewed in Cecchi and Boncinelli, 2000). In Emx2 mutants, the cerebral hemispheres and the olfactory bulbs are reduced in size (Pelligrini et al., 1996; Yoshida et al., 1997), and defects are evident in hippocampal and parahippocampal structures (Tole et al., 2000a). Reelin-expressing Cajal-Retzius cells of the marginal layer are absent from about E15.5 onward (Mallamaci et al., 2000a). In addition, changes in the patterning of molecular markers and area-specific connections between the cortex and thalamus suggest that arealization of the neocortex is disproportionately altered in Emx2 mutants in a manner predicted from its graded expression (Bishop et al., 2000, 2002; Mallamaci et al., 2000b). In contrast, Emx1 mutants exhibit relatively modest defects (Qui et al., 1996; Yoshida et al., 1997) dependent on the genetic background of the Emx1 mutation (Guo et al., 2000). Although Emx1 has a graded expression in the cortical neuroepithelium similar to Emx2 (Guilisano et al., 1996), unlike Emx2, Emx1 does not appear to play a role in conferring areal identity to cortical neurons (Bishop et al., 2002).
Because Emx1 and Emx2 are highly homologous (94% in the homeodomain at the amino acid level) and are co-expressed in the cortical neuroepithelium (Simeone et al., 1992a), it is possible that they act redundantly in some aspects of the development of the cerebral cortex and that analyses of Emx single mutants do not reveal the full function of these genes. In the present study, we test this idea by generating Emx1; Emx2 double mutant mice and comparing aspects of their cerebral cortical phenotype with those of Emx single mutants and wild-type mice. Our results demonstrate that defects in cortical development in Emx1; Emx2 double mutants are much more severe than in either single mutant. Emx double mutants exhibit a profound hypoplasia of the cerebral cortex that appears not to be explained by an increase in the death of cortical progenitors or a decrease in their proliferation. In the double mutants, early-born cortical neurons are reduced in number and fail to differentiate normally, leading in part to defects in lamination and in the development of the major input and output cortical projections. Laminar differentiation is abnormal, but expression of cortical markers persisst in the double mutant cortex, indicating that the EMX proteins are not required for this feature of cortical specification. These results suggest that the Emx genes act together in the development of the cerebral cortex and its connections and indicate novel roles for Emx1 and Emx2 in these processes.
MATERIALS AND METHODS
Mice
Emx2 (Pelligrini et al., 1996) and Emx1 (Qiu et al., 1996) mutant, wild-type, and heterozygous mice were generated and genotyped by polymerase chain reaction (PCR) as described. Emx2 mice were maintained on a C57/BL6 background and Emx1 mice on a 129/Sv background. To generate Emx1; Emx2 double mutant embryos, Emx1+/− mice were crossed with Emx2+/− mice. Offspring were genotyped, and Emx1+/−; Emx2+/− and Emx1−/−; Emx2+/− mice were bred with each other to obtain double mutant embryos and their littermates. Emx1 mutant mice are viable and fertile (Qiu et al., 1996). Emx2 mutant mice lack kidneys and a urogenital tract and die within a few hours after birth (Pelligrini et al., 1996; Yoshida et al., 1997). Emx1; Emx2 double mutant mice also die within a few hours after birth. Emx1; Emx2 double mutant embryos and their littermates were obtained at E12.5 for the cortical progenitor proliferation, differentiation, and cell death analyses and at E18.5 for all other analyses. Midday of the day of vaginal plug discovery was considered E0.5. All research and procedures carried out on mice in this study conform to NIH guidelines and have been approved by our Institution's animal care and use committee (Salk IACUC protocol approval #00-028).
Quantification of cortical area
To quantify the dorsal surface area of the cortex of Emx1; Emx2 double mutants and their littermates, E18.5 embryos were perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), and the brains were extracted and postfixed overnight. The brains were then photographed under a Leitz dissection microscope, slide images were digitized, and the dorsal surface area of the cortex was measured using NIH Image. Measurements were made blind to genotype.
Cell proliferation and apoptosis assays
Cortical cell proliferation and cell death assays were conducted at E12.5, early in the peak period of cortical neurogenesis (Bayer and Altman, 1991). To analyze cell proliferation in Emx double mutant cortices, pregnant mothers were given a single injection of bromdeoxyuridine (BrdU; 40 mg/kg I.P.) at 12.5 days of gestation. One hour later, embryos were collected, immersion fixed overnight in 4% PFA/0.1 M PB, cryoprotected in 30% sucrose, and cut in 10-μm sections on a cryostat. Immunohistochemistry was performed as described (see below). Sections were examined using a Leitz microscope connected to a Spot II digital camera. The density of M-phase nuclei was quantified in normal and mutant cortical proliferative zones by counting the number of phospho-Histone H3-positive cells in a 300 × 100-μm area in the lateral wall of the cortex. Cells were counted in sections every 50 μm through the telencephalon, except for the most rostral and caudal portions of the telencephalon, which were not analyzed. Cells in the ventricular zone (VZ) and the subventricular zone (SVZ) were counted separately. TUNEL labeling was performed using the ApopTag Peroxidase In Situ Cell Death Detection kit (Intergen, Purchase, NY). The distribution of BrdU-positive cells within the neocortical proliferative zones was analyzed by counting the number of BrdU-positive cells in a 300 × 50-μm box containing the ventricular zone and part of the subventricular zone of the lateral neocortex of E12.5 mice (see Table 1 legend for further details).
| Emx1+/−; Emx2+/− | Emx1−/−; Emx2+/− | Emx1+/−; Emx2−/− | Emx1−/−; Emx2−/− | Emx1−/−; Emx2−/− | |
|---|---|---|---|---|---|
| No. of BrdU+ cells | |||||
| Within 50 μm of the ventricular surface | 50 | 42 | 43 | 53 | 45 |
| 43 | 38 | 35 | 34 | 45 | |
| 47 | 56 | 34 | 46 | 30 | |
| 41 | 35 | 42 | 43 | 49 | |
| 44 | 37 | ||||
| 40 | |||||
| Mean | 45.2 ± 4 | 42.5 ± 7.3 | 38.5 ± 4.7 | 42.6 ± 7.5 | 42.2 ± 8.4 |
| Along the ventricular surface | 16 | 5 | 13 | 1 | 1 |
| 19 | 4 | 11 | 2 | 2 | |
| 13 | 6 | 12 | 2 | 1 | |
| 13 | 5 | 15 | 2 | 3 | |
| 10 | 6 | ||||
| 8 | |||||
| Mean | 15.2 ± 2.9 | 6.3 ± 2.2 | 12.7 ± 1.7 | 2.6 ± 1.9 | 1.7 ± 0.9 |
- 1 BrdU, bromodeoxyuridine.
- The distribution of BrdU-positive cells within the neocortical proliferative zones was analyzed by counting the number BrdU-positive cells in a box of 300 μm × 50 μm containing the ventricular zone and part of the subventricular zone of the lateral neocortex of E12.5 mice. Two measurements were made: (1) the total number of BrdU-positive cells located within the box; (2) the number of BrdU-positive cells aligned along the ventricular surface (i.e. cells that are either in or near M-phase). The analysis was performed on 4 to 6 coronal sections per brain, at an intermediate anteroposterior level. The results from each section counted, as well as the mean and standard deviation of these data are presented. The “VZ surface counts” in the bottom row are included in the “50 μm” top row. All embryos analyzed are littermates.
Immunohistochemistry and thionin staining
Immusnohistochemistry was performed as described previously (Tuttle et al., 1999; Yun et al., 2001). The antibodies used were as follows: mouse monoclonal anti-βIII tubulin (1:400; Promega, Madison, WI) used 1:400; rat monoclonal anti-BrdU (1:10; Harlan, Indianapolis, IN); rabbit polyclonal anti-calretinin (1:1,000, E12.5 embryos; Chemicon, Temecula, CA); rabbit polyclonal anti-calretinin (1:2,000, E18.5 embryos; Swant, Bellinzona, Switzerland); rabbit polyclonal anti-L1 (1:2,000; a gift of C. Lagenauer, University of Pittsburgh; Pittsburgh, PA); rabbit polyclonal anti-phospho-Histone H3 (1:400; Upstate Biotechnology, Lake Placid, NY) used 1:400; mouse monoclonal anti-Reelin G10 (1:500; a gift of A. Goffinet, University of Louvain Medical School, Brussels, Belgium); and rabbit polyclonal anti-TBR1 C1 (1:100; a gift of M. Sheng, Howard Hughes Medical Institute, Massachusetts Institute of Technology; Cambridge, MA). For immunofluorescence, Hoechst (Aldrich Chemicals, Milwaukee, WI) and thionin counterstaining were performed as described previously.
In situ hybridization
For in situ hybridization on sections, brains were fixed with 4% PFA/0.1 M PB, cryoprotected with 30% sucrose in PFA/PB, and cut at 20 μm in the sagittal plane on a cryostat. In situ hybridization using 35S-labeled riboprobes and counterstaining with bisbenzimide was performed as described (Liu et al., 2000). The following riboprobes were synthesized from cDNA templates: Emx1 (a gift from J. Chun, University of California, San Diego; La Jolla, CA); Emx2, Cad6 (a gift from S. Mah and C. Kintner, The Salk Institute; La Jolla, CA); Id2 (a gift from M. Israel, Laboratoire de Neurobiologie Cellulaire et Moleculaire, C.N.R.S.; Gif-sur-Yvette, France); RZRβ (a gift from M. Becker-Andre, Serono Pharmaceutical Research Institute S.A.; Plan-les-Ouates, Switzerland); p75 (a gift from K.-F. Lee, The Salk Institute, La Jolla, CA); ephrin-A5, and Lhx2 (a gift from L. Jurata, The Salk Institute, La Jolla, CA).
Tract tracing
E18.5 embryos were collected and perfused with 4% PFA/0.1 M PB. Brains were extracted and postfixed overnight. Axonal projections between the thalamus and cortex were labeled using crystals of the lipophilic dye 1,1′dioctadecyl-3,3,3′tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) (Honig and Hume, 1989a, b). A single crystal of DiI was centered either in the occipital cortex or the dorsal thalamus in littermate sets of Emx double mutant and wild-type embryos. Care was taken to equate crystal size and placement between sets of brains. For all cortical injections, care was taken to place the dye crystal in the cortical plate and not in the underlying subplate where the axon pathway lies. Brains were stored for 2 weeks at 30°C in fixative to allow for tracer diffusion. They were then sectioned sagitally at 100 μm on a vibratome, mounted in 4% PFA/ glycerol, and visualized and photographed with a Nikon fluorescent microscope. Placement and sizes of injection sites were verified in sections counterstained with bisbenzimide (Sigma, St. Louis, MO).
Figure preparation
Figures were prepared from digital images using Adobe Photoshop software and printed on a Fujix printer. Color adjustments were done to match the actual color of the material. Adjustments of contrast and brightness levels were also done to match the actual material. All adjustments were applied equally to all figures of the same type of material. No artifacts were removed from the images.
RESULTS
Disproportionate compensation between Emx1 and Emx2 in the development of the cerebral cortex and olfactory bulbs
We generated Emx double mutants by intercrossing the Emx1 and Emx2 single mutants. Emx1; Emx2 homozygous double mutants die a few hours after birth, as do Emx2 homozygous mutants (Pelligrini et al., 1996; Yoshida et al., 1997). Therefore, our late-stage analyses were done at late embryonic stages (E18.5), with the mice removed shortly before birth from pregnant Emx1 or Emx2 heterozygous females bred to Emx2 or Emx1 heterozygous males, respectively. Comparisons were done between littermates, blind to genotype. Although the Emx double mutant phenotype is more severe than either Emx1 or Emx2 single mutants, we did not observe major dosage effects in the compound heterozygotes; thus, no clear phenotype differences were observed among Emx1+/+; Emx2+/+, Emx1+/−; Emx2+/+, Emx1+/+; Emx2+/−, and Emx1+/−; Emx2+/− mutants (pooled as wild type), between Emx1−/−; Emx2+/+ and Emx1−/−; Emx2+/− mutants (pooled as Emx1 mutants), or between Emx1+/+; Emx2−/− and Emx1+/−; Emx2−/− mutants (pooled as Emx2 mutants).
At E18.5, the cortices of Emx double mutants are greatly reduced in size compared with wild types, Emx1 mutants, and Emx2 mutants (Fig. 1). Emx double mutant cortices are always smaller than single mutants; the cortex is reduced both in surface area (Fig. 1D) and in radial thickness (Fig. 2A′), although the penetrance of the reduction is variable (Fig. 1D illustrates an average example, and Fig. 2A′ is a fairly robust example, but the cortices of others are even smaller). Quantification of cortical dorsal surface area at E18.5 (Fig. 1E) indicates no significant difference between wild types (0.080 ± 0.008 cm2; Fig. 1A) and Emx1 mutants (0.078 ± 0.006 cm2; Fig. 1B). Emx2 mutant cortices (0.057 ± 0.005 cm2; Fig. 1C) are significantly reduced in size compared with wild type (P < 0.0001) by about one-third, similar to that reported elsewhere (Bishop et al., 2000). At E18.5, Emx double mutant cortices (0.044 ± 0.002 cm2; Fig. 1D) are significantly reduced in size compared with wild types and Emx1 mutants (P < 0.0001), being about one-half the size, and with Emx2 mutants (P < 0.001), being about three-fourths the size. These results indicate that Emx1 and Emx2 function together in regulating cortical size.
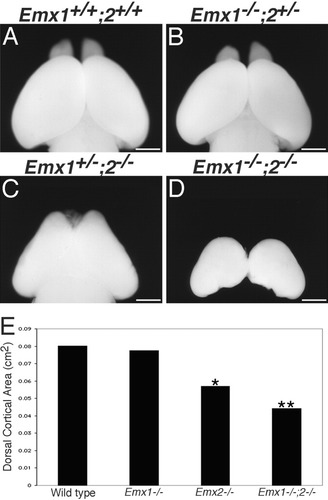
Cortices of Emx double mutants are significantly reduced in size. Dorsal surfaces of the cortex of E18.5 brains. Wild-type (A) and Emx1 (B) mutant cortices are similar in size. Emx2 mutant cortices (C) are slightly reduced and Emx double mutant cortices (D) are severely reduced in size compared with wild types. E: Quantification of dorsal cortical surface area. Emx2 mutant cortices are significantly reduced in size compared with wild types (*, P < 0.0001) and Emx double-mutant cortices being significantly reduced in size compared with wild types (P < 0.0001) and with Emx2 mutants (**, P < 0.001). See Results section for SEM values. Scale bars = 1 mm.
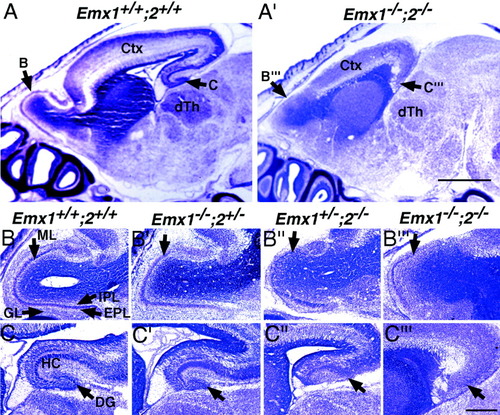
Developmental defects in Emx double mutants are confined to the dorsal telencephalon. Thionin-stained sagittal sections at E18.5 show that defects in Emx double mutants (A′) are confined to the dorsal telencephalon, compared with wild types (A). Sagittal sections through the olfactory bulbs illustrate the normal morphology and lamination of the olfactory bulbs in wild types (B). GL, glomerular cell layer; EGL, external granule cell layer; ML, mitral cell layer; IGL, internal granule cell layer. B′: Olfactory bulb morphology and lamination appear normal in Emx1 mutants. B″: Emx2 mutant olfactory bulbs show disorganized lamination, and outer layers (arrow marks the ML) are thinner than in wild-type olfactory bulbs. B‴: Emx double mutant olfactory bulbs are greatly reduced in size and show poor lamination. Arrow indicates the ML, which is very sparsely populated compared with wildtype. C: Sagittal section through the hippocampus (HC) and dentate gyrus (DG) shows their normal morphological appearance in wild-type brains. C′: In Emx1 mutants, the hippocampus appears relatively normal, but the dentate gyrus may be slightly reduced in size. C″: In Emx2 mutants, the dentate gyrus is morphologically absent and the hippocampus is reduced in size. C‴: In Emx double mutants, both the hippocampus and dentate gyrus are morphologically absent. Scale bars = 500 μm in A,A′; 250 μm in B–B‴,C–C‴.
Thionin-stained sagittal sections at E18.5 show that morphological defects in Emx double mutants are principally confined to the dorsal telencephalon, which is greatly reduced in length and thickness, and olfactory bulbs (Fig. 2). The ventral telencephalon, diencephalon (including thalamus), midbrain, and hindbrain regions appear grossly normal (compare Fig. 2A with A′).
The olfactory bulbs in E18.5 Emx double mutants are reduced in size and severely disorganized compared with wild types (Fig. 2B), Emx1 mutants (Fig. 2B′), and Emx2 mutants (Fig. 2B″). The mitral cell layer, external plexiform layer, and glomerular layer of the olfactory bulbs of Emx double mutants are thin and poorly organized, and the internal plexiform layer appears thickened (Fig. 2B‴). Preservation of Id2 expression provides evidence that mitral cells are produced (Fig. 3A,A′), and preservation of Cad6 expression provides evidence that some external granule layer cells are produced (Fig. 3B,B′). L1 expression indicates that the olfactory tract and the glomerular layer, which contains arborizations of the axons of the olfactory tract, are deficient in Emx double mutants (Fig. 3C,C′). Emx1 mutants exhibit no defects in olfactory bulb development (Yoshida et al., 1997; Fig. 2B′). In Emx2 mutants, the olfactory bulbs are slightly reduced in size and the mitral cell layer is disorganized (Pelligrini et al., 1996; Yoshida et al., 1997; Fig. 2B″).
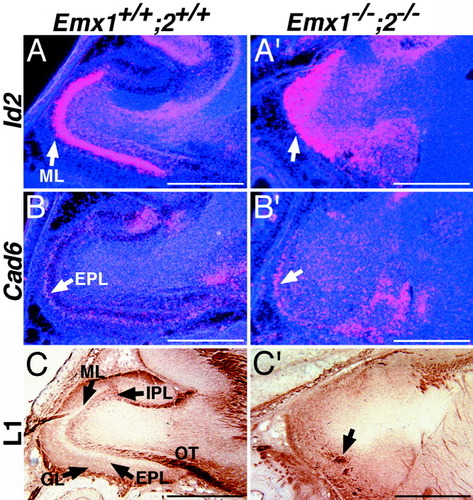
Expression of markers of olfactory bulb cell types and lamination in wild-type and Emx double mutants. In situ hybridization for Id2 and cadherin6 (Cad6) and immunohistochemistry for L1 on sagittal sections through the Emx1; Emx2 wild-type and double mutant olfactory bulb at E18.5. A: Id2 expression labels the mitral cell layer (ML) in wild-type olfactory bulbs. A″: Preservation of Id2 expression (arrow) provides evidence that mitral cells are produced in Emx double mutant olfactory bulbs but are poorly laminated. B: Cad6 expression labels the external plexiform layer (EPL) in wild-type olfactory bulb. B′: Preservation of Cad6 expression provides evidence that some external plexiform layer cells are produced (arrow) in Emx double mutant olfactory bulbs. C: L1 expression is strongest in the olfactory tract (OT) and glomerular layer (GL) of wild-type olfactory bulbs. C′: Absence of strong L1 staining in Emx double knockout olfactory bulbs indicates that the formation of the olfactory tract and the glomerular layer, where fibers of the olfactory tract terminate, is disrupted. Scale bars = 500 μm.
The extent of defects exhibited in the hippocampal formation of Emx double mutants is greater than in either Emx1 or Emx2 single mutants (Fig. 2C–C‴). In Emx1 mutants, the hippocampus is slightly smaller than in wild types (Yoshida et al., 1997; Fig. 2C′). In Emx2 mutants, the hippocampus is usually greatly reduced in size and a morphologically identifiable dentate gyrus is missing, although molecular features of dentate granule cells and hippocampal fields are present (Tole et al., 2000a; Fig. 2C″). However, in Emx double mutants, both the hippocampus and dentate gyrus are morphologically absent (Fig. 2C‴).
The histological defects in cortical structures are correlated with abnormalities in the major telencephalic commissures. The corpus callosum and hippocampal commissure are severely reduced in size or absent, and the anterior commissure is present but reduced in size and disorganized in Emx double mutants (data not shown).
In conclusion, the Emx double mutant has more substantial defects in forebrain development than either Emx single mutant, and the degree of a specific defect observed in Emx1 and Emx2 single mutants varies substantially from one another and from the double mutant. This finding indicates that Emx1 and Emx2 compensate for the loss of one another, but in a disproportionate manner.
Early growth of the cerebral cortex is reduced in Emx double mutants
The reduction in cortical size of Emx double mutants at E18.5 suggests that Emx1 and Emx2 may cooperate to regulate the rate of cell death and/or proliferation of cortical progenitors. To evaluate this hypothesis, we examined cortical size, as well as cell death and proliferation in the dorsal telencephalon in E12.5 embryos, a stage of robust neocortical neurogenesis when virtually all preplate neurons have been generated and the generation of cortical plate neurons is beginning. At E12.5, the size of the Emx double mutant cortex is already greatly reduced compared with wild types and Emx single mutants (Fig. 4A–A″″). Furthermore, a mediolateral gradient in severity is apparent. The medial cortical wall, which includes the primordium of the hippocampus, is almost absent in most double mutant embryos (Fig. 4A‴) and is not detected in the most severe cases (Fig. 4A″″). The lateral cortical wall is less affected, although it too is greatly reduced in size (Fig. 4A–A″″). As at E18.5, the penetrance of the hypoplasia is variable at E12.5 (compare Fig. 4A‴ with ″″). We detected no difference in the incidence of TUNEL labeling among E12.5 cortices of wild types, single mutants, or Emx double mutant embryos (data not shown), suggesting that the hypoplasia in the double mutants is not due to an exaggerated loss of cortical progenitors.
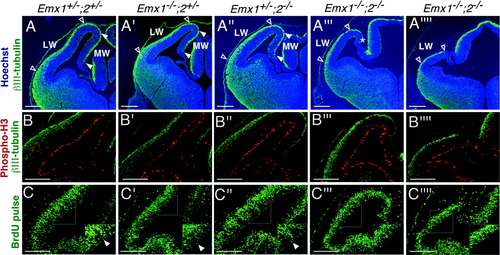
Relative sizes of cortical wall and markers of cell proliferation and cycle in early embryonic wild-type mice and Emx mutants. Immunohistochemistry on coronal sections through the telencephalon of E12.5 embryos. A–A″″: βIII-tubulin immunohistochemistry (green) and Hoechst counterstaining (blue). B–B″″: Phospho-Histone H3 (red) and βIII-tubulin (green) immunohistochemistry. C–C″″: BrdU immunohistochemistry (green). Sections from two different Emx double mutant embryos are shown, one with a mild phenotype (A‴,B‴,C‴) and another with a severe phenotype (A″″,B″″,C″″). In A–A″″, Open arrowheads delimit the lateral wall of the telencephalic vesicles and solid arrowheads the medial wall of the telencephalic vesicles. The lateral wall of the telencephalic vesicles is reduced in size in Emx double mutants (A‴,A″″) compared with Emx single mutant (A′,A″) and wild-type embryos (A). The medial wall of the telencephalic vesicles is severely reduced in size (* in A‴) or absent (A″″) in Emx2 double mutant embryos. A superficial layer of postmitotic cells (green anti-βIII tubulin-immunoreactive) is evident in all genotypes (A–A″″). In B–B″″, the distribution and density of phospho-Histone H3-positive cells (red) in the ventricular zone (VZ) and subventricular zone (SVZ) are not notably affected by either single or double Emx mutations. No significant difference in the number of phospho-Histone H3-positive cells in the cortical VZ is observed between genotypes: (wild types, 20.5 ± 1.7 cells [n = 3]; Emx1 mutants, 19.8 ± 2.1 cells [n = 3]; Emx2 mutants, 21.5 ± 0.9 cells [n = 2]; Emx double mutants, 21.2 ± 1.5 cells [n = 3]), nor was a significant difference observed in the number of phospho-Histone H3-positive cells in the cortical SVZ between genotypes (wild types, 4.4 ± 0.9 cells [n = 3]; Emx1 mutants, 4.3 ± 1.0 cells [n = 3]; Emx2 mutants, 4.6 ± 0.6 cells [n = 2]; Emx double mutants, 3.7 ± 0.8 cells [n = 3]). In C–C″″, the overall density of BrdU-positive cells (green) labeled by a 1-hour pulse in the VZ and SVZ was not clearly affected in Emx single mutants. However, as shown in the higher magnification views (boxes located in the lower right corners of C–C″″), a reduction in the number of BrdU-positive cells located near the ventricle (solid arrowheads) was observed in Emx double mutant embryos. LW, lateral wall; MW, medial wall. Scale bars = 200 μm.
We examined cortical cell proliferation by immunohistochemistry with an anti-phospho-Histone H3 antibody that labels cells in the M-phase of mitosis and by a brief BrdU pulse to label cells in the S-phase. Counts of phospho-Histone H3-positive cells in the cortical VZ and SVZ indicate that Emx deficiency does not significantly affect the density of mitotic cells in the E12.5 cortex (Fig. 4B–B″″; see figure legend for quantification). No significant difference in the number of phospho-Histone H3-positive cells in the cortical VZ is observed between the genotypes (Fig. 4; see figure legend for quantification)
A 1-hour pulse of BrdU, which labels cells in the S-phase, does not reveal a major change in the density of proliferating cells in the neocortical VZ and SVZ of E12.5 Emx double mutants compared with wild-type or intermediate mutant genotypes (Fig. 4C–C″″; Table 1). However, in Emx double mutants, we observed a reduction in the number of BrdU-positive cells that have their labeled nucleus near the ventricular surface (insets in Fig. 4C–C″″; Table 1). These results suggest that Emx mutations, single and double, do not affect the total number of BrdU-positive cells, whereas they imply that in Emx double mutants fewer BrdU-positive cells move to the ventricular surface within 1 hour of the BrdU pulse. This finding suggests a change in cell cycle kinetics in the cortical VZ of Emx double mutants, in particular a lengthening of the time in movement from the S-phase to M-phase, and therefore possibly an overall lengthening of the cell cycle. However, additional data would be required to make these findings conclusive.
Defects in cortical lamination and subplate in Emx double mutants
Comparisons of thionin-stained sagittal sections of E18.5 brains show that the cerebral cortex of Emx double mutants is abnormally thin and exhibits abnormalities of its laminar architecture (Figs. 2A,A′, 5A–A‴). In the double mutants, the marginal zone is thinner, the subplate is difficult to discern, and the immature cortical plate appears relatively homogeneous and lacks its nascent laminar differentiation of deeper layers. In addition, the intermediate and subventricular zones are also thinner. The double mutant defects are more severe than in the Emx1 and Emx2 single mutants (Fig. 5A′,A″)
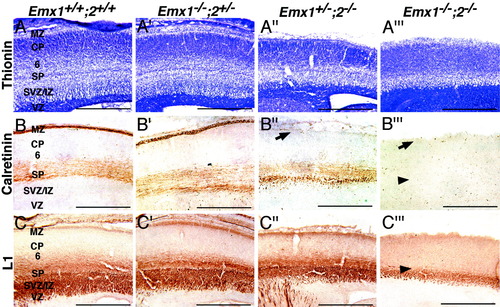
Cortical lamination is defective at late stages of embryonic cortical development in Emx double mutants. Thionin staining and immunohistochemistry on sagittal sections through the E18.5 somatosensory cortex. A–A‴: Thionin staining. B–B‴: Calretinin immunohistochemistry. C–C‴: L1 immunohistochemistry. A: In wild-type cortex, thionin staining illustrates the differential cellular composition of the layers of the cortex, including the cell-sparse marginal zone (MZ), the varying cellular densities of layers forming in the developing cortical plate (CP), including layer 6 (6), which is differentiated from the cortical plate, the cell-dense subplate (SP), the lightly stained intermediate and subventricular zone (SVZ/IZ), and the very darkly stained ventricular zone (VZ). A′: Laminar morphology of the Emx1 mutant cortex is similar to that of wild type. A″: The Emx2 mutant cortex is reduced in size, but laminar morphology appears normal. A‴: The morphological cellular composition of the Emx double mutant cortex is more homogeneous than that of wild type (compare with A). A cell-sparse MZ is evident, but it appears thin and disorganized. The CP is cellularly homogeneous, and the SP is sparsely populated. The IZ/SVZ is thin, but the VZ appears unchanged compared with wild type. B: Calretinin is a marker for preplate-derived MZ and SP cells in wild-type E18.5 cortex. B′: Calretinin staining is unchanged in Emx1 mutant cortices compared with wild types. B″: In Emx2 mutants, calretinin staining is present in the SP but is absent from the MZ (arrow). B‴: Calretinin expression is absent from both the MZ (arrow) and SP (arrowhead) in Emx double mutant cortices. There also appears to be an increase in calretinin-positive cells scattered through the CP of Emx double mutants. C: In wild-type cortices, L1 staining is concentrated in the IZ and the SP coincident with the paths of cortical efferents and thalamocortical axons (TCAs), respectively. Light diffuse staining is present dorsal to the SP where TCA branches begin to invade the CP. Cortical L1 staining is normal in Emx1 mutants (C′) and Emx2 mutants (C″). C‴: In Emx double mutant cortices, L1 staining is absent from the SP (arrowhead) and deeper cortical layers, being present only in the IZ/SVZ. Scale bars = 500 μ`m.
The substantial laminar defects in the Emx double mutants may be due in part to a loss of Cajal-Retzius neurons, which are required for the proper migration and settling of cortical neurons. To assess this issue, we used calretinin immunohistochemistry at E18.5. Calretinin is a marker for preplate-derived neurons, including Cajal-Retzius neurons, which are found in the marginal zone at E18.5 (del Rio et al., 1995; Alcantara et al., 1998), and subplate neurons (Fonseca et al., 1995; Zhou et al., 1999) (Fig. 5B). Calretinin staining is unchanged in Emx1 mutant cortices compared with wild types (Fig. 5B,B′). In Emx2 mutants (Fig. 5B″), cortical calretinin staining is present in the subplate but is absent from the marginal zone. This is consistent with the loss of Reelin-positive Cajal-Retzius cells in the marginal zone from about E15.5 onward in Emx2 mutants (Mallamaci et al., 2000a). Calretinin expression is absent from both the marginal zone and subplate in Emx double mutants (Fig. 5B‴). These defects are correlated with an apparent increase in calretinin-positive cells scattered through the cortical plate of Emx double mutants (Fig. 5B‴). This may be due to an ectopic positioning of preplate cells within the cortical plate, or, alternatively, an upregulation of calretinin expression in cortical plate cells.
Analysis of L1 immunohistochemistry provides additional support for the hypothesis that subplate cells fail to differentiate normally in Emx double mutants. In E18.5 wild-type cortices (Fig. 5C), L1 staining is concentrated in the intermediate zone, due mainly to L1 labeling of cortical efferent axons that accumulate in it and extend toward the internal capsule, and in the subplate, due in part to the labeling of thalamocortical axons (TCA), whose pathway is coincident with the subplate; lighter and more diffuse L1 staining is seen in the deep cortical plate (layer 6), due to its invasion by TCA branches (Chung et al., 1991; Fukuda et al., 1997). The pattern of cortical L1 staining appears normal in Emx1 mutants (Fig. 5C′) and Emx2 mutants (Fig. 5C″). However, in Emx double mutants, L1 staining is present in a layer that resembles the intermediate zone, but staining that is characteristic of the subplate and deep cortical layers is absent (Fig. 5C‴). This finding suggests a defective TCA projection, which we address in later sections.
Laminar expression of cortical markers
To address the extent to which different cortical cell types are specified in the Emx double mutant and to address further the issue of lamination, we performed an in situ hybridization analysis of a panel of five genes representative of a broad range of classes (Cad6, Id2, RZRβ, p75, and ephrin-A5; Fig. 6). These markers are normally expressed in layer-specific patterns, with each gene being expressed in a specific subset of layers. For example, Cad6 is primarily expressed in layers 4 and 5, Id2 is broadly expressed, RZRβ is primarily expressed in layer 4, p75 is primarily expressed in the subplate and layer 6, and ephrin-A5 is primarily expressed in layers 2–5 and the subplate. In Emx double mutants, the laminar expression patterns of these markers show a variable degree of diffuseness in the cortical plate relative to wild type, but their laminar patterns are not grossly different from those of wild type. Although these findings provide additional evidence for defects in cortical lamination in Emx double mutants, they also indicate that laminar molecular specification is at least partially intact even in the absence of both EMX1 and EMX2. In addition, the fact that these cortical markers are expressed in the reduced neocortex of Emx double mutants indicates that the Emx genes are not required to specify cortical neuronal fate per se, although our findings clearly show that they are required for determining cortical size, that is, the amount of tissue that will exhibit a cortical fate.
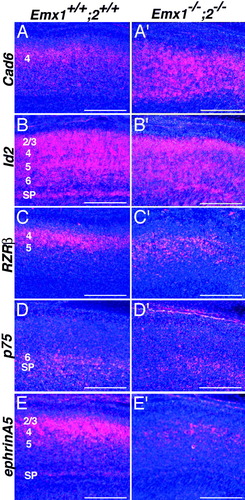
Laminar patterns of gene expression in wild-type and Emx double mutant neocortex. In situ hybridization for markers of cortical lamination on sagittal sections through wild-type (Emx1+/+; Emx2+/+) and Emx double mutant cortex (Emx1−/−; Emx2−/−) at E18.5. Genes that exhibit layer-specific patterns of expression in wild-type mice are also expressed in Emx double mutant neocortex, but in more diffuse patterns. Because the Emx double mutant cortex shows shifts in cortical area identity similar to those in Emx2 mutants (Bishop et al., 2002), sections are matched for area identity. Cadherin6 (Cad6) (A,A′), Id2 (B,B′), RZRβ (C,C′), p75 (D,D′), and ephrin-A5 (E,E′) are all expressed in the Emx double mutant cortex but in more diffuse laminar patterns. Scale bars = 500 μm.
Differentiation of preplate derivatives require EMX1 and EMX2 function
To determine whether defects in the differentiation of Cajal-Retzius and subplate neurons are evident at early stages in their development, we examined the differentiation of preplate neurons at E12.5, by which age virtually all are normally generated. For this analysis, we did double labeling of cortical sections using antibodies to βIII-tubulin and the transcription factor TBR1. βIII-tubulin is a general neuronal marker that is expressed early on in neuronal differentiation, whereas TBR1 expression in the cerebral cortex is largely limited to projection neurons, and therefore its expression is enriched in the population of early-born cells, which will form the deep layer projection neurons (Hevner et al., 2001). Reducing the dose of Emx gene expression leads to a progressive decrease in the thickness of the layer of βIII-tubulin/TBR1-positive cells that form the preplate (Fig. 7A–A″″). The reduction in preplate thickness correlates with the severity of the cortical size defect, being most severe in embryos with the smallest cortical surface area (Figs. 4A″″, 7A″″). The preplate layer also appears somewhat reduced in thickness in cortices of Emx2 mutants (Fig. 7A‴), whereas Emx1 mutants are indistinguishable from wild types (Fig. 7A,A′). Thus, although the number of preplate neurons is reduced in Emx double mutants, some aspects of their differentiation appear to be intact based on their expression of the transcription factor TBR1 (Fig. 7A–A″″), which is required for the proper differentiation of early-born cortical neurons (Hevner et al., 2001).
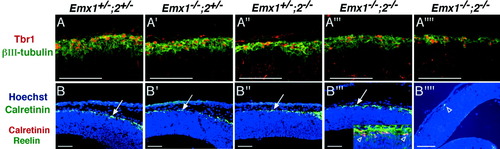
Expression of markers for the differentiation of preplate neurons in early embryonic neocortex of wild-type mice and Emx mutants. Immunohistochemistry on sagittal sections through the telencephalon of E12.5 embryos. A–A″″: TBR1 (red) and βIII-tubulin (green) immunohistochemistry. B–B″″: Calretinin immunohistochemistry (green) and Hoechst counterstaining (blue). Box in lower right corner of B‴: Calretinin (red) and Reelin (green) immunohistochemistry and Hoechst counterstaining (blue). Sections from two different Emx double mutant embryos are shown: A‴ and B‴ show an embryo with a mild phenotype; A″″ and B″″ show an embryo with a severe phenotype. A–A″″: Early born cortical neurons express βIII-tubulin (green) and TBR1 (red) in Emx double mutants embryos, although their number is greatly diminished, as indicated by the reduced thickness of the preplate (A‴,A″″). In addition, postmitotic cell layers appear slightly disorganized in Emx double mutant embryos (A‴,A″″). B–B″″: Subpial calretinin-positive cells corresponding to Cajal-Retzius cells (arrows) are present in a normal position and normal number in single mutant embryos (B′,B″). In Emx double mutant embryos, the number of these cells may be slightly reduced (B‴). Calretinin-positive cells (red) coexpress Reelin (green) in Emx double mutant embryos (open arrowheads; see box in B‴). However, in severely affected Emx double mutant embryos, a strong reduction in the number of calretinin-positive cells (red cells in B″″) is observed. Scale bars = 100 μm.
We also examined the expression of calretinin and Reelin at E12.5. Reelin is a marker specific for Cajal-Retzius neurons and is required for proper cortical lamination (D'Arcangelo et al., 1995; Ogawa et al., 1995). Calretinin expression at E12.5 marks preplate neurons (Fig. 7B–B″″), and Emx double mutants have fewer calretinin-positive cells in the preplate at this age (Fig. 7B‴,B″″). Similarly, fewer Reelin-positive Cajal-Retzius neurons are observed in the E12.5 Emx double mutants (Fig. 7B‴,B″″). Indeed, in some sections, we find no calretinin- or Reelin-positive cells, which is in sharp contrast to wild-type and single mutants. The number of cells expressing calretinin and Reelin is variably reduced, being most affected in brains that have the greatest reduction in cortical size (compare Fig. 7B‴ with B″″). Thus, reductions in Emx dosage lead to a progressive decrease in the thickness of the preplate, and a related decrease in the number of cells expressing markers of Cajal-Retzius and subplate neurons. The severity of these defects appears to correlate with the overall reduction in cortical area.
Cortical efferent and thalamocortical projections do not form in Emx double mutants
Analysis of lamination and subplate molecular markers shows that the differentiation of subplate cells is deficient in Emx double mutants. Subplate neurons pioneer the axonal pathway (i.e., internal capsule) between the cortex and thalamus (McConnell et al., 1989; De Carlos and O'Leary, 1992) and are suggested to be required for the pathfinding of cortical efferents (e.g., corticothalamic axons [CTAs]) and thalamocortical axons (TCAs) (Molnar and Blakemore, 1995; Molnar et al., 1998); they are also implicated in TCA target selection (Ghosh et al., 1990). Therefore, we studied whether cortical input and output projections form in the Emx double mutants by using L1 immunostaining, as well as anterograde and retrograde DiI labeling, in fixed brains from E18.5 wild-type and Emx double mutant littermates.
In wild type, L1-positive fascicles of cortical efferent axons and TCAs pass through the striatum (Fig. 8A). L1-positive TCAs can be followed along their pathway from dorsal thalamus, through the striatum, and into the cortex. L1-positive cortical efferent can be followed from the cortex, through the striatum, and into the cerebral peduncle (indicative of layer 5 efferents). In the Emx double mutant, L1-positive cortical axons fail to exit the cortex, and L1-positive TCAs fail to extend from striatum into the cortex (Fig. 8B,C). This results in a clear gap lacking L1-positive fascicles of axons in dorsolateral striatum beneath the cortex. In addition to failing to reach the cortex, a proportion of TCAs exhibit another pathfinding defect at a more proximal point in their pathway. A large fascicle of TCAs fails to turn dorsolaterally into the striatum and continues on an aberrant ventral trajectory (Fig. 8C). This particular defect appears similar to that in the Emx2 single mutant (data not shown; Mallamaci et al., 2000b; Lopez-Bendito et al., 2002).
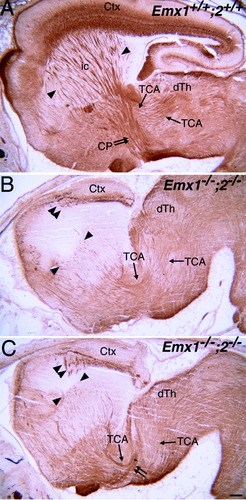
L1 immunostaining suggests that reciprocal connections between cortex and dorsal thalamus do not form in Emx double mutants. Sagittal sections through E18.5 brains show localization of L1 protein in wild-type (A) and Emx double mutant (B,C) brains. In all the panels, rostral is to the left. A: In wild-type brains, L1-positive fascicles of cortical efferent axons and TCAs pass through the striatum (outlined by arrowheads). TCAs can be followed along their pathway from the dorsal thalamus and through the striatum (arrows). Cortical efferents can be followed from the striatum into the cerebral peduncle (double arrow). B,C: In Emx double mutants, L1-positive cortical efferent axons fail to extend from the cortex into the striatum (double arrowheads in B and C), resulting in the lack of a cerebral peduncle. L1-positive TCAs can be traced in the diencephalon (arrows in B and C) and into the striatum, but they fail to extend into the cortex (outlined by arrowheads). In addition, a large fascicle of TCAs fails to turn dorsolaterally into the striatum and continues on an aberrant ventral trajectory (double arrow in C). Scale bar = 200 μm.
Anterograde and retrograde DiI labeling confirms and extends our findings obtained with L1 immunostaining. DiI injections into the cortex of wild-type mice labels cortical efferents, including CTAs; these axons normally accumulate in the intermediate zone, extend horizontally through it, exit the cortex into the striatum, and extend through it within the internal capsule (Fig. 9A). In contrast, similar cortical DiI injections done in Emx double mutants label axons that accumulate and extend horizontally within the cortical plate (Fig. 9A′). Growth cones are evident on the ends of many of these axons, indicating that they are still growing within the cortical plate. No cortical axons are seen to exit the cortex and enter the striatum. In addition, no back-labeled cells are observed in the cortex of Emx double mutants in which DiI was injected into the dorsal thalamus (data not shown)
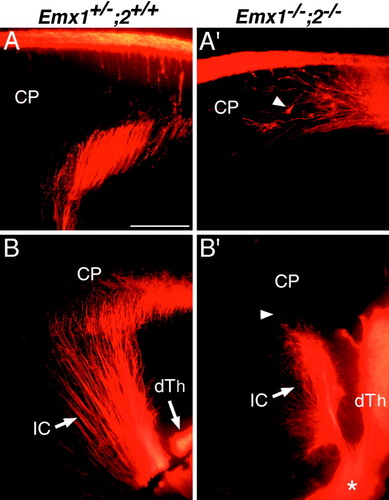
Axonal tracing with DiI confirms that connections between the cortex and thalamus do not form in Emx double mutants. Sagittal sections through E18.5 brains show axon tracing with DiI crystals placed in the cortex (A,A′) or dorsal thalamus (B, B′). A: Injections into occipital cortex label cortical efferent axons, including corticothalamic axons, leaving the cortex and entering the internal capsule in a wild-type brain. CP, cortical plate. A′: Injections into the occipital cortex also label cortical axons in Emx double mutants. However, in Emx double mutants, they wander within the cortical plate and do not exit the cortex. Growth cones are evident at the tips of many of these axons, indicating that they are growing within the cortical plate. B: Injections into the dorsal thalamus of wild-type brains label thalamocortical axons (TCAs) that leave the dorsal thalamus (dTh), turn dorsolaterally to travel through the internal capsule (IC), enter the cortex, and begin to invade the cortical plate. B′: Injections into the dorsal thalamus of Emx double mutants label TCAs that exit the dorsal thalamus, travel through the internal capsule, but do not enter the cortical plate. Instead, TCAs stop and many appear to turn back and bundle within the internal capsule. In addition, a large fascicle of DiI-labeled TCAs fail to turn dorsolaterally into the striatum (i.e., internal capsule) and continue on an aberrant ventral trajectory (marked by asterisk). Scale bars = 200 μm in A,A′; 500 μm in B,B′.
DiI injections into the dorsal thalamus of wild-type mice label TCAs that exit the thalamus, pass through the striatum within the internal capsule, and enter the cortex (Fig. 9B). Similar dorsal thalamic DiI injections in Emx double mutants also label TCAs that exit the thalamus and turn dorsolaterally to extend through the striatum within the internal capsule; however, these axons do not enter the cortex (Fig. 9B′). Instead, as the labeled TCAs approach the striatal-cortical boundary, they appear to stop or turn and double-back into the internal capsule. In addition, no back-labeled cells are evident in the thalamus of Emx double mutants in which DiI was injected into cortex (data not shown). As also seen in the L1-immunostained sections, in the Emx double mutants, a proportion of DiI-labeled TCAs fails to turn dorsolaterally into the ventral telencephalon and continues on an aberrant ventral trajectory (Fig. 9B′).
These findings using both L1 immunostaining and DiI axon tracing indicate that cortical efferent projections and TCA projections fail to form in the Emx double mutant. The defects in cortical efferent projections are either cell autonomous or are secondary to a failure of subplate axons to pioneer the internal capsule. Failure in TCAs to enter the cortex are probably secondary to defects in cortical differentiation, including defects in subplate neurons and the failure of cortical efferents to enter the striatum, or other properties intrinsic to the cortex that may influence TCA pathfinding. As described below, a distinct mechanism appears to account for the more proximal pathfinding defect.
Expression of Emx1 and Emx2 relative to the thalamocortical axon pathway
The TCA pathfinding defects in the Emx double mutants are greatly exaggerated compared with Emx single mutants. TCA pathfinding appears normal in Emx1 mutants (data not shown; Bishop et al., 2002), and relatively modest pathfinding defects are evident in the Emx2 mutant (data not shown; Mallamaci et al., 2000b; Lopez-Bendito et al., 2002). Therefore, we used in situ hybridization to examine the expression of Emx1 (Fig. 10A,B) and Emx2 (Fig. 10C–E) in structures along the TCA pathway. We focused on E13.5, an age when many TCAs have turned into the ventral telencephalon, but few if any have reached the neocortex (Braisted et al., 1999).
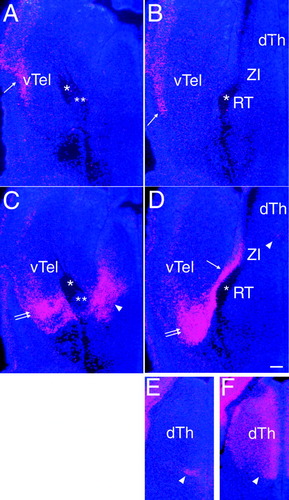
Expression of Emx1 and Emx2 in the dorsal thalamus and along the normal thalamocortical axon pathway. In situ hybridization for Emx1 (A,B), Emx2 (C–E), and Lhx2 (F) at E13.5. Coronal sections show the diencephalon and ventral telencephalon that surround the TCA pathway (asterisk). Midline is to the right. A and C are approximately 120 μm more rostral to B and D, respectively. E and F are at similar levels and approximately 120 μm more caudal to B and D. A,B: Emx1 is expressed in the lateral telencephalon (arrow) but not in dorsal thalamus or along the TCA pathway. C,D: Emx2 is expressed at a very low level in the medial ventral part of dorsal thalamus (dTh; arrowhead in D). Along the TCA path, Emx2 is not expressed in the prospective nuclei of ventral thalamus (zona incerta [ZI] or reticular nucleus [RT]). However, it is expressed at a more rostral level within diencephalon (arrowhead in C), which is within the lateral hypothalamus. This domain is located just rostral to the ventrally extending TCAs. In addition, Emx2 is expressed in a narrow strip of telencephalon immediately lateral to the TCA path (arrow in D). This is continuous with the Emx2-expressing domain in parts of the ventral telencephalon (vTh) located ventrolaterally to the TCAs (double arrows in C and D). These expression domains of Emx2 within the hypothalamus and ventral telencephalon are similar to those expressing Lhx1 and Lhx5 (Nakagawa and O'Leary, 2001) and are distinct from the Nkx2.1-positive domain that is part of the putative globus pallidus (double asterisk; Tuttle et al., 1999). The ventral telencephalic expression domain of Emx2 corresponds to where thalamocortical axons show ventrally turning defects in the Emx double mutants. E,F: Expression of Emx2 in the dorsal thalamus is highlighted. A small Emx2 expression domain is located just outside the ventricular zone in the medioventral part of the dorsal thalamus (arrowhead in E). This domain is located within the Lhx2-positive domain, which at this stage occupies a large part of the dorsal thalamus but not the adjacent ventral thalamus (Nakagawa and O'Leary, 2001). Scale bar = 100 μm.
Emx1 is expressed in lateral parts of the telencephalon, but we did not detect Emx1 expression in any structure along the path of TCA axons from the dorsal thalamus through the ventral telencephalon, including the striatum. This is consistent with previous descriptions of the limited expression of Emx1 (Simeone et al., 1992a, b; Briata et al., 1996; Guilisano et al., 1996; Gorski et al., 2002). Emx2 is not expressed in nuclei of the dorsal thalamus that gives rise to the TCA projection (data not shown; Fig. 10C–E). However, Emx2 is expressed in a very small domain in the medial part of the ventral aspect of the dorsal thalamus (Fig. 10E), as shown by its overlap with a dorsal thalamic marker, Lhx2 (Fig. 10F; Nakagawa and O'Leary, 2001). Emx2 is not expressed in the prospective nuclei of ventral thalamus (the zona incerta and reticular nucleus). However, Emx2 is highly expressed in more rostral diencephalon, within a domain in the lateral hypothalamus located just rostral to the path of TCAs extending ventrally within the diencephalon (Fig. 10C).
In addition, Emx2 is expressed in parts of the ventral telencephalon. A narrow domain of Emx2 expression is present in the medial telencephalon adjacent to the lateral edge of the TCA pathway positioned along the lateral edge of the diencephalon. This narrow band of expression is continuous with a larger domain of Emx2 expression located ventrolaterally to the pathway where TCAs turn into the ventral telencephalon and extend dorsolaterally toward the cortex (Fig. 10C,D). These expression domains within the lateral hypothalamus and ventral telencephalon are similar to those expressing Lhx1 and Lhx5 (Nakagawa and O'Leary, 2001) and are distinct from a Nkx2.1-positive domain that is part of the putative globus pallidus and is suggested to act as a template to guide TCAs (Tuttle et al., 1999). This ventral telencephalic expression domain of Emx2 corresponds to where TCAs show turning defects in Emx2 mutants and in Emx double mutants. Interestingly, within the cortex, Emx2 is only expressed in progenitor cells, with the exception of Cajal-Retzius neurons (Simeone et al., 1992a, b; Guilisano et al., 1996; Mallamaci et al., 1998), whereas the ventral telencephalic and dorsal thalamic domains of Emx2 expression that we describe are likely to be mainly, if not entirely, postmitotic neurons.
DISCUSSION
We have utilized a loss-of-function analysis to test the hypothesis that the homeobox transcription factors Emx1 and Emx2, which are highly homologous and expressed in overlapping domains in the cortical neuroepithelium (Simeone et al., 1992a), act cooperatively in aspects of cortical development. Shortly after submission of our paper, an excellent paper from Aizawa's group on Emx double mutants was published (Shinozaki et al., 2002). These two papers complement each other well. In instances of overlap between the two studies, the findings are in good agreement. Both groups also report distinct analyses. For example, Shinozaki et al. (2002) describe the important finding that tangential migration from the ganglionic eminence is disrupted in Emx double mutants. Their evidence indicates that this is a non-cell autonomous effect, and may be due to the lack of guidance substrates provided by cortical efferent and afferent axons. The distinct aspects of our paper include the examination of cortical efferent and afferent connections using axons tracers, correlating Emx2 expression domains with defects in TCA pathfinding and other data that argue for the handshake hypothesis, an analysis of cortical lamination and specification of cortical cell types using a panel of molecular markers, and analyses of the olfactory bulb and hippocampal formation.
Roles for Emx genes in regulating cortical size, cell proliferation, and the preplate
Based on the comparison of Emx1 and Emx2 single mutants with Emx1; Emx2 double mutants, we show that Emx1 and Emx2 have partially redundant roles in regulating cortical surface area and volume, as well as several important features of cortical differentiation, which lead to defects in cortical lamination and connectivity. Both Emx1 and Emx2 are expressed in cortical progenitor cells; therefore they are in a position to regulate their fate and proliferation directly, as well as to specify properties later exhibited by their differentiated progeny. Emx1 is also expressed in most neocortical neurons, except GABAergic interneurons; therefore it can also directly affect later differentiation events in these cells. In differentiating neurons, the direct influence of EMX2 would be limited to Cajal-Retzius cells, but this in turn would affect cortical migration and lamination.
The cortical surface area of E18.5 Emx double mutants is about half that of wild-type and Emx1 mutants and is approximately three-fourths that of Emx2 mutants. The reduced surface area is present as early as E12.5 and is not associated with an increase in apoptosis at that stage. The early appearance of this defect suggests that it is due in part to a defect in an earlier patterning event required for the proper allocation of domains of early telencephalic progenitors to a cortical fate. This defect could also be due to a change in proliferation, through several possible mechanisms such as increased cell cycle length, a reduced frequency of symmetrical progenitor cell mitoses, and/or premature differentiation. Our data do not support the premature differentiation mechanism, nor do they rule out an altered frequency of symmetrical progenitor cell mitoses.
On the other hand, evidence from our BrdU analysis suggests an increase in cell cycle length at E12.5 in Emx double mutants compared with wild-type or single Emx mutants. Emx double mutants have fewer BrdU-labeled nuclei near the ventricular surface 60 minutes following a BrdU pulse. The delay in nuclei moving from the S-phase zone to the M-phase zone could be due to an increase in the length of S or G2 phases. Additional studies will be needed to determine rigorously the cell cycle kinetics in these mutants. At later stages of cortical neurogenesis, the lack of TCA input to the Emx double mutant cortex may result in reduced proliferation, because in vitro thalamic explants release a diffusible mitogen for cortical precursors that reportedly shortens the total cell cycle duration via a reduction of the G(1) phase and facilitates the G(1)/S transition, leading to an increase in proliferation. In organotypic cultures, when they are devoid of TCAs, cortical explants show decreased proliferation rates (Dehay et al., 2001).
In addition to reduced surface area, Emx double mutants have reduced thickness of the preplate at E12.5 and the cortical plate at E18.5. These findings may reflect the combined effect of abnormalities in cortical neurogenesis, differentiation, migration, and process elaboration and growth. At later stages of embryonic development, the lack of afferent ingrowth, for example TCA and callosal inputs, will also contribute to the reduced cortical area and volume, due to the loss of space occupied by the afferents themselves, as well as their contributions to promoting the differentiation of their target neurons, including dendritic aborizations. In addition, interneurons generated in the ganglionic eminence fail to migrate into the cortex in Emx double mutants, which would also contribute to its reduced volume (Shinozaki et al., 2002).
Presently, it is uncertain whether the Emx genes regulate the numbers of cortical neurons produced, although the reduced thickness of the preplate at E12.5, in the absence of increased apoptosis, suggests that reduced neurogenesis contributes to this phenotype. Reduced size of the olfactory bulbs and loss of the hippocampus and dentate gyrus in Emx double mutants at E18.5 may also result from defects in neurogenesis in the dorsal telencephalic neuroepithelium at earlier ages (Tole et al., 2000a). Our observation that caudomedial cortical structures appear most severely affected in Emx2 mutants and Emx double mutants suggests that distinct regulatory genes, or sets of them, control the growth of different regions of the cortex. Recent evidence suggests that Emx2 primarily modulates growth of the caudomedial cortex (Muzio et al., 2002). Similarities in the graded expression of Emx1 and Emx2, and the more substantial reduction in cortical size in Emx double mutants compared with Emx2 mutants, suggests that the growth of caudomedial regions is more severely affected in the double mutants. However, a recent analysis of shifts in markers of cortical areas in Emx double mutants compared with Emx1 and Emx2 mutants indicates that the deletion of both Emx genes does not result in a complete loss of caudomedial cortical tissue (Bishop et al., 2002). Lhx2 may be partially redundant with the Emx genes in maintaining caudal cortical tissue (Nakagawa et al., 1999; Monuki et al., 2001). Other transcription factors expressed in gradients distinct from the Emx genes probably modulate the growth of other cortical regions. CoupTfI is a candidate for regulating growth of caudolateral cortical regions, based on its early expression pattern (Liu et al., 2000). Analysis of Small eye mutants suggests that PAX6 primarily modulates growth of rostrolateral cortex (Muzio et al., 2002).
Roles for Emx genes in regulating differentiation and maintenance of cortical neurons
Emx1 and Emx2 also appear to regulate differentiation and/or maintenance of cortical neurons, based on several features of the double mutant phenotype. First, Cajal-Retzius cells are formed (although in much reduced numbers based on calretinin and Reelin expression at E12.5 and the thin preplate), but they are not detected at later stages. This finding suggests that Emx function is required for the maintenance of this cell type. Second, subplate neurons are not detected in Emx double mutants based on histological (thionin staining, and calretinin and L1 expression) and hodological (lack of cortical efferents) criteria. Third, cortical axons do not leave the cortex, suggesting that subplate, layer 6, and layer 5 neurons fail to produce axons that are capable of pathfinding into the striatum. These results were surprising because the Emx1 mutant cortex appears to be normally differentiated and the Emx2 mutant cortex shows only a loss of marginal zone Cajal-Retzius cells starting relatively late in corticogenesis, at about E15.5 (Mallamaci et al., 2000a).
Our analysis also indicates the existence of defects in the specification of preplate-derived subplate cells in Emx double mutant cortex, as indicated by a loss of calretinin at E18.5. These defects were unexpected, based on the normal expression of calretinin in subplate cells of Emx1 and Emx2 single mutants. Our results suggest that the early differentiation of preplate cells is maintained in Emx double mutants, as indicated by the expression of TBR1, calretinin, and Reelin at E12.5. However, the number of preplate cells is greatly reduced in Emx double mutant cortex compared with wild types and Emx single mutants at this age. Two possible explanations for this defect seem likely. It is possible that subplate neurons are specified normally at E12.5, as indicated by the presence of TBR1- and calretinin-positive neurons in the preplate at this age, but are generated in reduced number. Subsequently, subplate neurons may die or change their molecular phenotype at intermediate stages, similar to how Cajal-Retzius cells are lost at E15.5 in Emx2 mutant cortex (Mallamaci et al., 2000a). However, it is also possible that subplate cells are mis-specified from their inception but that we are unable to detect this defect because there are no molecular markers to differentiate subplate neurons from marginal zone neurons co-mingled in the preplate at E12.5.
Emx genes are required for proper cortical lamination but not for the molecular specification of cortical plate cell types
Although many features of the cortex are defective in Emx double mutants, the reduced neocortex does express several genes and proteins characteristic of several classes of cortical neurons, such as Tbr1, L1, Id2, RZRβ, p75, ephrin-A5, and Cad6. This is also true in the olfactory bulb, where Id2 expression continues to mark presumptive mitral cells. In addition, most of these genes are normally expressed by layer-specific populations of cortical neurons (Chung et al., 1991; Suzuki et al., 1997; Mackarehtschian et al., 1999; Nakagawa et al., 1999; Rubenstein et al., 1999; Hevner et al., 2001). However, the POU domain transcription factor, SCIP (Brn-3.2), which is expressed by cortical plate neurons in wild-type mice (Frantz et al., 1994) and Emx2 mutants (Tole et al., 2000a), is absent in the Emx double mutant cortex (Shinozaki et al., 2002). Thus, our findings, interpreted in the context of those of Shinozaki et al. (2002), indicate that the molecular specification of basic cortical cell types and laminar identify is relatively intact in the reduced Emx double mutant, but some defects are evident.
Although Id2, RZRβ, p75, ephrin-A5, and Cad6 expression are preserved, their laminar expression patterns appear more diffuse than normal in the cortex and olfactory bulb in the Emx double mutants. Cortical cells reach their correct laminar position according to their birthdate, and their laminar identity is determined just prior to their generation (McConnell, 1995). Thus, the diffuse laminar distribution of neurons in the cortical plate of Emx double mutants may be due to defects in their progenitors. It is also possible that lamination defects are secondary to a loss of Reelin expression in Cajal-Retzius cells of the marginal zone, as in brains of Emx2 mutants (Mallamaci et al., 2000a). Our analysis indicates that Reelin is present in Emx double mutant preplate cells at E12.5 but that Cajal-Retzius cells, which express Reelin, are absent from the marginal zone by E18.5, and probably earlier. Marginal zone cellular defects in Emx double mutants are qualitatively similar to those of Emx2 mutants, but the early reduction in the number of preplate neurons, and probably Reelin-positive Cajal-Retzius cells, in Emx double mutants may partly account for the more severe lamination defects that they exhibit compared with Emx2 mutants. In addition, the lack of TCA input (present study) and GABAergic interneurons (Shinozaki et al., 2002) in Emx double mutants probably contributes to their cytoarchitectural defects in lamination.
Emx genes are required for pathfinding of cortical efferents and thalamocortical axons
Emx double mutants have major defects in the pathfinding of most cortical axons. All the major telencephalic commissures are hypoplasic (corpus callosum, hippocampal commissure, and anterior commissure), and the cortex fails to send axons into the internal capsule. These defects are much more substantial than the relatively minor defects in cortical efferent projections reported in Emx single mutants. This finding suggests that Emx function is essential for generating cortical neurons capable of producing axons that exhibit normal growth and pathfinding. In Emx double mutants, cortical efferent axons wander in the cortical plate, and none enter the internal capsule, in sharp contrast to their normal behavior of accumulating in the intermediate zone and showing a strong directed growth toward and into the internal capsule (Richards et al., 1997). Thus, layer 6 cortical projections to the thalamus, and layer 5 projections through the cerebral peduncle en route to the midbrain, hindbrain, and spinal cord, do not form. The inability of cortical efferent axons to pathfind properly may be a cell-autonomous defect or it may be secondary to a loss of instructive cues within the cortex. Because cortical efferents are heterogeneous, the mechanisms could be mixed; for example, the lack of a subplate projection through the internal capsule may be autonomous to subplate neurons, and the subsequent failure of other cortical efferents to develop may be secondary to the subplate defect (McConnell et al., 1989; DeCarlos and O'Leary, 1992; also see Koester and O'Leary, 1994; Rash and Richards, 2001; Shu and Richards, 2001).
Reciprocally, our findings show that in Emx double mutants, TCAs fail to enter the cortex. This is due to at least two major defects in their growth and pathfinding. TCAs exhibit a pathfinding defect near the junction of the diencephalon with the ventral telencephalon, where a large contingent of TCAs fail to turn dorsolaterally into the striatum and continue on an aberrant ventral trajectory. This defect resembles the aberrant TCA pathfinding in Emx2 single mutants (Mallamaci et al., 2000b; Lopez-Bendito et al., 2002). We show that Emx2 is highly expressed in the lateral hypothalamus and in specific ventral telencephalic structures located along the TCA pathway where this pathfinding defect occurs. Therefore, the loss of Emx2 in these structures may lead to their aberrant differentiation and in turn this particular TCA pathfinding defect. These structures are distinct from the Nkx2.1-positive domain that is part of the putative globus pallidus and suggested to act as a template to guide TCAs (Tuttle et al, 1999). In Mash-1 mutants, this population of Nkx2.1-positive cells is absent, and TCAs fail to turn dorsolaterally into the ventral telencephalon (Tuttle et al, 1999).
We observe a second major defect more distal in the TCA pathway near the junction of the dorsolateral striatum and the cortex. Specifically, TCAs fail to enter the cortex and instead they either stop or turn back into the internal capsule. This defect is not seen in either Emx single mutant (present study; Bishop et al., 2000, 2002; Mallamaci et al., 2000b; Lopez-Bendito et al., 2002). Because neither Emx1 nor Emx2 are expressed in TCA projection neurons or their progenitors, it is most likely that these pathfinding defects are non-autonomous to them. Because Emx1 is not expressed along the TCA pathway, the additional, and greatly exaggerated, defects in TCA pathfinding in Emx double mutants, compared with Emx2 single mutants, indicates that the failure of TCAs to enter the Emx double mutant cortex is not due to added effects of the Emx genes on the subcortical pathfinding of TCAs. Instead, because both Emx genes are highly expressed in the cortex, it is most likely that this defect is secondary to a cortical deficiency. Skaliora et al. (2000) have shown that before TCAs proceed into the cortex, they pause, and repeatedly retract and extend filopodia in different directions, at the striatal-cortical boundary region. Disruption of molecular signals from the cortex in this region may underlie the inability of TCAs to proceed past this decision point and enter the cortex in Emx double mutants.
An additional mechanism that may contribute to the failure of TCAs to reach the cortex in Emx double mutants could be the failure of cortical efferents to exit the cortex and extend into the striatum. It has been proposed that proper growth and pathfinding of TCAs and CTAs depend on their reciprocal interactions (“handshake hypothesis”; Molnar and Blakemore, 1995; Molnar et al., 1998). Recent evidence supporting this possibility comes from the Tbr1 and Gbx2 mutants (Hevner et al., 2001, 2002). Gbx2 expression in the dorsal thalamus is required for the production of a normal TCA projection (Miyashita-Lin et al., 1999). Despite the lack of Gbx2 expression in the cortex, corticothalamic axons fail to reach the dorsal thalamus in Gbx2 mutants (Hevner et al., 2002). Like Emx1 and Emx2, Tbr1 is expressed in the embryonic cortex and not in the dorsal thalamus, and, like the Emx double mutants, Tbr1 mutants fail to produce cortical axons that reach the dorsal thalamus and their TCAs fail to reach the cortex. However, because Tbr1 is expressed in domains near the TCA pathway, this could influence the trajectories of these axons. Additional studies are needed to establish more firmly whether or not pathfinding of TCAs depends on interactions with cortical axons.
Regulation of Emx expression and combinatorial action of Emx genes
The expression of Emx1 and Emx2 is lost or diminished in the forebrain of the extra-toes (Xt) mutant mouse, a naturally occurring mutant of the zinc finger transcription factor GLI3 (Thiel et al., 1999; Tole et al., 2000b). Recent studies indicate that Emx2 is a transcriptional target of Wnt and Bmp signaling, mediated by Tcf and Smad proteins (Ohkubo et al., 2002; Theil et al., 2002), and that Fgf8 represses Emx2 expression (Crossley et al., 2001). The effect of GLI3 on Emx expression is proposed to be indirect (Theil et al., 2002). Analysis of the Gli3Xt/Xt mutant shows the loss or diminished expression of certain Bmps and Wnts and the enhanced expression of Fgf8 (Theil et al., 1999). Thus, several mechanisms probably cooperate to account for the Emx deficiency in Gli3Xt/XT mutants.
Gli3Xt/XT mutants have some cortical defects in common with Emx double mutants: reduced cerebral hemispheres (Thiel et al., 1999), poor cortical lamination (Franz, 1994), loss of the hippocampus (Tole et al., 2000b), and defects in olfactory system development (Franz, 1994). However, as would be expected, cortical defects are much more severe in Gli3Xt/XT mutants than in Emx double mutants (compare our Fig. 2 with Fig. 2 of Thiel et al., 1999) because GLI3 regulates a number of genes that control not only Emx expression but also other factors important for dorsal telencephalic development.
Based on their overlapping and distinct functions in regulating cortical development, it is possible that EMX1 and EMX2 use partially overlapping sets of co-factors and regulate partially overlapping sets of target genes. Alternatively, EMX1 and EMX2 may regulate distinct sets of genes, which in turn cooperate to control specific mechanisms required for proper cortical development. In either scenario, the loss of function of both Emx genes would result in exaggerated defects compared with those resulting from the loss of either Emx gene alone, as we have observed.
Acknowledgements
We thank P. Gruss for the generous gift of breeding pairs of Emx2 mice, C. Lagenauer, A. Goffinet, and M. Sheng for gifts of antibodies, and various investigators for in situ hybridization plasmids (see Materials and Methods).




