Differential morphology of pyramidal tract-type and intratelencephalically projecting-type corticostriatal neurons and their intrastriatal terminals in rats
Abstract
Two types of corticostriatal projection neurons have been identified: 1) one whose intrastriatal arborization arises as a collateral of a projection to the ipsilateral brainstem via the pyramidal tract (PT-type); and 2) one that projects intratelencephalically to the cortex and striatum, in many cases bilaterally, but not extratelencephalically (IT-type). To assess possible functional differences between these two neuron types, we characterized their laminar location in the cortex, their perikaryal size, and the morphology of their intrastriatal terminals. IT-type neurons were retrogradely labeled by tetramethylrhodamine-dextran amine (RDA)3k injection into the contralateral striatum, whereas their intrastriatal terminals were labeled anterogradely by biotinylated dextran amine (BDA)10k injection into the contralateral motor or primary somatosensory cortex. To label PT-type neurons and their ipsilateral intrastriatal terminals retrogradely, BDA3k was injected into the pontine pyramidal tract. We found that IT-type neuronal perikarya are medium-sized (12–13 μm) and located in layer III and upper layer V, whereas PT-type perikarya are larger (18–19 μm) and most commonly located in lower layer V. At the electron microscopic level, the intrastriatal terminals of both corticostriatal neuron types made asymmetric synaptic contact with spine heads and less frequently with dendrites. IT-type axospinous terminals were characteristically small (0.4–0.5 μm) and regular in shape, whereas PT-type terminals were typically large (0.8–0.9 μm) and often irregular in shape. Perforated postsynaptic densities were common for PT-type terminals, but not IT-type. The clear differences between these two corticostriatal neuron types in perikaryal size and laminar location in the cortex, and in the size and shape of their intrastriatal terminals, suggest that they may differ in the nature of their influence on the striatum. J. Comp. Neurol. 457:420–440, 2003. © 2003 Wiley-Liss, Inc.
Widespread and functionally diverse areas of the cerebral cortex, including sensory, motor, and association regions, project to the striatum in mammals (Oka, 1980; Veening et al., 1980; Royce, 1982; Alexander et al., 1986; Goldman-Rakic and Selemon, 1986; McGeorge and Faull, 1989). This projection is known to be glutamatergic and to end as terminals that make asymmetric synaptic contacts primarily with the spines of striatal projection neurons, which are by far the most abundant type of striatal neuron (Kemp and Powell, 1971a, b, c; Fonnum et al., 1981; Gerfen, 1988; Albin et al., 1989). Retrograde labeling studies have shown that the corticostriatal projection arises from neurons whose somata are located in cortical laminae III and V, with minimal contributions from lamina II and VI (Hedreen, 1977; Schwab et al., 1977; Wise and Jones, 1977; Oka, 1980; Donoghue and Kitai, 1981; Royce, 1983; Fisher et al., 1984; Arikuni and Kubota, 1986; Tanaka, 1987; Wilson, 1987; McGeorge and Faull, 1989). In an early description of the corticostriatal projection based on Golgi material, Ramón y Cajal (1911) suggested that it arose as a collateral projection of the corticofugal fibers descending through the striatum via the internal capsule. Similar observations were later made by others in diverse mammalian species (Webster, 1961; Carman et al., 1965; Kemp and Powell, 1970, 1971c; DiFiglia et al., 1976).
More recent studies employing pathway tracing, intracellular labeling, and/or antidromic stimulation have made it clear that in each cerebrocortical region projecting to the striatum, two main types of corticostriatal projection neuron can be distinguished by their connections within the cortex and their projections to other subcortical areas (Kitai et al., 1976; Jones and Wise, 1977; Jones et al., 1977; Jinnai and Matsuda, 1979; Donoghue and Kitai, 1981; Royce, 1983; Gerfen, 1984, 1989; Royce and Bromley, 1984; Arikuni and Kubota, 1986; Donoghue and Herkenham, 1986; Wilson, 1987; McGeorge and Faull, 1989; Cowan and Wilson, 1994; Gerfen and Wilson, 1996; Kincaid and Wilson, 1996). One is, in fact, the type whose main axon projects extratelencephalically (brainstem-projecting neurons) identified by Ramón y Cajal, whereas the second is a type that projects to the basal ganglia and cortex but not to areas outside the telencephalon (intratelencephalically projecting neurons, or IT-type; Donoghue and Kitai, 1981; Landry et al., 1984; Goldman-Rakic and Selemon, 1986; Wilson, 1987; Cowan and Wilson, 1994; Levesque et al., 1996a, b; Levesque and Parent, 1998). Brainstem-projecting corticostriatal neurons are reportedly mainly found in lower cortical layer V, whereas intratelencephalically projecting corticostriatal neurons are reportedly mainly found in layer III and upper layer V (Wilson, 1987; Cowan and Wilson, 1994; Levesque et al., 1996a, b; Levesque and Parent, 1998).
The extratelencephalic axon of the type of corticostriatal neuron whose striatal projection arises as a collateral of the main descending axon travels, in most instances, to the ipsilateral pyramidal tract in the caudal pons via the cerebral peduncle. For this reason, we will refer to this type of corticostriatal neuron as the pyramidal-tract type (PT-type). The individual neurons of this type have been reported to give rise to an intrastriatal arborization that consists of one or more small, dense focal clusters of fine processes and terminals (about 250 μm in diameter per focal cluster) scattered over a 1–2-mm region of the striatum (Cowan and Wilson, 1994). The input of PT-type neurons to the striatum has attracted interest because of its potential for providing the striatum with a copy of the cortical motor signal transmitted to the brainstem and spinal cord (Donoghue and Kitai, 1981; Landry et al., 1984; Arikuni and Kubota, 1986; Tanaka, 1987; Levesque et al., 1996a, b). The other major type of corticostriatal projection neuron, the IT-type, projects to the contralateral (in many cases) as well as ipsilateral cortex and striatum, and neurons of this type are numerous in the agranular (i.e., motor) cortex (Wilson, 1987; Cowan and Wilson, 1994; Gerfen and Wilson, 1996; Kincaid and Wilson, 1996). In contrast to the scattered focal arborization of the PT-type neuron, the intrastriatal axon of individual IT-type neurons has been reported to give rise to an extended and uniform arborization that has sparse en passant terminals over a wide (about 1.5 mm in diameter) striatal expanse (Cowan and Wilson, 1994; Kincaid and Wilson, 1996).
The clear morphological differences between the PT-type and IT-type corticostriatal neurons in the laminar location of their cell bodies, the main regional target(s) of their extrastriatal axon, and the shape of their intrastriatal arborization suggest the possibility that these two types of neurons differ in the nature of the information they transmit to the striatum. Investigating this possibility is of importance for understanding how the cortical input to the striatum participates in the role of the basal ganglia in movement control. To address the possible differences between PT-type and IT-type neurons, we examined the differences between these two neuron types in the morphology of their intrastriatal terminals. We also re-examined the laminar location and size of the cell bodies of each type. To label each of these neurons types selectively, we took advantage of the differences between these two neuron types in the projection target of their main axon and of the sensitivity of dextran amines as pathway tracing agents (Veenman et al., 1992; Chen and Aston-Jones, 1998; Reiner et al., 2000). The intrastriatal terminals of IT-type neurons were anterogradely labeled by injection of 10,000 MW biotinylated dextran amine (BDA10k) into the contralateral cerebral cortex, whereas the intrastriatal terminals of PT-type neurons were collaterally labeled by injection of BDA 3,000 MW (BDA3k) into the pontine pyramidal tract. To label the perikarya of these neuron types selectively, tetramethylrhodamine-dextran amine 3,000 MW (RDA3k) was injected into the contralateral striatum, whereas BDA3k was injected into the pontine pyramidal tract.
MATERIALS AND METHODS
Animals and injection of dextran amines
Results from 18 Sprague-Dawley male rats (obtained from Harlan, Indianapolis, IN) are presented in this paper. All efforts were made to minimize animal suffering and to reduce the number of animals used, and all animal use was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Society for Neuroscience Guidelines, and University of Tennessee Health Science Center Guidelines.
Prior to the surgery required for the injection of the pathway tracing agents used, the animals were deeply anesthetized with 0.1 ml/100 mg of a mixture of ketamine (87mg/kg) and xylazine (13 mg/kg). To characterize and compare the perikarya of IT- and PT-type neurons, we injected the retrograde tracer RDA3k (anionic, lysine fixable; Molecular Probes, Eugene, OR) into the left striatum, as shown in Figure 1A, and injected BDA3k (anionic, lysine fixable; Molecular Probes) into the right pyramidal tract at caudal pontine levels, as shown in Figure 1B. Such dual injections were carried out in five cases (R27, R28, R29, R44, and R45), with RDA3k injected into the striatum contralateral (left) to the cortex analyzed (right) and BDA3k injected into the pyramidal tract ipsilateral to the cortex analyzed (right). Subsequent histological processing revealed that both injections were accurately targeted in four of the five rats (R27, R28, R44, and R45), whereas in one other of the five rats (R29) the striatal injection was accurate but the pyramidal tract injection was not. One additional rat (R36) successfully received only an injection of RDA3k into the left striatum. The approach of injecting RDA into the left striatum and BDA into the right pyramidal tract yields selective labeling of IT-type neurons with RDA and PT-type neurons with BDA in the right cortex, due to the unique differences between these two cell types in their contralateral and descending projections (Fig. 1).
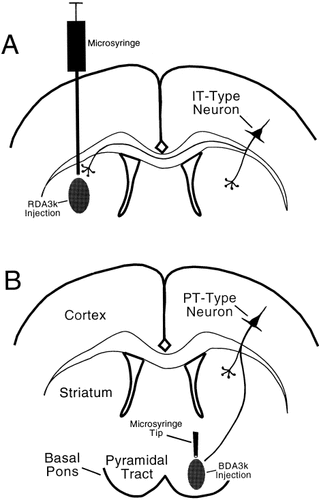
Schematic depiction of the strategies employed for selective retrograde labeling of intratelencephalically projecting (IT)-type (A) and pyramidal tract (PT)-type (B) corticostriatal neurons. To compare IT- and PT-type perikarya directly in the cortex of the right cerebral hemisphere, we labeled them in the same hemisphere by injecting tetramethylrhodamine-dextran amine (RDA)3k into the left striatum (A) and biotinylated dextran amine (BDA)3k into the right pyramidal tract at caudal pontine levels (B).
Stereotaxic methods were used for both injections, using coordinates from the Paxinos and Watson (1998) stereotaxic atlas of the rat brain to target the injections. Injections were made with a 1-μl Hamilton microsyringe, according to procedures previously described (Veenman et al., 1992; Reiner et al., 2000). For the RDA3k injection, 0.1 μl of 10% RDA3k in 0.1 M sodium citrate-HCl (pH 3.0) was used (Kaneko et al., 1996; Reiner et al., 2000); for the BDA3k injection, 0.15 μl of 10% BDA3k in 0.1 M sodium citrate-HCl (pH 3.0) was used. For sections in which both types of corticostriatal neurons were retrogradely labeled, the RDA was visualized by anti-rhodamine immunolabeling, whereas BDA was visualized by the ABC method, as detailed below. In sections in which only the BDA or RDA labeling was visualized, a brown diaminobenzidine (DAB) reaction was used. In tissue in which both neuron types were simultaneously visualized, two-color DAB was used, as detailed below.
To investigate the ultrastructural morphology of the intrastriatal terminals of IT-type neurons, eight rats received unilateral injections of BDA10k (anionic, lysine fixable; Molecular Probes) into the motor cortex (rats R20, R21, R30, and R31) or into the primary somatosensory cortex (rats R34, R80, R81, and R82) on the left side of the brain. In these cases, anterograde corticostriatal labeling in the right striatum would be limited to IT-type terminals, because PT-type corticostriatal neurons do not project to the contralateral striatum, but IT-type neurons do (Fig. 2A). A 1-μl Hamilton microsyringe was used to inject 5% BDA10k (Molecular Probes) in 0.1 M phosphate buffer (pH 7.4), using stereotaxic methods as described above. Among the four rats receiving injections into the motor cortex, in three (R20, R21, and R31) the injection site included both the primary motor cortex (M1, or as it is also called in rodents, the lateral agranular cortex) and the secondary motor cortex (also called the medial agranular cortex); in the remaining rat (R30), the injection was placed solely in the secondary motor cortex (M2). In all four rats with BDA10k injections into the somatosensory cortex, the injection was located in the primary somatosensory cortex.
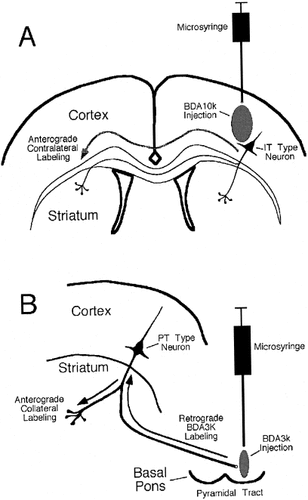
Schematic depiction of the strategies employed for selective anterograde labeling of the intrastriatal terminals of intratelencephalically projecting (IT)-type (A) and pyramidal tract (PT)-type (B) neurons. To label IT-type terminals in striatum selectively, we relied on the fact that this projection, but not the PT-type projection, is bilateral. Accordingly, BDA10k was injected into the right motor or sensory cortex, as shown in Figure 1B, and anterogradely labeled IT-type terminals were examined in the contralateral (i.e., left) striatum (A). To label PT-type terminals in the striatum selectively, the intrastriatal collaterals of the PT-type neurons were retrogradely labeled by injection of BDA3k into the ipsilateral pyramidal tract at caudal pontine levels (B).
To investigate the ultrastructural morphology of the intrastriatal terminals of PT-type neurons, the right pyramidal tract at caudal pontine levels was bilaterally injected with the retrograde tracer BDA3k in four rats (R6, R17, R18, and R19). Retrograde labeling of PT-type neurons by injection of BDA3k into the pyramidal tract yields selective labeling of the intrastriatal collaterals of these neurons (Fig. 2B). For these injections, 0.15 μm of 10% BDA3k (in 0.1 M sodium citrate-HCl, pH 3.0) was injected, using a 1-μl Hamilton microsyringe, according to procedures previously described (Veenman et al., 1992; Reiner et al., 2000). Stereotaxic coordinates for the injections were obtained from the atlas of Paxinos and Watson (1998). Subsequent histological processing revealed that the center of the BDA3k injection was located in the pyramidal tract at caudal pontine levels on both sides of the brain in two of the four rats (R6 and R19), whereas in the other two rats (R17 and R18), the injection on the right side of the brain was centered in the pyramidal tract, but the injection on the left side missed the pyramidal tract. The four rats with successful pyramidal tract injections were used to analyze the morphology of the intrastriatal terminals of PT-type corticostriatal neurons.
Tissue fixation and sectioning
Tissue processing for light microscopic examination.
After 7–10 days, the rats that had been injected with both BDA and RDA were deeply anesthetized with 0.8 ml of 35% chloral hydrate in avian saline and then perfused transcardially with 30–50 ml of 6% dextran in 0.1 M sodium phosphate buffer (PB; pH 7.4), followed by 400 ml of 4% paraformaldehyde/0.01 M DL-lysine/0.1 M sodium periodate in PB (pH 7.4). The brains were then removed and immersed overnight in 20% sucrose/10% glycerol/0.02% sodium azide in PB (pH 7.4). The brains were sectioned frozen on a sliding microtome at 40 μm in the transverse plane, and sections were serially collected in PB (pH 7.4) containing 0.01% sodium azide. The sections were then processed for RDA or BDA visualization (or both), as described in more detail below, and examined using light microscopy (LM).
Tissue processing for electron microscopic visualization.
After 7–10 days, the rats that had been injected with BDA for ultrastructural visualization of intrastriatal terminals were deeply anesthetized with 0.8 ml of 35% chloral hydrate in avian saline and then perfused transcardially according to procedures described by Anderson and Reiner (1991). The rats were first exsanguinated by perfusion with 30–50 ml of 6% dextran in PB, followed by 400 ml of a solution consisting of 3.5% paraformaldehyde/0.6% glutaraldehyde/15% saturated picric acid in PB (pH 7.4). The brain was then removed, postfixed overnight in the same fixative without glutaraldehyde, and then sectioned at 50 μm on a vibratome.
Following BDA visualization as described in the next section, the sections were rinsed in 0.1 M sodium cacodylate buffer (pH 7.2), postfixed for 1 hour in 2% osmium tetroxide (OsO4) in 0.1 M sodium cacodylate buffer, dehydrated in a graded series of ethyl alcohols, impregnated with 1% uranyl acetate in 100% alcohol, and flat-embedded in Spurr's resin (Electron Microscopy Sciences, Fort Washington, PA). For the flat-embedding, the sections were mounted on microslides pretreated with liquid releasing factor (Electron Microscopy Sciences, Fort Washington, PA). The Spurr's resin-embedded sections were examined light microscopically for the presence of BDA-labeled axons and terminals in the striatum. Pieces of embedded tissue were then cut from the striatum and glued to carrier blocks, and ultrathin sections were cut from these specimens with a Reichert ultramicrotome. The sections were mounted on mesh grids, stained with lead citrate and uranyl acetate using an LKB Ultrastainer, and finally viewed and photographed with a JEOL 1200 electron microscope (EM). One or more series from each case used for ultrastructural analysis were also processed for LM visualization of BDA labeling.
Visualization of dextran amines
Single labeling for BDA.
The sections were first pretreated with 1% sodium borohydride in 0.1 M PB for 30 minutes followed by incubation in 0.3% H2O2 solution in 0.1 M PB for 30 minutes. BDA was then visualized by using the ABC Elite kit (Vector, Burlingame, CA), from which a 5 ml volume of the ABC solution was prepared in 0.01 M PBS by adding 100 μl of avidin-DH and 100 μl of biotinylated horseradish peroxidase (HRP). Sections were incubated in this solution for 1–2 hours at room temperature or overnight at 4°C (Veenman et al., 1992; Reiner et al., 2000). After PB rinses, the sections were immersed for 10–15 minutes in 0.05% diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) in 0.1 M PB (pH 7.2) containing 0.04% nickel-ammonium sulfate. Hydrogen peroxide was then added to the solution to a final concentration of 0.01%, and the sections were incubated in this solution for an additional 15 minutes. The sections were subsequently washed six times in PB. Sections to be viewed by LM were mounted onto gelatin-coated slides, dried, and then dehydrated, cleared with Pro-par (Anatech Limited, Battle Creek, MI) or xylene, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). Tissue to be examined at the EM level was rinsed, dehydrated, and flat-embedded in plastic as described above.
Single labeling for RDA.
Several series of sections from each rat that received RDA3k injection into the striatum (with or without additional BDA3k injection into the pyramidal tract) were processed immunohistochemically for visualization of the RDA3k retrograde labeling. Sections were incubated for 72 hours at 4°C under constant agitation in a rabbit anti-Texas Red antibody (Molecular Probes), which also detects rhodamine, diluted 1:500 with PB (pH 7.2) containing 0.3% Triton X-100 and 0.01% sodium azide. The sections were then rinsed in PB (pH 7.2) and subsequently incubated in a donkey anti-rabbit IgG secondary antiserum (Jackson ImmunoResearch, West Grove, PA) for 1 hour and then (after PB rinses) in a rabbit peroxidase-antiperoxidase (PAP) complex (Sternberger, Baltimore, MD) for 1 hour. The secondary antiserum was diluted 1:50, and the PAP was diluted 1:100 with PB (pH 7.2) containing 0.3% Triton X-100 and 0.01% sodium azide. Sections were then rinsed twice in PB (pH 7.2) and twice in 0.05 M Tris-HCl buffer (pH 7.4), and the RDA immunolabeling was visualized by incubation in 0.05% DAB in Tris -HCl buffer (pH 7.4) for 10–15 minutes. Hydrogen peroxide was added to the solution to reach a final H2O2 concentration of 0.01%, and the sections remained immersed in the solution for 10–15 more minutes, producing a brown reaction product.
Double labeling for BDA and RDA.
Several series of sections from the rats that received injection of both RDA3k into the dorsal striatum and BDA3k into the pyramidal tract were processed for two-color DAB simultaneous visualization of cortical IT-type and PT-type neurons. Sections were first incubated in avidin-biotin complex (prepared as described above) for 1–2 hours at room temperature. The BDA labeling in the sections was then visualized using nickel-intensified DAB, as described above. This results in a dark, blue-black reaction product (Reiner et al., 2000). After rinsing, the sections were then incubated in the rabbit anti-Texas Red antiserum (Molecular Probes), diluted 1:500 with 0.1 M PB (pH 7.2) containing 0.3% Triton X-100/0.01% sodium azide, for 72 hours at 4°C under constant agitation. The sections were next rinsed in PB and subsequently incubated in secondary bridging antiserum and then PAP complex, as described above. The sections were subsequently rinsed twice in PB and twice in Tris-HCl buffer (0.05 M, pH 7.4), and the RDA3k was visualized with 0.05% DAB in Tris-HCl as described above, producing a brown reaction product that could be easily distinguished from the dark, blue-black, nickel-intensified DAB reaction product in the BDA3k-labeled PT-type neurons (Reiner et al., 2000). The distribution of retrogradely labeled neurons in the cortex in this material was charted using Neurolucida (microBright Field, Colchester, UT), and the diameters of the perikarya were measured by using the size measurement capabilities of Neurolucida and confirmed using NIH Image.
Image processing
Illustrations of BDA-labeled perikarya or terminals at the LM level were prepared from images captured using an Olympus BH-2 microscope, differential interference contrast optics, and a Newvicon video camera. Illustrations of BDA-labeled labeled terminals at the EM level were prepared from scanned images of photographic negatives taken using the JEOL 1200 electron microscope. The figures in this paper showing these images of the labeling at the LM and EM levels were prepared using Adobe Photoshop (version 4.0). Images were sharpened and contrast-enhanced, and minor imperfections were removed by using this image processing program.
RESULTS
Light microscope observations
Retrograde labeling of IT-type and PT-type perikarya in the cortex.
Among the six rats (R27, R28, R29, R36, R44, and R45) that received an RDA3k injection into the left striatum (with or without additional injection of BDA3k into the right pyramidal tract), the RDA injection in two of the cases (R36 and R45) was centered in the dorsal or dorsolateral striatum, whereas in the others it was located in the central striatum. In all six cases, RDA3k-labeled neuronal perikarya were highly abundant ipsilaterally in the motor areas and primary somatosensory area of the cerebral cortex and less abundant contralaterally in these same cortical regions (Fig. 3). In all six cases, the RDA3k-labeled perikarya in the cortex ipsilateral to the injection (i.e., left cortex) included large and medium-sized pyramidal cells located in layers III, V, and VI, with the greatest abundance being in layer V (Figs. 4, 5). In many cases, the proximal and secondary dendrites of these neurons were also labeled, thus making their pyramidal morphology unequivocal (Figs. 3, 4). Whereas RDA-labeled neurons were found within the same cortical layers in the contralateral (i.e., right) cortex as in the ipsilateral (i.e., left) cortex, on the contralateral side the RDA3k-labeled perikarya tended to be medium-sized and located in layers III and V, with those in layer V largely restricted to the upper part of layer V (Figs. 4, 5).
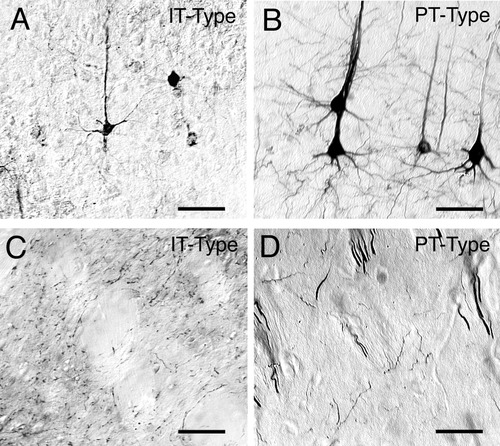
Images of intratelencephalically projecting (IT)-type and pyramidal tract (PT)-type perikarya in cortex (A,B) and of IT-type (C) and PT-type (D) axons and varicosities in the striatum. The IT-type perikarya were retrogradely labeled from the contralateral striatum with RDA3k. They are moderate in size (about 12–13 μm), as is evident in A, and they are largely localized to layer III and upper layer V (Fig. 5A). By contrast, the PT-type perikarya, retrogradely labeled by BDA3k injection into the ipsilateral pontine pyramidal tract, are largely localized to deep layer V of the cortex (D) and are slightly larger (about 18–19 μm) than the IT-type neuronal perikarya (B). IT-type terminals enter the striatum from the external capsule (C), whereas PT-type terminals arise as collaterals of corticofugal axons as they course through the striatum. Both give rise to varicosities within the striatal neuropil. Scale bars = 50 μm in A–C; 25 μm in D.

Line drawings showing the distribution and laminar location of intratelencephalically projecting (IT)-type and pyramidal tract (PT)-type perikarya in the cortex (A,B) in representative cases. The injections sites yielding the retrograde labeling are depicted (A,C). The IT-type perikarya were retrogradely labeled from the contralateral striatum with tetramethylrhodamine-dextran amine (RDA)3k. As is evident in A, they are largely localized to layer III and upper layer V. By contrast, the PT-type perikarya, retrogradely labeled by biotinylated dextran amine (BDA)3k injection into the ipsilateral pontine pyramidal tract, are largely localized to deep layer V (B). AC, anterior commissure; cc, corpus callosum; Cg, cingulate cortex; GI, granular insular cortex; M1, primary motor cortex; M2, secondary motor cortex; mlf, medial longitudinal fasciculus; n6, abducens nucleus; n7, facial motor nucleus; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; Sp5, spinal nucleus of the trigeminal nerve; V, lateral ventricle.
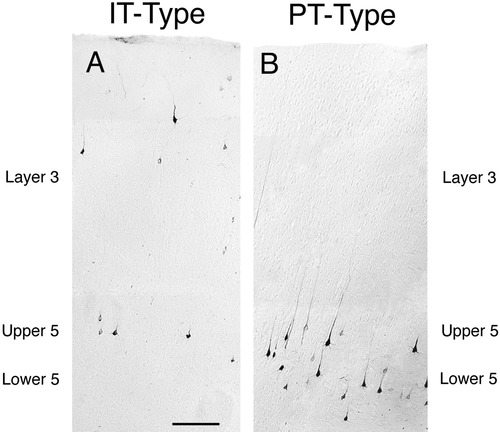
Low-power images of the laminar distribution in primary somatosensory cortex of intratelencephalically projecting (IT)-type (A) and pyramidal tract (PT)-type (B) perikarya in the same rat. The IT-type perikarya were retrogradely labeled from the contralateral striatum with RDA3k. As is evident in Figure 3A, they are moderate in size, and as is evident in A, they are largely localized to layer III and upper layer V. By contrast, the PT-type perikarya, retrogradely labeled by BDA3k injection into the ipsilateral pontine pyramidal tract, are largely localized to deep layer V of the cortex (B) and are larger than the IT-type neuronal perikarya (see Fig. 3B). Scale bar = 200 μm in A (applies to A and B).
Because both PT-type and IT-type neurons project to the ipsilateral striatum, whereas only IT-type neurons project to the contralateral striatum (Wilson, 1987; Cowan and Wilson, 1994), the difference in the ipsilateral versus contralateral laminar distribution of RDA-labeled perikarya after intrastriatal RDA3k injection can be explained by the labeling of both PT-type and IT-type ipsilaterally and the selective labeling of the IT-type contralaterally. Measurement of 192 IT-type neurons in the motor cortex contralateral to the striatal injection and 198 in the somatosensory cortex contralateral to the striatal injection in R36 and R44 (pooled together) indicated a mean perikaryal diameter of 12.2 ± 1.1 μm for this neuron type in the motor cortex and 12.3 ± 1.0 μm in the somatosensory cortex (Table 1). No major layer-specific differences in diameter were found among the IT-type perikarya, although there was a tendency for IT-type neurons of layers III and VI of the motor cortex to be slightly smaller than those in layer V (Table 1).
| Parameter | Layer III | Upper layer V | Lower layer V | Layer VI | All layers |
|---|---|---|---|---|---|
| IT-type Perikarya | |||||
| Motor cortex | |||||
| Diameter | 11.8 ± 0.9 | 12.5 ± 1.1 | 12.2 ± 0.9 | 10.7 ± 0.8 | 12.2 ± 1.1 |
| Number | 34 | 94 | 58 | 6 | 192 |
| Somatosensory cortex | |||||
| Diameter | 12.5 ± 1.1 | 12.1 ± 1.0 | 12.4 ± 0.9 | 12.3 ± 0.6 | 12.3 ± 1.0 |
| Number | 65 | 86 | 42 | 5 | 198 |
| PT-type Perikarya | |||||
| Motor cortex | |||||
| Diameter | None measured | 16.8 ± 1.7 | 18.5 ± 1.7 | None measured | 18.2 ± 1.7 mm |
| Number | 0 | 12 | 83 | 0 | 95 |
| Somatosensory cortex | |||||
| Diameter | None measured | 19.3 ± 1.6 | 19.1 ± 2.1 | 16.7 ± 0.6 | 19.1 ± 2.0 |
| Number | 0 | 13 | 89 | 4 | 106 |
- 1 Diameter are mean ± SD. IT, intratelencephalically projecting; PT, pyramidal tract. Data are for two of the IT-type cases pooled (R36 and R44) and three of the PT-type cases pooled (R6, R17, and R44).
In four of the rats in which we attempted to label both the IT-type and PT-perikarya in the cerebral cortex of the right hemisphere and in the rats used in ultrastructural studies of PT-type terminals in the striatum, BDA3k injected into the right pyramidal tract at caudal pontine levels yielded labeling of numerous perikarya in the ipsilateral cortex (Figs. 3-5). The apical dendrite and the primary and secondary branches of the basal dendrites of these neurons tended to be well labeled, confirming their pyramidal identity. Because many if not all neurons projecting into the pyramidal tract give rise to a striatal collateral (Donoghue and Kitai, 1981; Wilson, 1987; Levesque et al., 1996), most if not all of the BDA3k-labeled neurons in the ipsilateral cortex in these rats are likely to be PT-type corticostriatal projection neurons. We found that these BDA3k-labeled presumptive PT-type neurons commonly possessed larger cell bodies than did the RDA3k-labeled IT-type perikarya and were typically located deeper in layer V than were IT-type perikarya. Simultaneous examination of PT-type and IT-type perikarya in the two-color DAB double-labeled tissue confirmed that the PT-type and IT-type perikarya have overlapping but distinct laminar distributions in the cortex, with the IT-type typically located more superficially in the cortex than the PT-type neuronal perikarya (Figs. 3-5). Measurement of 95 IT-type neurons in the motor cortex and 106 in the somatosensory cortex in rats R6, R17, and R44 (pooled together) indicated a mean perikaryal diameter of 18.2 ± 1.7 μm for this neuron type in the motor cortex and 19.1 ± 2.0 μm in the somatosensory cortex (Table 1). No major layer-specific differences in diameter were found among the PT-type perikarya, but those of upper layer V in the motor cortex were slightly smaller than those in lower layer V of the motor cortex and those in all layers of the somatosensory cortex (Table 1).
Two cases with only PT-type neuron labeling (R6 and R17), one with only IT-type neuron labeling (R36), and one with simultaneous labeling of both neuron types (R44) were used to characterize the laminar distribution of PT-type and IT-type perikarya in the motor (M1 and M2) and primary somatosensory cortex (Fig. 6; Table 2). The results confirmed the qualitative observations described above. Overall for these cases, nearly 80% of the PT-type perikarya were located in lower layer V, and nearly 20% were found in upper V, with the small remainder located in layer VI. The distribution pattern of PT-type perikarya, however, was slightly different for motor than for primary somatosensory cortex, with lower layer V being more the predominant site of PT-type neurons for the somatosensory cortex, and upper layer V being a more common site for the motor cortex than for the somatosensory cortex (Table 2). For the IT-type neurons, about 35% of the perikarya were in layer III, 40% in upper layer V, and nearly 20% in lower layer V, with the small remainder located in layer VI. The distribution pattern of IT-type perikarya was also slightly different for the motor (M1 and M2) than for primary somatosensory cortex, with upper and lower layer V being more the predominant site of IT-type neurons for the motor cortex and layer III and upper layer V being a more common site of IT-type neurons in the primary somatosensory cortex (Table 2). Direct comparison of PT-type neurons and IT-type neurons for perikaryal diameter shows that across all layers of the motor and somatosensory cortices, the PT-type neurons were consistently 5–7 μm larger than the IT-type perikarya (Fig. 7; Table 1).
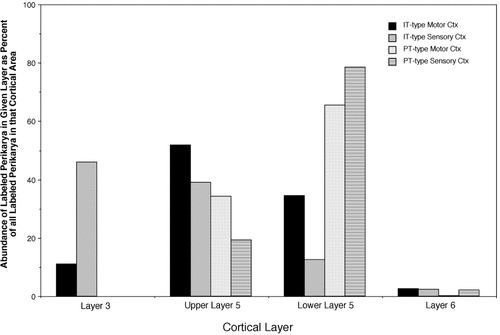
Frequency histogram showing the relative laminar abundances of pyramidal tract (PT)-type and intratelencephalically projecting (IT)-type perikarya in layers III–VI of the motor and somatosensory cortices, expressed as the percent of all labeled neurons of the given type (IT or PT) in a given cortical field (sensory or motor cortex) found in a given layer or sublayer within that field.
| Parameter | Layer III % | Upper layer V % | Lower layer V % | Layer VI % | No. of neurons |
|---|---|---|---|---|---|
| IT-type Perikarya | |||||
| Motor cortex | 11.1 | 51.8 | 34.4 | 2.7 | 477 |
| Sensory cortex | 46.0 | 39.1 | 12.6 | 2.4 | 1,011 |
| Motor and sensory cortex combined | 34.8 | 43.1 | 19.6 | 2.5 | 1,488 |
| PT-Type Perikarya | |||||
| Motor cortex | 0.0 | 34.2 | 65.5 | 0.3 | 345 |
| Sensory cortex | 0.0 | 5.9 | 90.2 | 3.9 | 388 |
| Motor and sensory cortex combined | 0.0 | 19.2 | 78.6 | 2.2 | 733 |
- 1 For abbreviations, see Table 1 footnote. Data are for two of the IT-type cases pooled (R36 and R44) and three of the PT-type cases pooled (R6, R17, and R44).
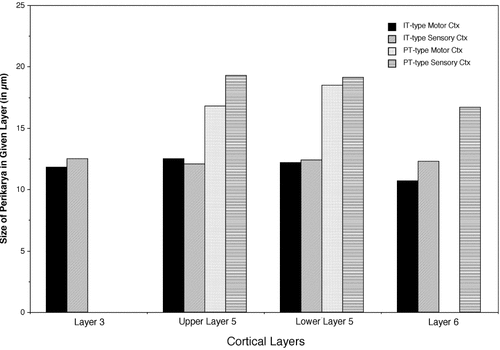
Comparison of pyramidal tract (PT)-type and intratelencephalically projecting (IT)-type perikarya size in layers III, V, and VI in the motor and somatosensory cortex (ctx), with upper and lower layer V considered separately.
Anterograde labeling of IT-type axons and terminals in the striatum.
Following BDA10k injection into the right primary somatosensory (S1) or motor cortex (M1 and/or M2), anterograde labeling of fibers and varicosities was observed along much of the rostrocaudal extent of the ipsilateral striatum, with progressively less labeling at increasingly more caudal levels. Anterograde labeling was also present in these cases in the contralateral (left) striatum (Fig. 3), with the abundance of the contralateral anterograde labeling depending on the extent of the cortical BDA deposit. The contralateral intrastriatal anterograde labeling was, however, consistently less than in the ipsilateral striatum, and was more abundant for the M1/M2 injections than for the S1 injections. Among the eight rats in which cortical BDA10k injection yielded contralateral anterograde labeling in the striatum, the somatomotor and somatosensory cortices were both observed to target the dorsolateral sector of both the ipsilateral and contralateral striatum (Table 3). The intrastriatal axons of IT-type neurons coursed to the contralateral side of the brain in the corpus callosum, approached the striatum from within the external capsule, and departed obliquely from the external capsule to enter the striatum. BDA10k-labeled axons and varicosities were evident in the striatum on both sides of the brain.
| Animal ID no. | Target of BDA 10k injection | Area of left striatum showing anterograde labeling | BDA+ terminals | BDA+ terminals not making synaptic contact in plane observed | BDA+ terminals making synaptic con | |||
|---|---|---|---|---|---|---|---|---|
| No. | Mean size (μm) | No. | Mean size (μm) | No. | Mean size (μm) | |||
| R20 | Right cortex M1/M2 | Dorsolateral | 70 | 0.50 | 23 | 0.46 | 47 | 0.50 |
| R21 | Right cortex M1/M2 | Dorsolateral | 16 | 0.45 | 6 | 0.45 | 10 | 0.45 |
| R30 | Right cortex M1/M2 | Lateral | 15 | 0.47 | 2 | 0.4 | 13 | 0.48 |
| R31 | Right cortex M1/M2 | Dorsolateral | 27 | 0.32 | 20 | 0.31 | 7 | 0.36 |
| R34 | Right cortex S1 | Dorsolateral | 17 | 0.42 | 11 | 0.43 | 6 | 0.41 |
| R80 | Right cortex S1 | Lateral | 31 | 0.35 | 8 | 0.30 | 23 | 0.37 |
| R81 | Right cortex S1 | Lateral | 25 | 0.32 | 17 | 0.29 | 8 | 0.39 |
| R82 | Right cortex S1 | Lateral | 24 | 0.25 | 11 | 0.18 | 13 | 0.33 |
- BDA, biotinylated destran; IT, intratelencephalically projecting; PT, pyramidal tract.
Retrograde collateral labeling of PT-type axons and terminals in the striatum.
After injections of BDA3k into the pyramidal tract at caudal pontine levels, the intrastriatal collaterals of the retrogradely labeled PT-type neurons were labeled. These intrastriatal axons are unlikely to represent anterogradely labeled axons from neurons residing within the pyramidal tract vicinity, because the site of injection was far caudal to any major source of input to the striatum (Heimer et al., 1985; Reiner et al., 1998). Within the striatum, numerous BDA-labeled axons were observed coursing within the pencil bundles that traverse the striatum as they course between the cortex and striatum (Fig. 3). Within the striatal neuropil itself, BDA-labeled axons and varicosities were additionally observed (Fig. 3), representing the selective labeling of the PT-type input to the striatum ipsilateral to the injected pyramidal tract. The BDA3k-labeled intrastriatal collaterals were located in the dorsolateral part of the striatum in cases yielding retrograde labeling of PT-type neurons uniformly throughout the ipsilateral cortex, and more in the intermediate striatum in cases in which retrograde labeling of PT-type neurons was more prominent in temporal cortical fields (Table 4). Whereas the labeled corticofugal axons in the pencil bundles were of a relatively thick caliber, the intrastriatal collaterals were finer in diameter and possessed numerous varicosities along their length (Fig. 3).
| Animal ID no. | Target of BDA 3k injection | Region of cortex showing PT-type neuron retrograde labeling | Area of ipsi-striatum showing anterograde labeling | BDA+ terminals in ipsi-striatum | BDA+ terminals in ipsi-striatum not making synaptic contact in plane observed | BDA+ terminals in ipsi-striatum making synaptic contact in plane observed | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | Average size (μm) | No. | Size (μm) | No. | Size (μm) | ||||
| R6 | Right pyramidal tract | Uniformly throughout cortex | Right dorsolateral striatum | 28 | 0.93 | 7 | 0.75 | 21 | 0.99 |
| R6 | Left pyramidal tract | Throughout cortex, but most abundant in temporal cortical fields | Left striatum widespread | 40 | 0.73 | 18 | 0.64 | 22 | 0.82 |
| R17 | Right pyramidal tract | Throughout cortex, but most abundant in motor and somatosensory cortical fields | Right dorsolateral striatum | 42 | 0.83 | 28 | 0.79 | 14 | 0.92 |
| R18 | Left pyramidal tract | Throughout cortex, but most abundant in temporal cortical fields | Left intermediate striatum | 28 | 0.69 | 11 | 0.59 | 17 | 0.76 |
| R19 | Right pyramidal tract | Throughout cortex, but most abundant in temporal cortical fields | Right intermediate striatum | 34 | 0.70 | 13 | 0.58 | 21 | 0.78 |
| R19 | Left pyramidal tract | Throughout cortex, but most abundant in temporal cortical fields | Left intermediate striatum | 30 | 0.74 | 11 | 0.67 | 19 | 0.77 |
- 1 For abbreviations, see Table 3 footnote.
Electron microscopic observations
Ultrastructure of the BDA-labeled IT-type corticostriatal terminals.
BDA10k-labeled terminals (identified by the presence of synaptic vesicles) were observed at the EM level in the striatum contralateral to the motor cortex or somatosensory cortex injections. Most of these IT-type terminals were observed making contact with spine heads of striatal neurons, and some with dendrites. For all eight cases analyzed, the IT-type terminals tended to be small and regular in shape (Fig. 8; Table 3). Of the 225 BDA10k-labeled IT-type terminals observed contacting spines in the eight cases analyzed, the mean diameter was 0.39 ± 0.03 μm (± SEM; Table 5). IT-terminals arising from the motor cortex appeared to be somewhat larger (0.44 ± 0.05 μm) than those arising from the somatosensory cortex (0.34 ± 0.04 μm). Slightly more than half of the BDA10k-labeled IT-type terminals were observed to form synaptic contact with a spine head (based on the presence of a postsynaptic density) in the plane of section viewed. The BDA10k-labeled terminals that did not make evident synaptic contact with a spine head were likely to have been captured in a plane of section that did not reveal the synaptic contact. IT-type terminals making synaptic contact with a spine appeared to be similar in shape but were slightly larger (0.41 ± 0.02 μm for all eight cases) than those not observed to make synaptic contact in the plane of section viewed. The larger size of the terminals making an observable synaptic contact is consistent with the premise that the plane of section through these terminals tended to capture them more at their midpoint, where the synaptic specialization is more likely to be in evidence. The IT-type synaptic terminals arising from the motor cortex also appeared to be slightly larger (0.45 ± 0.04 μm) than those arising from the somatosensory cortex (0.38 ± 0.02 μm).
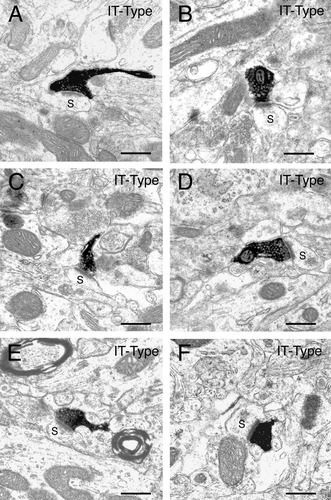
A–F: Examples of the BDA10k-labeled intrastriatal terminals of intratelencephalically projecting (IT)-type corticostriatal neurons at the EM level. The terminals in the six images shown in each case make asymmetric synaptic contact with a spine (s), presumably that of a striatal projection neuron. Note that the terminals are round, largely regular in shape, and about 0.5 μm in diameter. Scale bars = 0.5 μm in A–F.
| Type of terminal | Mean size (±SEM) of all terminals contacting a dendritic spine (μm) | Mean Size (±SEM) of terminals contacting a spine but not making a synaptic contact (μm) | Mean Size (±SEM) of terminals contacting a spine and making a synaptic contact (μm) |
|---|---|---|---|
| IT-type terminals | |||
| Arising from motor cortex (n = 4) | 0.443 ± 0.05 | 0.405 ± 0.04 | 0.448 ± 0.04 |
| Arising from somatosensory cortex (n = 4) | 0.335 ± 0.04 | 0.300 ± 0.06 | 0.375 ± 0.02 |
| Motor and sensory cortex combined (n = 8) | 0.388 ± 0.03 | 0.353 ± 0.04 | 0.411 ± 0.02 |
| PT-type terminals (n = 6) | 0.768 ± 0.04 | 0.670 ± 0.04 | 0.824 ± 0.04 |
- a For abbreviations, see Table 1 footnote.
Ultrastructure of the BDA-labeled PT-type corticostriatal terminals.
BDA3k-labeled terminals (identified by the presence of synaptic vesicles) were observed at the EM level in the striatum ipsilateral to the pyramidal tract injections. Most of these PT-type terminals were observed to make contact with spine heads of striatal neurons. BDA3k-labeled PT-type terminals were also observed to contact dendrites, and, irrespective of postsynaptic target, form exclusively asymmetric synaptic contacts. Unlike the IT-type axospinous terminals, the PT-type terminals contacting striatal spines tended to be large, with many being about 1 μm or more in diameter, and irregular in shape (Table 4). In the case of some of these large PT-type terminals, the spines targeted by PT-type terminals also appeared to be larger than in the case of the spinous targets of the IT-type neurons. In these instances, the presence of spine apparatus and the absence of mitochondria and microtubules indicated the targets of the PT-type neurons to be nonetheless spines (Pasik et al., 1976). Additionally, the synaptic zone postsynaptic to PT-type terminals (as judged by the postsynaptic density) in many cases tended to be lengthy and perforated near its center, and some of the PT-type terminals possessed extensions by which they appeared to envelop their postsynaptic target spine (Fig. 9).
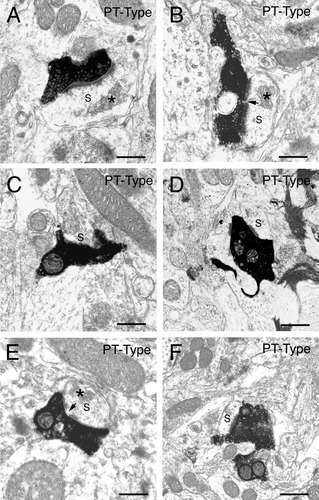
A–F: Examples of the BDA3k-labeled intrastriatal terminals of pyramidal tract (PT)-type corticostriatal neurons at the EM level. The terminals in the six images shown in each case make asymmetric synaptic contact with a spine (s), as revealed by their size and the presence of spine apparatus (asterisk), presumably that of a striatal projection neuron. Note that the terminals shown are typically large, irregular in shape, and in some cases envelop their postsynaptic target structure and are often associated with a perforated postsynaptic density (arrow). Scale bars = 0.5 μm in A–F.
Although the size and morphology of the PT-type terminals did not differ greatly among the six pyramidal tract injection cases analyzed (Table 4), the mean size of the terminals was slightly greater in cases in which many PT-type neurons in the motor and primary somatosensory cortex were intensely retrogradely labeled. Of the 200 BDA10k-labeled PT-type terminals observed contacting spines in the six cerebral hemispheres analyzed, the mean diameter was 0.77 ± 0.04 μm (± SEM; Table 5). Slightly more than half of the BDA3k-labeled PT-type terminals were observed to form synaptic contact with a spine head (based on the presence of a postsynaptic density) in the plane of section viewed. The BDA10k-labeled terminals that did not make evident synaptic contact with a spine head were likely to have been captured in a plane of section that did not reveal the synaptic contact. PT-type terminals synaptically contacting a spine head appeared to be similar in shape but slightly larger (0.82 ± 0.04 μm for all six hemispheres) than those not observed to make synaptic contact in the plane of section viewed. The larger size of the terminals making an observable synaptic contact is consistent with the premise that the plane of section through these terminals captured them more at their midpoint, where the synaptic specialization is more likely to be in evidence. A frequency histogram comparing the size of PT-type and IT-type terminals making axospinous synaptic contact in the striatum for all analyzed terminals pooled shows clearly that the two types of terminals typically differ in size, with PT-terminals being considerably larger (Fig. 10).
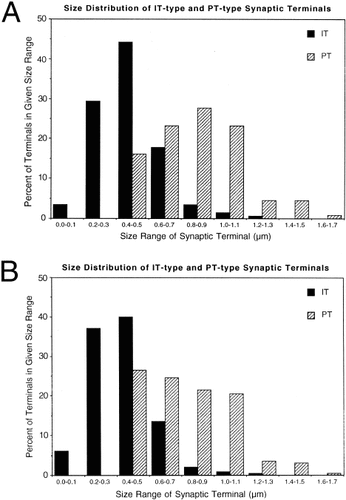
Frequency histogram comparing the size distributions of pyramidal tract (PT)-type and intratelencephalically projecting (IT)-type terminals making axospinous synaptic contact (A) in the striatum for all terminals analyzed pooled together, and for all axospinous terminals irrespective of the presence of a synaptic contact (B) pooled together. The results show that although PT-type and IT-type terminals overlap somewhat in their size range, the two types of terminals appear to be typically different in size, with PT-type terminals considerably large than IT-type terminals.
DISCUSSION
Two main types of corticostriatal projection neurons have been identified by their connections within the telencephalon and their projections to subtelencephalic sites (Cowan and Wilson, 1994). One of these types projects bilaterally to the striatum as well as to the contralateral cortex but does not project outside the telencephalon (Oka, 1980; Wilson, 1987; Cowan and Wilson, 1994; Wright et al., 2001). We have termed these the intratelencephalically projecting (IT)-type corticostriatal neurons. The second type gives rise to an extratelencephalic projection to brainstem sites, and its striatal projection arises as a collateral from this descending axon (Oka, 1980; Donoghue and Kitai, 1981; Cowan and Wilson, 1994; Levesque et al., 1996a, b; Levesque and Parent, 1998; Wright et al., 1999, 2001). The brainstem targets include the thalamus, hindbrain, and spinal cord, with the axons of this type descending to the latter two sites coursing in the pyramidal tract. We have termed these the pyramidal tract (PT)-type corticostriatal neurons. Using strategies for selectively labeling the perikarya and/or intrastriatal terminals of one or the other type of corticostriatal neuron, we have in the present study found that they differ in their perikaryal size, in their laminar distribution in cortex, and in the typical size and shape of their intrastriatal terminals. These findings and their implications, which reinforce notions of functional differences between these two corticostriatal neuron types, are discussed in greater detail below.
Technical considerations
IT-type perikarya.
The approach of injecting RDA into the left striatum and BDA into the right pyramidal tract yields selective labeling of IT-type neurons with RDA in the right cortex and PT-type neurons with BDA in the right cortex, due to the unique differences between these two cells types in their contralateral and descending projections (Fig. 1). The existence of IT-type neurons was first clearly noted in studies using retrograde labeling with horseradish peroxidase (Hedreen, 1977; Hedreen and McGrath, 1977; Wise and Jones, 1977; Veening et al., 1980; Royce, 1982; Goldman-Rakic and Selemon, 1986; Wilson, 1987; McGeorge and Faull, 1989), and later studies revealed further details about the size, laminar location, regional distribution, and intrastriatal arborization of this corticostriatal neuron type (Wilson, 1987; Cowan and Wilson, 1994; Kincaid and Wilson, 1996). Of current methodological interest was the discovery that whereas neurons of this type throughout the cortex project to the ipsilateral striatum (Hedreen, 1977; Hedreen and McGrath, 1977; Wise and Jones, 1977; Veening et al., 1980; Wilson, 1987; McGeorge and Faull, 1989), IT-type neurons in some regions of the cortex (such as the motor cortex and to a lesser extent the somatosensory cortex) project to the contralateral striatum as well (Oka, 1980; Wilson, 1987; Kincaid and Wilson, 1996; Wright et al., 2001). Because corticostriatal neurons with descending projections (which includes the PT-type) have an exclusively ipsilateral projection to the striatum (Wilson, 1987; Cowan and Wilson, 1994), injecting a retrograde tracer such as RDA3k into the left striatum yields selective RDA3k labeling of IT-type neurons within the right cortex. This labeling, however, is limited to those IT-type neurons with a contralateral striatal projection, which raises the possible concern that the traits of those IT-type neurons labeled by our methods may not be exactly representative of all IT-type neurons. Prior studies, however, have shown that IT-type neurons projecting contralaterally are highly similar in their morphology and physiology to those not having a significant contralateral projection (Oka, 1980; Fisher et al., 1984; Wilson, 1987; Cowan and Wilson, 1994; Kincaid and Wilson, 1996; Wright et al., 2001).
PT-type perikarya.
Our approach for selectively labeling PT-type neuronal perikarya also requires acknowledgement of some caveats. First, it must be noted that in retrogradely labeling cortical neurons projecting into the pyramidal tract, we are not directly labeling cortical neurons projecting to the striatum. In principle, it is possible that some or many cortical neurons projecting into the pyramidal tract do not send a collateral into the striatum. Arguing against this possibility are several published reports that the vast majority of, if not all, cortical neurons projecting to the brainstem, including those projecting into the pyramidal tract, give rise to a striatal collateral (Donoghue and Kitai, 1981, 1986; Wilson, 1987; Levesque et al., 1996a, b; Levesque and Parent, 1998). Thus most, if not all, the BDA-labeled neurons in the ipsilateral cortex in rats with pyramidal tract injection of BDA3k are likely to be PT-type corticostriatal projection neurons. An additional concern is the possibility that PT-type neurons with a contralateral striatal projection exist. Arguing strongly against this possibility, however, is the absence of evidence for this possibility in single-cell filling studies of this neuron type (Wilson, 1987; Cowan and Wilson, 1994). This possibility is also inconsistent with the size of the cortical perikarya that we observed labeled contralaterally to striatal injection of RDA3k, which were consistently smaller than was characteristic of PT-type neuronal perikarya.
IT-type terminals.
To label the intrastriatal terminals of IT-type neurons selectively for the purpose of determining their ultrastructural traits, we again relied on the bilateral nature of the projections from the motor cortex and somatosensory cortex to the striatum, as well as the unilateral nature of the PT-type neuron projection. Following unilateral BDA10k injection into the motor (M1/M2) or primary somatosensory cortex, anterograde corticostriatal labeling in the contralateral striatum is limited to IT-type terminals (Fig. 2A). This labeling, however, is restricted to those IT-type neurons with a contralateral striatal projection, which raises the possible concern that the traits of those IT-type terminals labeled by our methods are not representative of all IT-type terminals. Although this possibility needs to be kept in mind in interpreting the generality of our findings, IT-type neurons projecting contralaterally are highly similar in their morphology and physiology to those not having a significant contralateral projection (Oka, 1980; Fisher et al., 1984; Wilson, 1987; Cowan and Wilson, 1994; Kincaid and Wilson, 1996; Wright et al., 2001). Thus, there is no demonstrated reason to believe that IT-type terminals arising from the contralateral motor or somatosensory cortex differ significantly in their traits from those arising from the ipsilateral cerebral cortex.
PT-type terminals.
To label the intrastriatal terminals of PT-type neurons selectively, we relied on the ability of BDA3k to label collaterals of retrogradely labeled neurons (Chen and Aston-Jones, 1998; Reiner et al., 2000) and on the fact that IT-type neurons do not have extratelencephalic projections (Wilson, 1987; Wilson and Cowan, 1994). The disadvantage of this approach is that we could not be sure of the cortical region giving rise to any particular labeled PT-type terminal. By limiting our examination to the dorsolateral sector of striatum, which receives prominent input from somatomotor-somatosensory cortex (Goldman-Rakic and Selemon, 1986; Kincaid and Wilson, 1996; Alloway et al., 1998; Hoffer and Alloway, 2001), we were able to overcome this limitation to some extent. A further caveat associated with our selective labeling of PT-type neurons is that we cannot at present know the extent to which the terminal morphology of this type of corticostriatal neuron is also characteristic of those brainstem-projecting corticostriatal neurons (if there are any) that project to regions anterior to the pons, such as the thalamus and midbrain, but do not also project to the hindbrain and spinal cord (Levesque et al., 1996a, b; Levesque and Parent, 1998).
IT-type and PT-type neuronal perikarya
Laminar distribution of IT-type perikarya.
Numerous earlier studies in rats reported retrograde labeling of a large and widespread population of neurons in ipsilateral upper layer V following HRP injection into the striatum (Hedreen, 1977; Hedreen and McGrath, 1977; Wise and Jones, 1977; Veening et al., 1980). Use of the more sensitive retrograde tracer tetanus toxin in rat revealed additional corticostriatal neurons in ipsilateral cortical layer III (Schwab et al., 1977). It is now evident that these HRP-labeled or tetanus toxin-labeled perikarya in layers III and V mainly represented IT-type corticostriatal projection neurons (Wilson, 1987; Cowan and Wilson, 1994). A relatively insensitive retrograde tracer such as HRP would be likely to label many neurons of this type preferentially even in the ipsilateral striatum because individual neurons of this type typically give rise to a more uniform and widespread intrastriatal arborization than do individual PT-type neurons (Wilson, 1987; Cowan and Wilson, 1994; Wright et al., 2001). Additionally, because layer III corticostriatal neurons only include the IT-type, according to the current data and previously published data (Wilson, 1987; Cowan and Wilson, 1994), ipsilateral labeling of layer III corticostriatal neurons after striatal tracer injection would necessarily be limited to the IT type.
In the present study, we confirmed that IT-type corticostriatal neurons are mainly found in layer III and in upper layer V, and we provided further details on the relative distributions of IT-type neurons across layer III, upper and lower layer V, and layer VI in rats. We found that the laminar distribution of perikarya was slightly different for the motor (M1 and M2) than for primary somatosensory cortex. In the somatosensory cortex, the vast majority of IT-type perikarya were found in layer III and upper layer V, with the neurons being nearly equally abundant in the two. By contrast, in the motor cortex, many more perikarya were found in lower layer V than in layer III. Nonetheless, upper layer V was still the predominant location of IT-type perikarya in the motor cortex. These findings on the laminar location of the perikarya of IT-type neurons agree with the findings of prior studies in rats (Wilson, 1987; Cowan and Wilson, 1994). The laminar distribution of IT-type neurons may, however, differ in different mammalian species. For example, in cats, layer III seems to be the more prevalent location of retrogradely labeled neurons in the ipsilateral cortex after intrastriatal injection of HRP (Oka, 1980; Royce, 1982). Monkeys, however, appear to be more similar to rats, because upper layer V has been reported to be the predominant location of retrogradely labeled neurons in the ipsilateral monkey cortex after intrastriatal HRP injection (Jones and Wise, 1977; Jones et al., 1977; Goldman-Rakic and Selemon, 1986).
Laminar distribution of PT-type perikarya.
Although Ramón y Cajal had noted in his studies of Golgi-stained material that corticofugal axons appeared to give rise to an intrastriatal collateral as they coursed through the striatum (Ramón y Cajal, 1911), retrograde labeling studies with HRP seemed to dispute the notion that corticofugal pyramidal neurons project to the striatum (Jones and Wise, 1977; Jones et al., 1977; Hedreen, 1977; Hedreen and McGrath, 1977). These studies were rather taken to suggest that those neurons we have termed IT-type neurons were the main or only type of neuron in the cortex projecting to the striatum. As noted by Wilson (1987), however, the IT-type neuron by itself lacks several of the known characteristics of the corticostriatal projection considered as a whole, such as the dichotomy found among corticostriatal projection neurons in conduction velocity (Kitai et al., 1976; Jinnai and Matsuda, 1979; Wilson et al., 1982), and so it cannot account for the entire projection. Single-cell physiology and cell-filling studies have clearly shown that the PT-type neuron represents the type of corticostriatal neuron with a fast conduction velocity and that it possesses a different laminar distribution than does the IT-type neuron (Kitai et al., 1976; Donoghue and Kitai, 1981, 1986; Cowan and Wilson, 1994; Turner and DeLong, 2000). Cortical neurons projecting to the brainstem include those projecting to such diverse targets as the thalamus, midbrain, hindbrain, and spinal cord, with the large pyramidal neurons of deep layer V being a major subset whose axons course to the hindbrain and spinal cord via the pyramidal tract (Wise and Jones, 1977; Killackey et al., 1989).
Several studies have shown by morphological and/or electrophysiological means that all or nearly all cortical pyramidal neurons projecting to the brainstem and/or spinal cord send a collateral into the striatum (Donoghue and Kitai, 1981, 1986; Cowan and Wilson, 1994; Levesque et al., 1996a, b; Levesque and Parent, 1998). On this basis, then, it would seem likely that the distribution of retrogradely labeled neurons observed by us in the ipsilateral cortex after pyramidal tract injection of BDA3k represents the distribution of PT-type corticostriatal neurons, with minimal labeling of pyramidal tract neurons not having a striatal collateral. As reported to be true of pyramidal tract neurons projecting to the hindbrain/spinal cord (Wise and Jones, 1977; Killackey et al., 1989), we found that across the cortex the vast majority of the PT-type neurons were located in lower layer V. We did, however, observe a slight difference between the motor cortex and primary somatosensory cortex in this regard, with about 90% of the PT-type neurons of the somatosensory cortex located in deep layer V, but only 65% of the PT-type neurons of the motor cortex situated in deep layer V. Most of the PT-type neurons not in deep layer V were located in upper layer V. Our findings therefore extend previous observations that the predominant locus of PT-type neurons in cortex is in deep layer V (Cowan and Wilson, 1994).
IT-type and PT-type neuron perikaryal size.
Wise and Jones (1977) reported the mean perikaryal diameter for several different types of layer V neurons in the somatosensory cortex in rat. They found that corticospinal neurons, which are situated in the deep half of layer V, have a mean somal diameter of 21.3 ± 2.9 μm, whereas corticomedullary projection neurons were reported as 18.8 ± 4.9 μm in diameter. Because it is now known that most of these neurons also send a collateral into the striatum, these findings suggest that PT-type neurons are 18–21 μm in diameter. Neurons referred to as corticostriatal neurons by Wise and Jones (1977) were reported to be medium-sized (15.2 ± 2.8 μm) and pyramidal in morphology. As noted above, it is now evident that these labeled perikarya mainly represent the IT-type corticostriatal projection neurons but may include some unknown number of PT-type neurons. Similar sizes for these two corticostriatal neuron types have also been reported for monkeys (Jones and Wise, 1977; Jones et al., 1977). Thus, prior studies have suggested that PT-type neurons tend to be 3–6 μm larger in diameter than IT-type neurons.
Our current findings suggest that this difference is even greater than prior data indicate. The mean size we observed for PT-type neurons was 18–19 μm. These neurons clearly corresponded in size, shape, and laminar location to the large pyramidal neurons of the rat cortex previously reported to project to the hindbrain and spinal cord. By contrast, we observed that the IT-type neurons, although still pyramidal in morphology, tended to have a more pear-shaped perikaryon, less well-developed basal dendrites than PT-type neurons, and a cell body in the 12–13-μm-diameter range. Although it is possible that these traits may only be true of the IT-type neurons we labeled from the contralateral striatum, our findings for the size and shape are consistent with the apparent traits reported for this same cell type in the primary somatosensory cortex labeled from the ipsilateral striatum (Wright et al., 2001) and with the IT-type cells shown in McGeorge and Faull (1989). It may be that prior reports in rats that corticostriatal neurons labeled from the ipsilateral striatum (among which IT-type neurons would predominate) had a mean diameter of 14–15 μm may have included some PT-type neurons among the corticostriatal neurons measured. Single-cell labeling studies have not reported on the size of the IT-type neurons, other than to indicate that they are clearly smaller than PT-type neurons (Wilson, 1987; Cowan and Wilson, 1994). Our results therefore suggest the conclusion that IT-type corticostriatal neurons are substantially smaller than PT-type corticostriatal neurons.
Topographic organization of PT and IT neurons.
Restricted parts of the cerebral cortex have been shown to have bilateral projections to the striatum in a variety of mammalian species (Carman et al., 1965; Kemp and Powell, 1970, 1971b; Künzle, 1975; Jones et al., 1977; Cospito and Kultas-Ilinsky, 1981; Tanaka et al., 1981; Fisher et al., 1984; Tanaka and Sakai, 1985; Ferino et al., 1987; Wilson, 1987; McGeorge and Faull, 1989; Tehovnik et al., 1989; Ebrahimi et al., 1992; Cowan and Wilson 1994). Both anterograde and retrograde labeling studies show that the cingulate and motor cortices in rats and other mammalian species appear to be a prominent source of the contralateral corticostriate projection (Donoghue and Wise, 1982; Schwartz and Goldman-Rakic, 1982; Arikuni and Kubota, 1986; McGeorge and Faull, 1989). Although the primary somatosensory cortex also projects to the contralateral striatum, this projection is less robust than that of the motor and cingulate cortices, at least in rats (Kincaid and Wilson, 1996; Kincaid et al., 1998; Wright et al., 1999, 2001). Neurons projecting to the brainstem, however, appear to be present in diverse cortical areas and are especially prominent in somatosensory and motor cortices (Jones, 1984; Jones and Wise, 1977; Wise and Jones, 1977; Killackey et al., 1989; Levesque et al., 1996a, b; Levesque and Parent, 1998). Because these brainstem-projecting neurons are all or nearly all likely to have a striatal collateral (Levesque et al., 1996a, b; Levesque and Parent, 1998), PT-type neurons are likely to be present in diverse regions of the cortex.
The present study confirms that IT-type corticostriatal projection neurons projecting to the contralateral striatum are most common in motor cortices and less common in other cortical areas and suggests that presumptive PT-type neurons are found throughout the cortex. The possibility that IT-type neurons lacking a contralateral projection are more widespread than those projecting contralaterally cannot be ruled out.
Morphology of IT-type and PT-type synaptic terminals
Cowan and Wilson (1994) examined the morphology of the intrastriatal axonal arborization of IT-type and PT-type corticostriatal neurons in the medial agranular cortex in rat by filling single corticostriatal cells with biocytin. They reported that the PT-type neurons, identified by antidromic activation from the pyramidal tract, give rise to one or more small and dense focal arborizations (about 250 μm in diameter) scattered over a 1–2-mm region of the striatum (Cowan and Wilson, 1994; Kincaid and Wilson, 1996). By contrast, the intrastriatal axon of intratelencephalically projecting (IT-type) corticostriatal neurons was reported to give rise typically to an extended and uniform arborization that has sparse en passant terminals over a wide (about 1.5 mm in diameter) region of the striatum (Cowan and Wilson, 1994; Kincaid and Wilson, 1996). The arborization style of PT-type neurons has been termed focal and that of IT-type neurons diffuse or extended (Cowan and Wilson, 1994; Wilson, 1995; Wright et al., 1999, 2001). Few details have been available on the morphology of the intrastriatal terminals of these two types of corticostriatal projection neurons. Kincaid et al. (1998) used anterograde labeling and EM methods to show that corticostriatal terminals arising from the ipsilateral cingulate and motor cortex in rat as a group range in diameter from 0.2 to1.0 μm, with a mean size of about 5 μm. They were not, however, able to distinguish between PT-type and IT-type neuron terminals in their methods.
The work of Wright et al. (1999, 2001) sheds light on the differential morphology of the intrastriatal terminals of IT-type and PT-type corticostriatal neurons located in the barrel field of the primary somatosensory cortex of rat. In brief, their findings are consistent with those reported here that intrastriatal PT-type terminals differ in several respects from IT-type terminals. In their study, they used two anterograde pathway tracers, PHA-L and BDA, and found that two distinct types of corticostriatal pathways arise from the barrel cortex. One system was reported to give rise to a nontopographic projection to the striatum with an intrastriatal arborization that was termed a “diffuse” system (Wright et al., 1999). This projection was found to arise largely from neurons located between barrel columns that also project contralaterally to the striatum (Wright et al., 2001). The illustrations of these corticostriatal neurons in Wright et al. (2001) suggest that these neurons resemble the IT-type neurons described in the present paper in their size, shape, and preferential localization in upper layer V. The other corticostriatal projection arising from the barrel cortex was reported to give rise to a topographically ordered projection that was termed the “discrete pathway.” This corticostriatal projection arose as collaterals of corticofugal axons descending through the striatum and only projected to the ipsilateral striatum. Moreover, the intrastriatal arborization of the discrete system gave rise to patches of dense focal innervation. Because of these various features of these two projection systems, the authors concluded that the diffuse system appeared to arise from the IT-type corticostriatal projection neurons previously described by Wilson and his coworkers (Wilson, 1987; Wilson and Cowan, 1994), whereas the discrete system appeared to arise from the PT-type corticostriatal projection neurons previously described by Wilson and his coworkers (Wilson, 1987; Wilson and Cowan, 1994).
Wright et al. (1999) also examined the LM and EM morphology of the two types of intrastriatal terminals they had observed arising from the barrel cortex. The fibers of the diffuse pathway were noted to be fine caliber, and at the EM level the terminals of this pathway were described as small, with a mean area of 0.32 μm2 (indicating a mean diameter of approximately 0.2–0.4 μm, assuming a rounded profile). These terminals were reported to make asymmetric synaptic contacts with the dendritic spines of striatal projection neurons, and the contacts were described as usually possessing a simple (i.e., uniform) postsynaptic density. By contrast, the discrete system was made of thicker axons that gave rise to large terminals, whose mean area was 0.79 μm2 (indicating a mean diameter of about 0.8–1.0 μm, assuming a rounded profile). The terminals of the discrete system were also reported to make asymmetric synaptic contacts with the dendritic spines of striatal projection neurons, but these contacts were described as complex and the spines as typically possessing a perforated postsynaptic density. At the EM level, we observed that axospinous IT-type intrastriatal terminals are characteristically small (0.41 μm mean diameter), and rounded, whereas PT-type terminals are large (0.82 μm mean diameter), and complex in shape. Moreover, the IT-type terminals formed simple asymmetric synaptic contacts with spines, whereas the PT-type terminals in many cases formed asymmetric synaptic contacts with spines possessing a perforated postsynaptic density.
Thus, although only one prior study has raised the possibility that PT-type and IT-type neurons differ in the size and shape of their intrastriatal terminals, the findings of this study are consistent with our own. Recently, however, a population of brainstem-projecting corticostriatal neurons (possibly not projecting to caudal pontine levels within the pyramidal tract) was detected whose intrastriatal collateral gives rise to an extended and uniform arborization closely resembling that previously reported for the IT-type corticostriatal neuron (Zheng and Wilson, 2001). Additionally, some IT-type neurons may possess ipsilateral and contralateral projections to the striatum that give rise to focal arborization within the patches constituting the striosomal compartment of the striatum (Kincaid and Wilson, 1996; Levesque and Parent, 1998). As a consequence, we cannot yet be sure that the differences observed in intrastriatal terminal size for the two types of corticostriatal neurons studied here are true for all intratelencephalically projecting corticostriatal neurons and for all brainstem-projecting neurons. Our findings, together with those of Wright et al. (1999, 2001), suggest, nonetheless, that corticostriatal neurons projecting into the pontine pyramidal tract give rise to intrastriatal terminals that are considerably larger and more complex than the corticostriatal neurons of the motor and sensory cortex that project only within the telencephalon.
Functional considerations
Corticostriatal terminals make excitatory synaptic contacts on striatal spiny neurons (Gerfen, 1988, Wilson, 1992). Striatal projection neurons themselves show low spontaneous spike discharge, as well as membrane fluctuations between a more and less hyperpolarized state similar to that observed in corticostriatal neurons, with action potentials only occurring in the “up” state (Wilson, 1995). The resting membrane fluctuations of striatal neurons and the action potentials generated depend on the level of activity in the cortical input to individual striatal projection neurons (Wilson, 1992, 1995). The differences observed here between PT-type and IT-type terminals suggest that these two types of corticostriatal projection neurons may differ in their impact on striatal neurons in at least two ways, the specific type of neuron targeted and the efficacy with which target neurons are activated.
First, it is possible that IT-type and PT-type corticostriatal neurons target different types of striatal projection neurons. Two major types of striatal projection neurons, which are all γ-aminobutyric acid (GABA)ergic, have been identified, those containing the neuropeptides substance P and dynorphin, which project to the entopeduncular nucleus (the internal pallidal segment in primates) and the substantia nigra, and those containing enkephalin, which project to the globus pallidus (the external pallidal segment in primates; Anderson and Reiner, 1990; Besson et al., 1990; Graybiel, 1990; Reiner and Anderson, 1990; Gerfen, 1992). The former are thought to be involved in promoting desired movement and in models of basal ganglia function have been referred to as the source of the direct pathway, whereas the latter are thought to be involved in inhibiting unwanted movement and are referred to as the source of the indirect pathway (Albin et al., 1989; DeLong, 1990).
It may be the case that PT-type and IT-type neurons differ in which of these two types of striatal projection neurons they target. Although this might provide an explanation for why the target spines of the two types of corticostriatal terminals examined here differ in size, there is currently no available evidence that the two major types of striatal projection neurons differ in their dendritic spine morphology (Pasik et al., 1976; Wilson et al., 1983; Freund et al., 1984; Izzo et al., 1987), and there is no published evidence that PT-type and IT-type terminals differ in targeting direct-type and indirect-type striatal projection neurons (Hersch et al., 1995). Nonetheless, the notion that PT-type and IT-type neurons may target different types of striatal projection neurons remains of interest and requires further examination. The target neurons of the PT-type terminals is of particular interest, because this projection may provide striatal neurons with a copy of the cortical motor signal (Endo et al., 1973; Miller, 1975; Oka and Jinnai, 1978; Jinnai and Matsuda, 1979; Kitai et al., 1976; Donoghue and Kitai, 1981, 1986; Landry et al., 1984). Knowing which striatal neurons receive such information will help clarify their role in basal ganglia function.
Second, it may be the case that the size difference and the synaptic morphology difference between PT-type and IT-type neurons have an impact on synaptic transmission between these two types of corticostriatal neurons and their synaptic targets. Our work and that of Wright et al. (1999) show that the PT-type input to striatum typically ends as large terminals on spines with a perforated postsynaptic density. Although the functional significance of such perforations has not been established, it has been suggested that they augment synaptic efficacy, either because of the increased postsynaptic density surface area created by the perforation or by the tighter coupling between pre- and postsynaptic membranes at the perforation (Herrera et al., 1985; Geinisman et al., 1987a, b; Geinisman, 1993; Sulzer and Pothos, 2000). The large size of the PT-type terminals may also facilitate synaptic transmission (Herrera et al., 1985; Geinisman, 1993; Sulzer and Pothos, 2000). If the PT-type terminals also selectively contact a particular type of striatal projection neuron, they might then be able to activate these target spiny striatal neurons robustly. If enkephalinergic neurons are, for example, a preferential target of the PT-type input, it might explain why enkephalinergic striatal neurons appear more responsive to cortical input than the substance P/dynorphin-containing neurons (Uhl et al., 1988; Parthasarathy and Graybiel, 1997). On the other hand, the small size of the IT-type terminals and the diffuse nature of the projections of IT-type neurons may require greater synchrony of firing of the IT-type neurons projecting to a given set of spiny striatal neurons to activate them (Wilson, 1992, 1995).
Of further interest, perforated postsynaptic densities have been noted to be associated with synaptic sites at which learning (notably long-term potentiation) has occurred (Geinisman et al., 1991, 1996; Geinisman, 1993; Sulzer and Pothos, 2000; Topni et al., 2001). Spines with perforated postsynaptic densities are common in the striatum (Roberts et al., 1996), long-term potentiation has been observed in striatal neurons following tetanic activation of the cortical input (Calabresi et al., 1992), and some form of motor learning is thought to be involved in the normal role of the basal ganglia in movement control (Saint-Cyr et al., 1988; Knopman and Nissen, 1991; Marsden and Obeso, 1994; Graybiel and Kimura, 1995; Gabrieli, 1995). Thus, PT-type terminals and their target striatal projection neurons may be more prominently associated with motor learning in the basal ganglia than is the case for IT-type neurons and their striatal cellular target neurons. Identification of the specific types of striatal neurons targeted by the two types of corticostriatal neurons studied here may therefore provide some further insight into the workings of the basal ganglia in motor function.
Acknowledgements
We thank Drs. C. Meade, Y.P. Deng, and Z. Sun for their helpful comments during the course of this study, and we are grateful to S.L. Cuthbertson for technical assistance and advice.




