Rearrangement of GUV-confined actin networks in response to micropipette aspiration
Abstract
Although diverse actin network architectures found inside the cell have been individually reconstituted outside of the cell, how different types of actin architectures reorganize under applied forces is not entirely understood. Recently, bottom-up reconstitution has enabled studies where dynamic and phenotypic characteristics of various actin networks can be recreated in an isolated cell-like environment. Here, by creating a giant unilamellar vesicle (GUV)-based cell model encapsulating actin networks, we investigate how actin networks rearrange in response to localized stresses applied by micropipette aspiration. We reconstitute actin bundles and branched bundles in GUVs separately and mechanically perturb them. Interestingly, we find that, when aspirated, protrusive actin bundles that are otherwise randomly oriented in the GUV lumen collapse and align along the axis of the micropipette. However, when branched bundles are aspirated, the network remains intact and outside of the pipette while the GUV membrane is aspirated into the micropipette. These results reveal distinct responses in the rearrangement of actin networks in a network architecture-dependent manner when subjected to physical forces.
1 INTRODUCTION
In cells, actin networks are capable of specialized tasks such as bearing external forces, changing cell mechanics, and exerting physical forces, enabled by their ability to rearrange and assemble into diverse networks via numerous actin-binding proteins (Blanchoin et al., 2014; Fletcher & Mullins, 2010). Given the mechanical milieu that cells experience while navigating their environment is constantly changing, this demands cells to adapt by reassembling the cytoskeleton. Whether stretching, compressing, or locally indenting, actin self-assembles in response to the mechanical stimuli. For instance, locally indenting a cell using microneedle probes revealed a quick response in the dynamics of actin cortex to reinforce the cell membrane (Hu et al., 2019). Others have also investigated responses of actin networks by probing actin-binding protein localization. Under micropipette aspiration-induced loading, it was discovered that myosin and alpha-actinin localization were sensitive to area dilation induced by aspiration, while filamin had a pronounced response to high-shear regions (Luo et al., 2013). Similarly, using numerous cell types, many have characterized the dynamics of actin networks in response to physical forces. However, although collective responses of actin dynamics to mechanical cues have been investigated in cells, it remains unclear how specific actin architectures respond differentially to applied forces. There is convincing evidence that loading remodels reconstituted dendritic actin network architecture in the absence of a membrane-bound compartment (Bieling et al., 2016). Given the nature of membrane-cytoskeleton interactions, investigating differential dynamics of actin architectures assembled by various actin-binding proteins to applied forces in a confined membrane space is vital for dissecting the mechanical responses in cells.
The challenge of investigating cellular processes in a modular manner is the intrinsic complexity of the whole cell. Prior studies characterizing the mechanical properties of actin have tackled this challenge by leveraging bottom-up reconstitution using giant unilamellar vesicle (GUV)-based minimal cell models. For example, studies have investigated GUV shape change due to forces exerted by actin networks (Dürre et al., 2018; Litschel et al., 2021; Liu et al., 2008). Furthermore, many perturbation methods used for mechanophenotyping biological samples have been implemented to mechanically perturb GUV-based minimal cell models (Wubshet & Liu, 2023). Using some of these perturbation methods, studies have investigated compressibility modulus (Schäfer et al., 2013), viscoelastic response (Perrier et al., 2019), and deformability of actin network-encapsulating GUVs (Wubshet et al., 2023). However, to date, how isolated actin architectures in a cell-like confinement dynamically respond to physical forces remains to be explored.
In this work, we aim to investigate the reorganization of actin networks assembled by different actin-binding proteins and whether they exhibit differential dynamic responses to loading. To do this, we reconstitute bundled networks and branched-bundled actin networks in GUVs and subject them to external force using micropipette aspiration. Micropipette aspiration is a well-established technique originally developed for making mechanical measurements in membrane biophysics. Here, instead of making direct measurements of membrane physical quantities, we repurposed micropipette aspiration to study dynamic responses of membrane-confined actin networks. A similar approach of using a PDMS-based aspiration system to study force-induced responses of FtsZ networks encapsulated in GUVs has been reported (Ganzinger et al., 2020). Using micropipettes, we induce a negative pressure to aspirate on single GUVs. In F-actin GUVs, we observed that actin filaments resist deformation and withstand forced entry into the micropipette. Interestingly, we find that fascin-bundled networks rearrange and align parallel to the flow axis of the micropipette. However, addition of Arp2/3 complex, which assembles branched-bundled networks, inhibits bundle alignment, subsequently preventing the network from entering the micropipette.
2 MATERIALS AND METHODS
2.1 Reagents
Purified actin and Arp2/3 complex were purchased from Cytoskeleton, Inc. ATTO 488 actin was purchased from Hypermol. General actin buffer (G-buffer) and actin polymerization buffer (F-buffer) were prepared as directed by Cytoskeleton, Inc. Fascin and hexa-histidine-VCA (His6-tag VCA) were purified as detailed in Wubshet et al. (2021); 10 mg/mL stock concentration of actin was diluted to 1 mg/mL and further serially diluted as needed to working concentrations using G-buffer + 0.5 mM DTT + 0.2 mM ATP. 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (nickel salt) (DGS-NTA(Ni)), and cholesterol were purchased from Avanti Polar Lipids. Optiprep, a density gradient medium, was purchased from Sigma Aldrich.
2.2 Micropipette aspiration
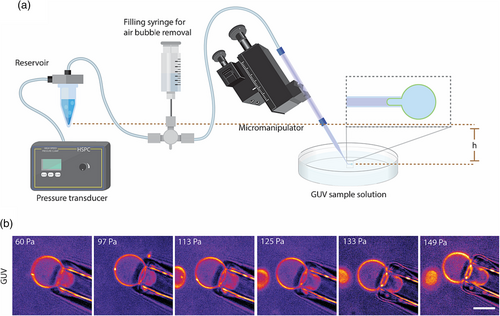
The micropipette aspiration setup consists of a pulled and cut micropipette tip mounted on a micropipette holder, a pipette micromanipulator, a liquid reservoir, a filling syringe, and a pressure transducer.
To prepare micropipettes, standard glass capillaries (WPI) were pulled using a Sutter P-87 pipette puller (Sutter Instruments). After pulling a parallel-walled pipette tip, pipettes were manually cut to an acceptable size in the range of 4–8 μm using a heated glass rod. A glass rod is heated using a Bunsen burner and brought to contact with the pulled capillary at the tip. Instantly after contact, the glass rod is pressed through the pipette in a swift motion to result in a clean pipette cut. This is principally similar to cutting pipettes using microforges. Next, pipettes were submerged in a 1% BSA solution to prevent GUV adhesion. Integration of the micropipette aspiration setup, shown in Figure 1a, is as follows: (1) Pipettes are mounted and secured into a pipette holder. (2) A three-way valve connects a fluidic line between the filling syringe, the micropipette, and the fluid reservoir. (3) The reservoir is pneumatically connected to a high-speed pressure clamp (HSPC, ALA Scientific Instruments). The filling syringe, containing a solution osmotically matched to the encapsulated solution in GUVs, is used to fill the micropipette line and reservoir. Elimination of all air bubbles is vital for performing micropipette aspiration experiments. Once the sample is ready for imaging and aspiration, a slight positive pressure should be maintained either by keeping the reservoir at a slightly higher altitude or applying a minimal positive pressure using the pressure transducer. This should be maintained until the GUV of interest is located under the microscope. Finally, using a micromanipulator, the pipette will be brought to close proximity. This should result in a slight pushing of the GUV from the positive pressure coming from the pipette tip. Once GUV is located, the pressure transducer is used to apply an appropriate negative pressure to induce aspiration of the GUV.
2.3 Actin encapsulation inside GUVs
First, inner solutions of the desired actin/actin-binding protein composition were prepared. To reconstitute fascin-bundled actin networks inside GUVs, fluorescent filamentous actin was reconstituted by incubating 5.3 μM actin, 0.53 μM of ATTO 488 actin in F-buffer supplemented with 3 mM ATP on ice for 15 min. Next, fluorescent filamentous actin was brought to room temperature, and fascin, at a 1:5 ratio to actin, was added to the solution to initiate the formation of bundles. To reconstitute actin cortex into GUVs, filamentous actin was prepared identical to the above followed by the addition of 500 nM Arp2/3 and 500 nM His6-tag VCA; 7.5% Optiprep is included in the final volume of inner solutions to facilitate GUV sedimentation as a result of a density difference between inner and outer solutions. After the addition of crosslinker proteins, the inner solution is immediately emulsified in a lipid/oil mixture via pipetting the solution up and down to create volumetric confinement to actin networks before reaching full network assembly. Droplet emulsions are then dispensed in the rotating cDICE chamber to form GUVs. Prior to the addition of emulsified inner solution, in a rotating cDICE chamber, we add an outer aqueous solution (similar osmolarity to the inner solution of ~200 mOsm) and an oil/lipid mixture. These two solutions are planarly layered due to centrifugal forces. Oil phase in our oil/lipid mixture contains 20/80% (v/v) mineral oil/silicon oil, and our standard lipid composition uses DOPC/cholesterol at 70/30 mol/mol. Details of actin encapsulation inside GUVs are as described in Bashirzadeh et al. (2021).
2.4 Imaging and analysis
Micropipette aspiration of actin GUVs (of a diameter of ~15–40 μm) was observed using Olympus IX-81 inverted microscope equipped with a spinning disk confocal (Yokogawa CSU-X1), OBIS LS/LX lasers (Coherent), and an iXON3 EMCCD camera (Andor Technology). Metamorph was used to control the above components and acquire images; 40×/1.3 NA objective was used for imaging samples. Fluorescence images of actin networks were acquired to visualize networks by exciting ATTO 488 actin at 200 ms exposure, while low-light brightfield images were simultaneously taken to capture and track the micropipette tip as it was operated using a micromanipulator. To record actin dynamics in response to changes in aspiration pressure, timelapse images were taken every 300 ms. Images were processed using ImageJ. After acquiring line-scan data from ImageJ, using Python's matplotlib library, we plotted intensity profiles and bar graphs. Statistical analysis between peak means of each two groups, pre- and post-aspiration of bundles and branched bundles, was done using Student's t-test with p = 0.05 as the level of significance.
3 RESULTS
3.1 Actin filaments inside GUVs display resistance to aspiration
To validate our micropipette aspiration setup (Figure 1a), empty 70% DOPC/30% cholesterol GUVs with a trace amount of fluorescent lipid were aspirated at incremental pressure points (Figure 1b). After filling all fluidic tubings with glucose solution, a net positive pressure is applied at the tip of the micropipette by slightly raising the reservoir with respect to the tip to assure air bubbles are not drawn into the tip when the micropipette tip is not submerged into the sample solution containing GUVs (Figure 1a). Once the micropipette is positioned, using the micromanipulator, in the correct frame and plane where GUVs are, we use the motorized microscope stage to locate a GUV of interest with respect to the micropipette tip, which is in a fixed frame. Once aspirated, GUVs instantly seal the pipette and protrude. At each pressure increment, we observed aspirated protrusions elongating (Figure 1b). We can also see that there is not a visible decrease in the GUV size outside of the pipette despite an increase in elongation of protrusion. This is consistent with prior observations (Olbrich et al., 2000) and is attributed to area dilation due to the elastic stretching of the GUV lipid bilayer. Following validation of the micropipette aspiration setup, we investigated the mechanical response of GUVs encapsulating actin filaments.
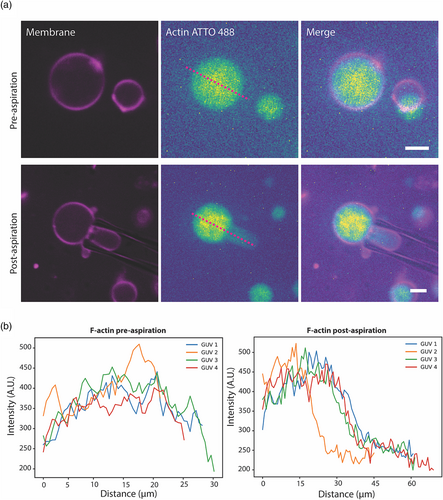
As the major determinants of cellular mechanics, we became interested in how actin filaments encapsulated inside GUVs respond to loads by using micropipette aspiration. We reconstituted actin filaments by polymerizing 5.3 μM globular actin using F-buffer. Similar to empty GUVs, actin filaments were encapsulated in DOPC/cholesterol (70/30) GUVs. When unaspirated, fluorescence images of F-actin GUVs display uniform distribution of filaments throughout the GUV lumen (Figure 2 top). Interestingly, we saw that this symmetry/uniformity breaks when actin-GUVs are aspirated (Figure 2 bottom). The actin density is lower throughout the protrusion compared to the GUV lumen outside of the pipette. Figure 2b displays line scan profiles of crossecting lines similarly shown in Figure 2a pre- and post-aspiration, showing the diminished F-actin fluorescence in the micropipette post-aspiration. Actin filaments are flexible polymers with lengths ranging from submicron to a few microns in length. Our findings suggest that polymerized filaments at 5.3 μM behave in a connected manner, possibly through entanglement (Gardel et al., 2003), to resist aspiration and remain outside of pipette constriction. Transforming actin filaments into more complex networks, for example via branching, could potentially increase network resistance thus diminishing actin fluorescence in the micropipette.
3.2 Fascin-bundled actin aligns along the axis of the flow in response to aspiration
Following our observation that entangled actin filaments resist entering the micropipette upon aspiration, we continued to investigate how other actin architectures reorganize in response to aspiration-induced loading. First, we encapsulated fascin-bundled actin networks inside GUVs as illustrated in the schematic in Figure 3a. We reconstituted actin filaments by polymerizing 5.3 μM actin using F-buffer followed by adding fascin at 1:5 ratio to actin to bundle actin filaments. Encapsulation using the modified cDICE technique was done promptly before the complete formation of the bundled network. As has been shown previously (Bashirzadeh et al., 2020; Wubshet et al., 2021), fascin, at 1:5 ratio to actin, assembles stiff protrusive bundles in random orientations. These protrusive bundles do not reorient with respect to the GUV via diffusion but rather maintain a fixed architecture. Often, there is a dominant bundle that highly deforms the GUV resulting in the longest axis of the GUV, which we refer to as the dominant bundle. We observed, upon applying negative pressure via a micropipette of a GUV initially at a distance from the pipette tip, the GUV as a whole reoriented in a manner where the dominant actin bundle aligned with the axis of flow as it moved near the tip of the pipette. We show, in Figure S1, the reorientation of GUV with respect to the dominant bundle. Hydrodynamically, this alignment is energetically favorable to minimize drag against fluidic current in a manner where the smallest cross-sectional area is perpendicular to the current, akin to how ideal shapes of water transport systems such as boats and ships do not sail on their side.
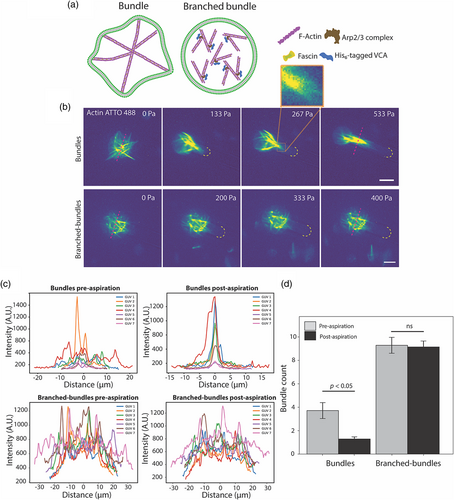
For GUVs that have a network of fascin-bundled actin, we observed dynamic reorganization under micropipette aspiration (Figure 3b top). Once aspiration began, at the first instance of pressure increment, fascin-bundled networks almost instantly reorganized and started to align by converging at the pipette tip (Figure 3b), and the opposite ends of bundles that were in the unaspirated region of the GUV remained oriented in random directions. We call this the alignment initiation stage. Such an arrangement is reminiscent of a matcha whisker. Fascin GUVs equilibrate in this arrangement exhibiting resistance for further insertion into the micropipette. Further increasing aspiration pressure, however, begins to align the fascin bundles along the axis of aspiration, in part due to energetically unfavorable bent fascin-bundled filaments. At the final aspiration pressure of 533 Pa, initially randomly reoriented bundles, were fully aligned into one thick bundle fully constricted by the walls of the capillary. Line scans of bundle networks pre-aspiration show random reorientation of bundles as indicated by the multipeak profile (Figure 3c top left) and alignment of bundles at the final stage of aspiration is shown by the single-peak line profiles (Figure 3c top right). Figure 3d presents a statistical analysis of multiple GUVs where we compared peak-counts in the intensity profile plots which represent the number of resolved bundles pre- and post-aspiration. Note that these may not represent the true number of bundles in a strict sense as we are interested in thick bundles that protrude the GUVs or provide major resistance to alignment that can be easily identified as an intensity peak. Our initial mechanistic hypothesis for this observation was that these bundles were not crosslinked to one another, and the increase in membrane tension exerts forces on the protruded bundles may facilitate bundles to slide and rearrange.
To support this idea, we performed an experiment where we encapsulated bundles that are branched (i.e., the bundles are physically linked to one another). We achieved this architecture by co-encapsulating fascin with Arp2/3 complex along with its activator VCA. From our prior work, we have demonstrated that co-encapsulation of Arp2/3 with fascin suppresses protrusive bundles via branching and shortening fascin bundles. When aspirated, branched bundled actin GUVs (Figure 3b bottom) exhibited an entirely different response where there was no observed alignment of bundles. Since these bundles are not protrusive, membrane forces are not directly coupled to the bundles to rearrange them. These architectures continued to resist inserting into the micropipette until the aspiration exceeded the area dilation threshold resulting in GUV rupture. Line scans support our observation where multipeak profiles pre-aspiration (Figure 3c bottom left) remain at the final stage of aspiration (Figure 3c bottom right). A comparison of the number of bundles along the crossecting line shows that bundles converged from multi-peak pre-aspiration to a single-peak aligned bundle post-aspiration (Figure 3d). In contrast, branched bundles showed no difference between pre- and post-aspiration conditions. This suggests that physical crosslinking between bundles and their interaction with membranes have a strong influence on the reorganization in response to aspiration load.
4 DISCUSSION
Recent work has investigated the impacts of elevated membrane tension on the organization of reconstituted actomyosin cortex during GUV adhesion and spreading (Sakamoto et al., 2023). Using the natural increase of membrane tension of adhesion and spreading of GUVs, it was found that adhesion-induced membrane tension dramatically reorganized the F-actin cytoskeleton. Here we used micropipette aspiration to induce local stresses and uncovered distinct responses in the reorganization of F-actin, bundled, and branched-bundle networks. Although further experiments are required to investigate the mechanism by which the flow of actin filaments is reduced during aspiration, there are logical mechanisms we believe may contribute to this phenomenon. Probable cause for this dynamic response of actin filaments to micropipette aspiration can be attributed to the synergetic effect of stress stiffening (Chaudhuri et al., 2007) and entanglement (Gardel et al., 2003). Prior studies have reported that actin filaments are intertwined long-chain polymer strands and are also tightly packed thus having minimal bending fluctuations (Uchida et al., 2008). Translating this to our observation, first, entanglement of actin filaments, which enhances its mechanical property, could be creating resistance of filamentous actin to micropipette-induced deformation. Second, given filamentous actin solution is a non-Newtonian fluid and has been known to show stress stiffening and relaxation (Falzone et al., 2015), it is possible that, due to elevated lumenal stress in response to aspiration, entangled actin filaments stiffen and subsequently become unyielding to enter the constricted micropipette region. The roles of passive and active stresses of actin networks and membranes in governing network dynamics and membrane shape changes will be investigated in the future as a continuation of this work. Although not performed here, encapsulating an Arp2/3-branched lumenal network in a GUV is expected to further entangle and stiffen actin filaments and result in a similar behavior as entangled filament networks. It will also be interesting to investigate how membrane-attached actin networks would remodel following GUV aspiration in the future.
So how do protrusive bundles seamlessly reorient into alignment of a larger bundle under elevated membrane tension? First, we believe fascin bundles cannot be crosslinked to one another, otherwise bundles cannot reorient independently of one another. Second, the stiff bundles must be protrusive such that the enveloping membrane can exert a force to facilitate rearrangement without membrane or bundle buckling when aspiration forces are applied. This is enabled by the intrinsic fluidic property of lipid bilayers. Due to the nominal friction between membrane and bundles, sliding of protrusive ends of bundles along the membrane is possible without necessarily creasing or buckling the membrane. Previously, membrane-induced merging of membrane-bound actin filaments to form thicker filopodia has been proposed (Liu et al., 2008), thus supporting this as a plausible mechanism. Thus, our model is that a lack of crosslinking between bundles and fluidity of membrane are operating jointly to assist the alignment of randomly oriented protrusive bundles into one single thick bundle. The contributions of these mechanisms should be further investigated.
In summary, the micropipette aspiration setup is a great tool to mechanically characterize biological samples such as GUV-based minimal cell models. It also mimics physical constrictions cells may experience in their native environment, thus making it an ideal tool to study how actin networks reorganize differentially under load. Our findings reveal distinct dynamics of actin networks under load depending on their initial architecture. Polymeric entanglement due to the nature of actin filaments and stress stiffening may endow a network resistance to deformation. Similarly, branching by actin-binding proteins also reinforces structural integrity by withstanding loads. Protrusive bundles, similar to those assembled by fascin, on the other hand, rearrange to adapt to the new mechanical environment. Although these findings are insightful in understanding actin mechanics, it is also important to acknowledge that these are simplified systems with no actin turnover or reassembly machineries and thus do not entirely capture cellular events.
AUTHOR CONTRIBUTIONS
N.H.W. and A.P.L. designed research. N.H.W. and C.J.Y. performed research. N.H.W. analyzed data. N.H.W. and A.P.L. wrote the paper.
ACKNOWLEDGMENTS
This work is supported by the National Science Foundation (MCB-2201236). N.H.W. was supported by National Institutes of Health's Microfluidics in the Biomedical Sciences Training Program (NIH NIBIB T32 EB005582). A.P.L. acknowledges support from the National Institutes of Health (R01 EB030031) and the National Science Foundation (EF1935265).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




