Exposure assessment of severe acute respiratory syndrome coronavirus 2 and norovirus genogroup I/genogroup II in aerosols generated by a municipal wastewater treatment plant
Abstract
The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in wastewater and its potential as an airborne transmission source require extensive investigation, particularly in wastewater treatment plants (WWTPs), where few studies have been conducted. The aim of this study was to investigate the presence of SARS-CoV-2 and norovirus (NoV) RNA in wastewater and air samples collected from a municipal WWTP. In addition, the study assessed the potential risk of viral exposure among WWTP employees. In both the summer and winter campaigns of this study, SARS-CoV-2 and NoV RNA were quantified in wastewater/sludge samples other than effluent. Viral RNA was not detected in any of the air samples collected. The exposure risk assessment with the SARS-CoV-2 RNA concentrations in the influent pumping station of this study shows a lower risk than the calculation with the historical data provided by AquaVall, but both show a low-to-medium exposure risk for the WWTP workers. The sensitivity analysis shows that the result of the model is strongly influenced by the SARS-CoV-2 RNA quantification in the wastewater. This study underscores the need for extensive investigations into the presence and viability of SARS-CoV-2 in wastewater, especially as a potential airborne transmission source within WWTPs.
1 INTRODUCTION
In December 2019, a new coronavirus of the genus Betacoronavirus was isolated for the first time from a cluster of individuals with unrecognizable acute pneumonia in the city of Wuhan, China.[1] The newly recognized virus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was quickly detected in almost every country and territory in the world.[2, 3] The World Health Organization (WHO) declared the new SARS-CoV-2 a public health emergency of international concern[4] and a few weeks later a global pandemic.[5]
Transmission of SARS-CoV-2 has been thought to occur primarily through close contact with infected respiratory droplets,[6] and since the beginning of the current pandemic, it has been debated whether or not airborne is a route of transmission.[7-9] Several studies have suggested the possibility of airborne transmission.[8-11] Today, airborne transmission of SARS-CoV-2 is widely accepted[12] and is mainly associated with crowded and poorly ventilated indoor environments, such as schools, restaurants, offices, and healthcare facilities. The risk of infection in outdoor settings is still unclear, but a number of factors, such as wind speed, temperature, and relative humidity,[13] could increase the risk of transmission in these environments.[14, 15]
The presence of SARS-CoV-2 RNA in fecal samples from infected patients has been observed, with an estimated 2%–10% of confirmed positive patients presenting with gastrointestinal symptoms, including diarrhea.[16] Several studies have reported the presence of SARS-CoV-2 RNA in wastewater[17-20] and contaminated water bodies.[21, 22] When considering possible airborne sources, wastewater treatment plants (WWTPs) should be considered.[23, 24] Noroviruses (NoVs) are the major cause of acute gastroenteritis and cause several outbreaks worldwide.[25-27] They are genetically diverse and are divided into 10 genogroups, with NoV genogroup I (GI) and genogroup II (GII) containing most strains capable of infecting humans and causing 75%–90% of all NoV outbreaks.[28] NoV spreads via the fecal–oral route and is, therefore, one of the most common human viruses found in wastewaters,[29] even after treatment.[29, 30] The primary modes of NoV transmission include the consumption of contaminated water or food and contact with infected individuals or contaminated surfaces.[31] However, there is also some evidence indicating the potential for airborne transmission.[32-34]
Studies have shown that wastewater treatment processes produce aerosols of less than 1–2 µm, which are in the range of respirable aerosols.[35, 36] The main emission sources of aerosols in WWTPs include aeration tanks, sludge dewatering units, and mechanical agitation systems.[37] Some studies have reported a frequent occurrence of respiratory symptoms among employees of WWTPs.[38, 39]
Despite the significance of this topic, the presence of SARS-CoV-2 in aerosols generated during wastewater treatment has been primarily addressed by only one study, conducted by Gholipour et al.[23] This study underscored the potential risk of COVID-19 transmission through wastewater aerosols. It is important to highlight that fecal bioaerosols may contain both viable and nonviable pathogens and viruses, presenting a significant public health risk, particularly for individuals exposed to them, such as residents of buildings, sewage treatment plant workers, neighboring residents, and farmers, among others.[40] The SARS-CoV-1 outbreak in Hong Kong in 2003 highlighted the role of wastewater-associated bioaerosols as a significant transmission mechanism for the virus.[41, 42] Researchers emphasized that the virus’ persistence in sewage, primarily due to inadequate disinfection, increased the risk of transmission.[43] The infectivity of SARS-CoV-2 in wastewater has not been conclusively proven.[44] Although studies indicate that the likelihood of SARS-CoV-2 transmission through aerosols from wastewater is low, the possibility cannot be entirely ruled out. Due to this knowledge gap, it remains crucial to ascertain the detectability of SARS-CoV-2 RNA in aerosols to gain a comprehensive understanding of the potential risks involved in future epidemics.
A recent study has reported that the survivability rates of Delta and Omicron variants of concern in wastewater pose a low risk of fecal–aerosol transmission.[45] In addition, and although NoV transmission is mainly via the fecal–oral and vomit–oral routes, airborne transmission has also been suggested in recent years, but this route has not yet been consistently demonstrated.[26, 33, 34] The objective of this study is to investigate the presence of SARS-CoV-2 and NoV RNA in wastewater and air samples obtained from a municipal WWTP. Furthermore, this study utilizes historical data from SARS-CoV-2 wastewater-based epidemiology as a framework to infer the potential presence of viruses in aerosols. This study contributes to the understanding of exposure risk associated with aerosolized viral particles in wastewater treatment settings.
2 MATERIALS AND METHODS
2.1 Collection of wastewater and air samples
The WWTP of Valladolid in Spain (41.6463° N, 4.7249° W) serves a population of 570 000 and is designed with a maximum flowrate treatment capacity of 3 m3 s−1 (1000 000 population equivalents). It consists of a water treatment line composed of solid removal and degreasing, primary treatment, A2O biological treatment, and secondary clarification. It also has a sludge treatment line that receives the settled (primary) sludge and the excess activated (secondary) sludge from clarification. After homogenization, mixed sludge is thickened and anaerobically digested before dewatering.
Two sampling campaigns were carried out, one on August 1, 2021 (summer campaign) and the other on November 30, 2021 (winter campaign). According to data obtained from the Spanish State Meteorological Agency (AEMET), the weather conditions on the 1st August were as follows: The average temperature recorded was 18.4°C, with a relative humidity of 42%. There was no precipitation, and the wind speed was measured at 5.4 km h−1. On the 30th November, the weather conditions were as follows: The average temperature was 3.7°C, whereas the relative humidity increased to 84%. Similar to the previous date, there was no recorded precipitation, and the wind speed remained calm at 3 km h−1.
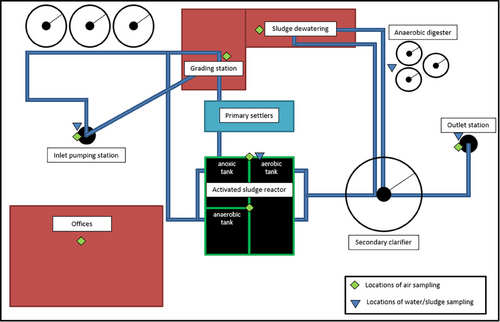
Grab wastewater samples were collected from different areas of the WWTP as detailed in Table 1. A total of eight wastewater/sludge samples (four samples from each collection campaign) were collected in 250 mL plastic sterile bottles and immediately transferred to the laboratory in an insulated box with cooling packs. Air samples were collected using a Coriolis Compact (Bertin Instruments) according to da Silva et al.[8] A cross section sampling was performed at the WWTP, and the information of each sampling site is detailed in Table 1. Air sampling was performed for 30 min in each sampling site, with an airflow rate of 50 L min−1 (total of 1.5 m3) and collected on dry medium, with 4 mL of sterile PBS (biotechnology grade with a purity of ≥99%) (Fisher Scientific) added to the collection cones after sampling. All samples were stored in an insulated box with cooling backs before being transferred to the laboratory. In the laboratory, samples were kept at 4°C, and RNA was extracted within 24 h. A schematic representation of the WWTP of Valladolid and the sampling locations is summarized in Figure 1.
| Summer sampling | Winter sampling | ||||
|---|---|---|---|---|---|
| Sampling location | Sample type | Setting | Sampling location | Sample type | Setting |
| Inlet pumping station | Air | Outdoor | Inlet pumping station | Air | Outdoor |
| Activated sludge reactor | Air | Outdoor | Activated sludge reactor | Air | Outdoor |
| Activated sludge reactor (middle) | Air | Outdoor | Activated sludge reactor (middle) | Air | Outdoor |
| Pretreatment | Air | Indoor | Pretreatment | Air | Indoor |
| Sludge dewatering | Air | Indoor | Sludge dewatering | Air | Indoor |
| Outlet wastewater plant discharge | Air | Outdoor | Outlet wastewater plant discharge | Air | Outdoor |
| Offices | Air | Indoor | Offices | Air | Indoor |
| Inlet pumping station | Wastewater | Outdoor | Inlet pumping station | Wastewater | Outdoor |
| Activated sludge reactor (aerobic) | Wastewater/Sludge | Outdoor | Activated sludge reactor (aerobic) | Wastewater/Sludge | Outdoor |
| Anaerobic digester | Wastewater/Sludge | Outdoor | Anaerobic digester | Wastewater/Sludge | Outdoor |
| Outlet wastewater plant discharge | Wastewater | Outdoor | Outlet wastewater plant discharge | Wastewater | Outdoor |
2.2 Wastewater concentration
Before each concentration step, 20 µL of mengovirus (3.2 × 103 copies µL−1) was added to 70 mL of each sample as an internal control of the concentration process (BioMérieux). Samples of wastewater were homogenized and centrifuged at 700 × g for 10 min to remove large particles and organisms (pellet). An aliquot of 70 mL of the resulting supernatant was used for concentration with centrifugal ultrafiltration devices with a cutoff of 10 kDa (Centricon Plus-70). Briefly, 70 mL of sample was added to a Centricon Plus-70 and centrifuged at 4000 × g for 40 min at 4°C. Elution was done by centrifugation at 700 × g for 40 min. The resulting concentrate was used for nucleic acid extraction. Sludge samples were centrifuged at 10 000 × g for 3 min, and the resulting pellet was used for nucleic acid extraction.
2.3 Extraction of viral DNA and RNA
The AllPrep PowerViral Kit (Qiagen) was used for the isolation of viral nucleic acids from 200 µL of wastewater and air samples and from 200 µg of sludge, according to the manufacturer's instructions to obtain 100 µL of isolated DNA and RNA. For all samples, a known concentration of mengovirus RNA (3.2 × 103 copies µL−1) was added as an internal control for nucleic acid isolation and to check potential for possible PCR inhibitory effects intrinsic to the samples. Briefly, the viral extraction efficiency was controlled using the Mengovirus Extraction Control Kit (BioMérieux) by adding 20 µL of a known amount of mengovirus (strain VMC0) before nucleic acid extraction. For wastewater samples, mengovirus was added before viral concentration to both control the concentration step and nucleic acid isolation. For each nucleic acid isolation procedure, a negative control of isolation (NCI) was included containing 200 µL of nuclease-free water (Type II 18-megohm filtered) (Thermo Fisher) instead of sample. For each RNA isolation procedure, an NCI was included containing only buffers.
2.4 Molecular detection of mengovirus, SARS-CoV-2, and norovirus GI/GII
Mengovirus was quantified using nucleic acid amplification by one-step RT-qPCR on a QuantStudio1 Real-Time PCR system (Applied Biosystems) using the Mengovirus Extraction Control Kit (BioMérieux) (Reference number KMG), with a final volume per reaction of 25 µL (20 µL of Mastermix and 5 µL of RNA). The cycling conditions for mengovirus were reverse transcriptase at 45°C for 10 min, polymerase activation at 95°C for 10 min, 45 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 45 s (limit of quantification = 100 copies L−1).
SARS-CoV-2 and NoV GI/GII were also quantified using nucleic acid amplification by one-step RT-qPCR on a QuantStudio 1 Real-Time PCR system (Applied Biosystems). Quantification of SARS-CoV-2 was done by targeting the gene N1 and using N2 for confirmation (IDT Technologies) (Reference number 10006713) using TaqPath 1-Step RT-qPCR Master Mix (Applied Biosystems) with a final volume per reaction of 20 µL for each gene (15 µL of Mastermix and 5 µL of extracted RNA). The cycling conditions for SARS-CoV-2 were reverse transcriptase at 50°C for 15 min, polymerase activation at 95°C for 2 min, 45 cycles of denaturation at 95°C for 3 s, and annealing/extension at 55°C for 30 s (limit of quantification = 383 copies L−1).
The quantification of NoVs GI and GII was done by targeting NoV GI capsid protein gene and NoV GII RNA polymerase gene using NoVs GI and GII One-Step RT-qPCR Kit (NZYTech) (Reference number MD04051), with a volume per reaction of 20 µL per gene (15 µL Mastermix and 5 µL of extracted RNA). The cycling conditions for NoV were reverse transcriptase at 50°C for 20 min, polymerase activation at 95°C for 2 min, and 50 cycles of denaturation at 95°C for 4 s and annealing/extension at 60°C for 30 s (limit of quantification = 231 copies L−1).
The fluorescence thresholds were manually set for each gene for both SARS-CoV-2 and NoVs GI and GII according to the amplification curves. For mengovirus control, the threshold was set at 0.2. Non-template control (NTC) was added at each reaction to monitor potential contamination in the qPCR reagents during pipetting. A positive control (PC) for each gene was added in each qPCR assay to monitor each amplification. Each positive amplification curve was manually checked, and only curves showing a significant slope increase, in contrast with the negative control curves (NTC and NCI), were considered positives. Each reaction was performed in triplicate, including the used controls (NTC, NCI, and PC).
PCs for each N1 and NoV were 10-fold serially diluted to 1 × 109 and assayed for each gene in triplicate. Standard curves were obtained by plotting Ct values for each dilution against the log10 expected copy number (Figure S1). The standard curves were used to quantify the tested samples.
2.5 Quantitative microbial exposure assessment of SARS-CoV-2 aerosol exposure
The probability of exposure of WWTP workers by aerosolized particles containing SARS-CoV-2 was calculated using a slightly modified version of the model by Gholipour et al. using SARS-CoV-2 RNA concentration in untreated wastewater instead of COVID-19 prevalence.[23]
3 RESULTS AND DISCUSSION
3.1 Detection of SARS-CoV-2 and NoV RNA in wastewater/sludge samples
qPCR-based methods are the most used and offer a rapid way to detect low concentrations of viruses, but their high sensitivity and the complexity of environmental samples, such as the large number of inhibitory substances, pose some challenges. To avoid false results and cross contamination, the use of controls in each reaction is crucial.[52, 53] In the current study, controls have been used to avoid these issues (Table S1). The NCI and NTC added to monitor each sampling run were negative, ruling out the possibility of contamination during RNA extraction and quantification with RT-qPCR. The Ct values of the mengovirus control RNA varied only slightly among samples (less than 1 Ct value), confirming that the RNA isolation and—in the case of wastewater samples—the concentration step were almost optimal and that there was little or no inhibition during nucleic acid amplification. The PCs added to the RT-qPCR reactions for each gene behaved as expected with very low inter-reaction variation.
The detection of viruses in wastewater has been used as a complementary method to clinical testing. It is an early warning indicator of the spread of viruses in communities and detects both symptomatic and asymptomatic cases. Several studies report the detection and quantification of SARS-CoV-2 RNA in wastewater during the current COVID-19 pandemic.[17, 18, 20, 54] NoV has also been detected and quantified in wastewater.[29, 55, 56] In this study, both SARS-CoV-2 and NoV RNA were detected in untreated wastewater collected at the inlet pumping station and in sludge collected at the activated sludge reactor and anaerobic digester, but RNA was not detected in treated water at the outlet station (Table 2 and Figure 2). Other studies have reported similar results with SARS-CoV-2 RNA not being detected or being detected sporadically in effluent samples.[57-59] Studies investigating the removal of NoV during wastewater treatment also showed that NoV RNA is often not detected in wastewater effluent.[60, 61] On the other hand, a monitoring study conducted for 1 year has reported that NoV was reduced but still often found in effluent samples.[62] Another similar study has also reported the presence of NoV in effluent samples.[61] In addition, studies have shown the detection of NoV in receiving water bodies downstream of WWTPs.[48, 63] A possible explanation can be due to the size of the population served by the WWTP, as there might be a greater chance of higher concentrations of NoV in WWTPs serving larger populations. On the other hand, a higher population would result in higher fecal matter being excreted. The prevalence of NoV in the population that is served by the WWTP might be another important factor, as in non-epidemic periods, NoV is shed by a small percentage of the population.[61] The effect of different wastewater treatment technologies is also known to be an important factor in the elimination and reduction of pathogenic viruses.[64] No detection of SARS-CoV-2 and NoV RNA in effluent samples may also be a consequence of the viral concentration methods and further research and optimization of concentration methods is highly needed. Studies evaluating the removal of human pathogenic viruses during wastewater treatment have been conducted,[65] but there are still few long-term studies evaluating the removal of SARS-CoV-2.[57, 58]
| Target genes for SARS-CoV-2 | Target gene for NoV | |||
|---|---|---|---|---|
| Sampling location | N1 | N2 (+ or −) | NoV GI and GII | |
| Summer campaign | Inlet pumping station | 7.26 × 104 ± 8.04 × 103 | + | 2.76 × 104 ± 2.95 × 103 |
| Outlet wastewater plant discharge | – | − | – | |
| Activated sludge reactor (aerobic) | 3.79 × 103 ± 1.90 × 103 | + | 2.55 × 104 ± 2.06 × 103 | |
| Anaerobic digester | 4.38 × 103 ± 1.92 × 103 | + | 7.02 × 104 ± 4.74 × 103 | |
| Winter campaign | Inlet pumping station | 6.78 × 103 ± 3.93 × 102 | + | 2.28 × 104 ± 2.61 × 103 |
| Outlet wastewater plant discharge | – | − | – | |
| Activated sludge reactor (aerobic) | 4.03 × 103 ± 1.90 × 103 | + | 6.31 × 104 ± 1.14 × 104 | |
| Anaerobic digester | 1.90 × 103 (only 1/3 reactions amplified) | + | 1.67 × 105 ± 3.13 × 104 | |
- Note: Results show copies L−1 for the inlet pumping station wastewater sample and copies g−1 in the aerobic and anaerobic digester samples. Presence (+) and absence (−) of SARS-CoV-2 N2 gene are used for confirmation.
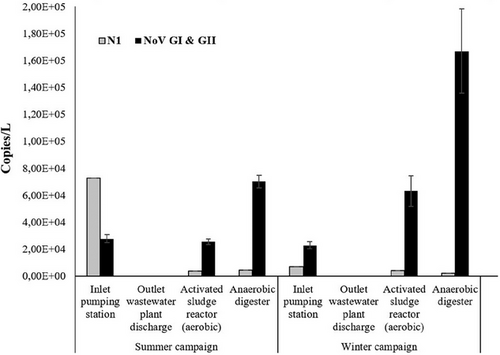
The concentrations of NoV RNA at the inlet pumping station were 2.76 × 104 in summer and 2.28 × 104 in winter (Table 2). The observed concentrations are at the lower end of those previously reported in other studies. Nordgren et al. have shown NoV concentrations in untreated wastewater ranging from 1 × 104 to 6 × 106 copies L−1.[62] The same study showed a significant increase of NoV concentrations in winter, which has not been observed in the current study. Grøndahl-Rosado et al. also reported concentrations of NoV ranging from 2.5 × 105 to 67.4 × 105 copies L−1.[31] The concentration of SARS-CoV-2 RNA in raw wastewater collected at the inlet pumping station was about 7.26 × 104 copies L−1 in the summer campaign and 6.78 × 103 copies L−1 in the winter campaign (Table 2), which is in accordance with other studies.[16, 17, 23, 66]
3.2 Detection of NoV and SARS-CoV-2 RNA in aerosols
Out of the 14 air samples collected during both summer and winter campaigns, no amplification was detected for N1 or N2 gene of SARS-CoV-2. Similarly, none of the air samples showed amplification for NoV G1 capsid protein gene and NoV GII RNA polymerase gene, indicating the absence of NoVs GI and GII. It is important to interpret these reported results cautiously, as the samples may have yielded false negatives. This could be attributed to either low concentrations of viral RNA falling below the methodological limit of detection or the presence of inhibitors during the RT-qPCR process. To ensure meticulous monitoring and mitigation of false negatives, rigorous adherence to optimal laboratory practices was maintained. To avert the detrimental effects of thawing and unthawing cycles, extracted RNA was promptly aliquoted. Comprehensive control measures, outlined in the methods section, were systematically incorporated at each procedural stage, featuring an internal control. Notably, the outcomes of the internal control and supplementary controls revealed an absence of indications for false negative results. Additionally, each sample was subjected to triplicate application and subjected to a 10-fold dilution within each RT-qPCR reaction, with no discernible signs of inhibition identified.
There are few studies performing detection and/or quantification of SARS-CoV-2 and NoV in outdoor air, and even fewer studies conducted in aerosols generated by WWTPs.[16, 67] Gholipour et al. report the detection of SARS-CoV-2 RNA in 6 out of 15 air samples collected in a WWTP serving a population with a higher COVID-19 prevalence compared to the population in this study.[23] NoV has been detected in air samples collected near open sewers and polluted surface waters in La Paz, Bolivia.[68] A Danish study also showed the occurrence of NoV in aerosols generated by WWTPs at concentrations that may pose an occupational health risk and increase the incidence of gastrointestinal diseases in workers.[69] The risk of infection from bioaerosols for residents living near biosolid application sites was assessed and showed a high risk of infection from coxsackievirus A21.[70] A 2014 study showed that adenovirus RNA was present in all air samples collected from a WWTP in summer and in 97% of the samples in winter. NoV was detected in only 3 of 123 air samples, and hepatitis E was not detected in any sample.[71] In another study, hepatitis E was detected in aerosols from WWTPs generated in active sludge tanks.[72] NoV was also detected in 9 out of 16 samples at a WWTP in Japan. In the same study, adenoviruses were detected in 4 out of 16 samples, and F-specific RNA bacteriophages (FRNA bacteriophages) and enteroviruses in 3 out of 16 samples.[73]
A study conducted in northern Italy reported the presence of SARS-CoV-2 RNA associated with particulate matter (PM10) in air samples collected in an industrial area.[74] Similarly, another study reported different detection rates of SARS-CoV-2 in outdoor samples from northern and southern Italy.[75] A recent study has shown the detection of SARS-CoV-2 RNA in WWTP aerosols with a detection rate of 40% (6/15 samples).[23] The study was conducted when the prevalence of COVID-19 was very high in the region, whereas the current study was conducted with a low prevalence of COVID-19 (62 201 and 69 856 confirmed cases, in August and November, respectively). Gholipour et al. showed that the RdRp gene was the most frequented gene detected, whereas N and ORF-1ab genes amplified in few samples. However, the analytical sensitivity of RT-qPCR assays for SARS-CoV-2 reported in different studies is dissimilar. In one study, ORF-1ab exhibited the highest frequency of amplification when compared with N and E genes,[22] whereas Ahmed showed a high frequency of amplification of N gene.[17] The sampling strategy might also be a key factor influencing the detection. Gholipour et al. collected wastewater aerosols using portable pumps with a total collected volume of 3.5–4 m3, whereas in this study, a total of 1.5 m3 was collected per sample.
Although the viral RNA of SARS-CoV-2 has been detected in various environments—including wastewater, surface water, biosolids, groundwater, sediments, and indoor and outdoor air—its infectivity in these matrices has not been confirmed or thoroughly investigated.[22, 44, 76-78] Viable SARS-CoV-2 was isolated from the feces of six patients.[79, 80] Considering the high efficiency of most WWTPs in reducing viral load,[24, 44, 81] several studies suggest that fecal–oral transmission of SARS-CoV-2 via wastewater is likely to be low compared to the predominant person-to-person transmission via respiratory droplets and aerosols.[44] Although the possibility of low infectivity in wastewater has not been proven, it cannot be completely ruled out. In addition, some studies suggest that in areas without adequate sanitation infrastructure, high levels of SARS-CoV-2 load could enter water bodies and remain active there for extended periods of time.[82-84] Low seasonal temperatures may further increase the risk of waterborne transmission of SARS-CoV-2, leading to concerns about the spread of COVID-19 via aerosolized contaminated water and wastewater. This emphasizes the importance of SARS-CoV-2 RNA detection for occupational risk assessment, especially for workers in environments where they may be exposed to contaminated water or aerosols.[82]
3.3 Exposure assessment of SARS-CoV-2 in WWTP workers
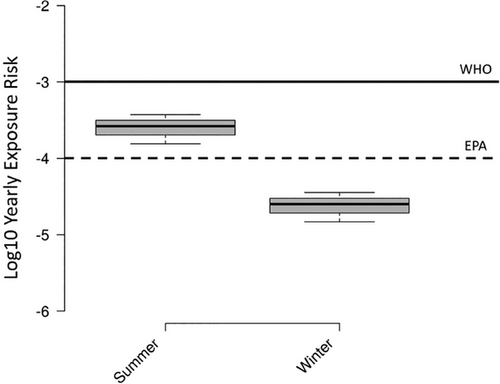
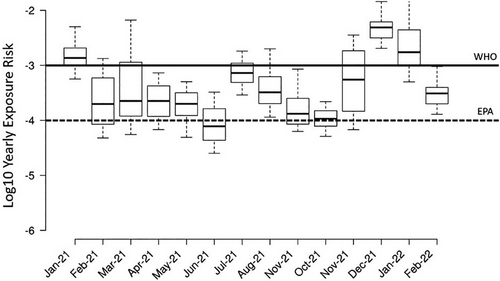
Wastewater contains large concentrations of human pathogens, including viruses, bacteria, and parasites, and the treatment process in WWTPs can lead to the aerosolization of these pathogens. Employees of WWTPs and the surrounding communities have been considered potential risk groups due to the bioaerosols generated in WWTPs.[33, 85] There are few studies assessing the risk of viral infection in WWTP workers. In this study, a microbial exposure assessment developed by Gholipour et al. was employed using historical data acquired from the Spanish national wastewater-based epidemiology program (https://aquavall.es/sistema-de-alerta-temprana-para-la-deteccion-de-covid-19/), and the results were compared with reference levels of WHO and the US Environmental Protection Agency (EPA) (Figure 3).[23, 86] The results summarized in Figure 3 show that there was a higher exposure risk per person per year in summer than in winter. In this study, the exposure risk herein estimated in summer was higher than the reference level of EPA, but in both sampling periods, the risk was lower than the WHO reference level. The exposure risk observed was significantly lower than that reported by Gholipour et al.[23] The difference was likely due to the lower prevalence of COVID-19 at the time of sampling compared to the high prevalence of the previous study. The herein observed exposure risk in summer was also slightly higher than the tolerable exposure risk of 5.5 × 10−4 recommended for SARS-CoV-2.[87] The same microbial exposure analysis was performed with gene copies of N1 from wastewater samples provided by AquaVall for each month of 2021 (Figure 4). When comparing the exposure risk calculated with the SARS-CoV-2 RNA concentrations in the inlet pumping station of this study to the exposure risk calculated with AquaVall data, the calculations using concentrations of this study showed a slightly lower exposure risk (winter and summer (Figure 4) correspond to August and November (Figure 4), respectively). As seen in Figure 4, the exposure risk was higher than the EPA reference and lower than the WHO reference in most of the months. The highest exposure risk was observed in December 2021 and January 2022. The exposure risk follows the prevalence of COVID-19 in the catchment community. A previous study suggested a reduction of working hours with the aim to reduce the exposure time and thus decrease the exposure risk.[23] On the other hand, both the current study and the study from Gholipour et al. did not consider the effect of vaccination and protective material, as well as the fact that workers are typically not 7.5 h in the near proximity of areas with the possibility of aerosol generation. Although SARS-CoV-2 RNA has been widely detected and quantified in wastewaters and aerosols, there is no evidence that the infectious virus is present, and exposure to fecal material and wastewater is not a known transmission vector.[88]
Sensitivity analyses were performed (Table 3) to investigate the influence of the input parameter on the estimated exposure risk. The sensitivity analysis shows that the risk of exposure to SARS-CoV-2 is highly affected by the SARS-CoV-2 genomic copies detected in wastewater, followed by the CF of genomic copies to TCID50. Other important factors are the microbial-to-air portioning coefficient (PCwa) and the IR. The sensitivity analysis shows very similar results to those of Gholipour et al., where the number of COVID-19 cases was the input variable with the highest contribution to the model output. The results of the present study report the possibility of exposure to viral RNA and do not confirm or suggest wastewater and aerosol transmission in wastewater. Risk of infection was not shown, and exposure risk is low; however, the use of appropriate personal protective equipment and frequent hand washing by WWTP workers is suggested. AquaVall has already implemented preventive measures for exposure to biological agents since 2018.
| Variable | Correlation coefficient |
|---|---|
| Concentration of SARS-CoV-2 RNA copies L−1 (GC) | 0.651 ± 0.087 |
| Conversion factor of genomic copies to TCID50 (CF) | −0.385 ± 0.066 |
| Microbial-to-air portioning coefficient (PCwa) | 0.229 ± 0.001 |
| Inhalation rate (IR) | 0.201 ± 0.001 |
4 CONCLUSIONS
This study provides a snapshot of the occurrence of NoV and SARS-CoV-2 RNA in wastewater and sludge samples along the wastewater treatment line of Valladolid WWTP. Interestingly, viral RNA was not found in aerosols generated by the WWTP. The results supported the idea that viral RNA concentrations in outdoor spaces are typically low and that the risk of exposure is lower than in poorly ventilated indoor spaces.
The results showed a moderate-to-low exposure risk in the studied WWTP during low COVID-19 prevalence in the catchment population. Although viability and infectivity have not been shown, the results are important to assess the risk management measures employed and highlight the need to keep good sanitary practices to avoid the risk of exposure in WWTP employees. More studies with more samples need to be performed to understand the infection risk of aerosols generated by WWTPs because viral viability data in both wastewater and aerosols remains scarce due to resource limitations and the need for biosafety level three laboratories. Coordinated studies using detection and viability methods, as well as with experts in environmental and biological sciences, are needed to be able to understand the risk of infection to workers of highly aerosol-generating areas.
ACKNOWLEDGMENTS
This work was funded by the Marie Skłodowska-Curie Actions Postdoctoral Fellowship (project PLASMARISE – 101151154). This work was performed with financial support from the Regional Government of Castilla y León and the FEDER program (Projects CLU 2017-09, CL-EI-2021-07, UIC315, and VA266P20). This work was funded by Fundação para a Ciência e a Tecnologia (FCT, Portugal), through the strategic projects UIDB/04292/2020 (https://doi.org/10.54499/UIDB/04292/2020) and UIDP/04292/2020 (https://doi.org/10.54499/UIDP/04292/2020) granted to MARE—Marine and Environmental Sciences Centre, and the project LA/P/0069/2020 (https://doi.org/10.54499/LA/P/0069/2020) granted to the Associate Laboratory ARNET—Aquatic Research Network. Agua de Valladolid E.P.E.L (AquaVall) is also gratefully acknowledged for providing the samples and preliminary data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




