Biological Treatment of Landfill Leachate at Elevated Temperature in the Presence of Polyurethane Foam of Various Porosity
Abstract
Application of sequencing batch reactors (SBR) for treatment of leachate from old, stabilized landfills, could be still an economically viable solution, as far as nitrogen removal is concerned. Very low amounts of accesible organic compounds requires an unconventional approach to treatment technology. In this paper the authors proposed utilisation of polyurethane (PU) foam of various porosity as a biomass carrier, hydraulic retention time (HRT) extended to 2 days and temperature elevated to 37°C, to stimulate simultaneous nitrification and denitrification (SND). The porosity of PU carriers had no effect on the efficiency of organic compounds removal, whilst the ammonium nitrogen (N-NH4+) removal efficiency was highest in the reactor (SBR2) equipped with PU cuboid-shape carriers of 30 pores per inch (ppi), reaching 77.6%. In the reactor SBR3 filled with 15 ppi foam, the N-NH4+ removal efficiency was much lower (54.2%), but still better than observed in reference reactor, SBR1, operated with suspended activated sludge only (46.3%). The average free ammonia concentration in the effluents ranged from 140.18 to 347.98 mg/L at pH 9.6, depending on the reactor. Neither nitrites nor nitrates were found in the effluents, and the kinetics of N-NH4+ removal proceeded according to the first-order reaction.
Abbreviations
-
- AOB
-
- ammonium oxidizing bacteria
-
- BOD
-
- biochemical oxidation demand
-
- COD
-
- chemical oxidation demand
-
- DO
-
- dissolved oxygen concentration
-
- FAN
-
- free ammonia concentration
-
- HRT
-
- hydraulic retention time
-
- NLR
-
- nitrogen loading rate
-
- N-NH4+
-
- ammonium nitrogen
-
- N-NO2−
-
- nitrite nitrogen
-
- N-NO3−
-
- nitrate nitrogen
-
- NOB
-
- nitrite-oxidizing bacteria
-
- OLR
-
- organic loading rate
-
- ppi
-
- pores per linear inch
-
- PU
-
- polyurethane
-
- SND
-
- simultaneous nitrification and denitrification process
-
- SBR
-
- sequencing batch reactors
-
- TAN
-
- total ammonia concentration
-
- TSS
-
- total suspended solids
-
- VSS
-
- volatile suspended solids
1 Introduction
Landfilling is currently still the most common method of waste management, used for the disposal of municipal waste. However, landfill sites pose numerous environmental threats; one of the most important is related to infiltration of surface and underground water, as well as from external sources such as atmospheric precipitation, to landfill beds. Excess water, not absorbed by the waste, forms leachates, which at first fill empty spaces in the bed profile, in the form of the so-called suspended water, and then running down and accumulate at the landfill bottom. Landfill leachate pose a direct threat to ground water even on properly designed and operated landfills 1, 2. Treatment of leachates from municipal waste landfills is a complex problem due to changeable composition, depending on the landfill age or variable content of disposed waste. Additionally, both quantity and quality of leachate can vary at different sampling sites on the same landfill. Apart from organics and other hazardous substances, landfill leachates, according to the literature 3-5, contain also ammonium nitrogen (NH4+) in high concentrations.
Biological treatment of leachate is an economically viable solution as far as nitrogen removal is concerned. It is usually carried out as the nitrification and denitrification process occurring in separate tanks 6. In the nitrification process, aerobic autrotrophic nitrifiers oxidize NH4+ first to nitrite (NO2−) and then to nitrate (NO3−), whereas, denitrification consists in reduction of nitrates to gaseous nitrogen by heterotrophic bacteria in anoxic conditions 7-9. However, both of the above mentioned processes can also occur in a single aerobic reactor. This phenomenon is described in the literature as simultaneous nitrification and denitrification (SND) process 9-11. Apart from improved wastewater treatment economy, SND offers higher nitrogen removal efficiency at low C/N ratios because more carbon is available to heterotrophic bacteria 12. It is estimated that the carbon demand in the SND process is by 22–40% lower and about 30% less sludge is generated compared to conventional nitrogen removal methods 11, 13. There are many factors affecting the efficiency of the SND process, e.g., C/N ratio 14, sludge retention time 15, concentration of dissolved oxygen (DO) 12, and temperature 16.
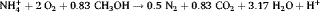 (1)
(1)One of the most important environmental factors affecting the SND process is the temperature. It influences metabolic activity of microorganisms 18 and the rate of biological reactions 16. Nitrification and denitrification in a highly ammonium-loaded landfill leachate is stopped at temperature 10°C, and the maximum nitrogen removal efficiency is observed within the temperature range of 22–37°C 9.
High biomass concentration and prolonged sludge age in the biological reactor allow to making wastewater treatment more effective. The application of biomass carriers causes that sludge is not flushed out from the reactor. This increases the biomass amount and prolongs its age. Research aimed at increasing nitrification efficiency was also carried out with the use of combined methods – active sludge with a biological membrane developing on the fill (movable or immovable carriers) 19. Different biomass carriers are currently available, such as Kaldnes (polyethylene media), Liapor (ceramic media), Linpor (plastic media with high porosity), and polyurethane (PU) foam cubes 20. Lim et al. 21 report that suspended biomass plays an important role in N-NH4+ oxidation, as the biomass colonizing inner surfaces of PU foam is used as a carbon source in the denitrification process.
The objectives of the study were: (i) utilization of widely used PU filtration media, as a biomass carrier for biological sequencing batch reactors (SBR) in process of landfill leachate treatment, (ii) estimation of PU surface area, (iii) determination the influence of PU porosity on the removal efficiency of organics and nitrogen, and (iv) an attempt to determine the nature of the process by calculation of the kinetics of the ammonium nitrogen removal.
The results may prove to be useful in the search for the most efficient and economically viable conditions of the landfill leachate treatment process.
2 Materials and methods
2.1 Experimental setup
The leachate used in the present study was taken from a 16-years-old and currently still operated waste landfill in Kozodrza by Rzeszów (south-eastern Poland, subcarphatian province), constituting a regional waste processing facility that receives municipal waste other than hazardous. Leachates were drained out to a retention reservoir with a capacity of 2295 m3 from eight already closed quarters, undergoing the process of reclamation, and one that is currently operated. Raw leachate test samples were taken from the retention reservoir outlet into 10-L plastic containers and then transported to the laboratory where they were stored at 4°C for further analyses.
Studies on technological processes used for leachate treatment were carried out in three independent SBRs, hereinafter referred to as SBR1, SBR2, and SBR3, with an active capacity of 2 L each, equipped with a mechanical stirrer (Heidolph) and fine bubble aeration system (DO = 2 mg/L). The tests were carried out at 37°C. Prior to dosing, each daily portion of leachate was left overnight in a water bath for temperature compensation (Aquarius). The reactors were equipped with electric heaters, temperature probes, and temperature stabilization system (WIGO, MS-11HS). The working cycle of each SBR lasted 24 h and comprised 4 phases: Filling (15 min), aeration (23 h), settling (30 min), and decant (15 min), and the adopted hydraulic retention time (HRT) was 2 days. One of the reactors (SBR1) was operated with the activated sludge alone, while the other two were additionally filled with biomass carriers in the form of rectangular cuboids with an apparent volume of 0.27 L made of PU foam (six blocks with dimensions 3 × 3 × 5 cm each); SBR2 was filled with foam blocks with the porosity of 30 pores per linear inch (ppi) as declared by the manufacturer, while a 15 ppi foam was used in SBR3. PU foam is a material which, due to its mechanical durability and resilience, is commonly used in the transport industry, and for its good hydrodynamic parameters and large developed surface it is commonly utilized in filtration of gases and liquids, as well as the biological filter in aquaculture. There are also biodegradation mechanisms developed for this material 22, 23. The production process allows to obtain foams within a wide range of porosity, usually described by manufacturers as a ppi parameter. This is the reason why PU foam is a material worth consideration also from the point of view of possible applications in wastewater treatment technology wherever; because of unfavorable wastewater composition, it is necessary to ensure effective retention of biomass in the system and maintain the fluid flow through the bed despite the trend for deposition of mineral compounds on its surface, observed in case of municipal waste landfill leachates. Filter media utilized in the experiment were an open cell polyether based PU foams. According to the manufacturer information those foams are suitable for the mechanical but also biological filtering of air and water, e.g., in aquariums, as they are non-toxic for water organisms. This product is characterized by fairly reproducible cell size, with diameter between the range of 1600–2200 μm for 30 ppi foam and 2800–4500 μm for 15 ppi. However, the data used in calculations were based on own measurements. The detailed characteristics of PU foams are shown in Tab. 1.
| Reactor | SBR2 | SBR3 |
|---|---|---|
| Foam cuboids area [cm2] and volume [cm3] | 468/270 | |
| Declared ppi parameter [pores per linear inch] | 30 | 15 |
| Measured mean strut length [mm] | 1.2527 (±0.3039) | 2.1446 (±0.6168) |
| kA coefficient | 0.2718 | 0.2873 |
| Estimated single cell volume [cm3] | 0.0151 | 0.0756 |
| Est. single cell surface area [cm2] | 0.088 | 0.2728 |
| Est. total inner surface area [cm2] | 1578.49 | 974.77 |
| Est. specific surface area [cm2/cm3] | 5.8463 | 3.6103 |
| Est. total outer surface area [cm2] | 226.5371 | 249.3084 |
| Est. total area of biomass carriers [cm2] | 1805.03 | 1224.082 |
To start up the reactors and increase the biomass in the initial stage of operation, each SBR was inoculated with 2.5 g/L (total suspended solid) of activated sludge. The inoculate was composed of 70 vol% of sludge taken from the laboratory reactor working at 37°C, and 30% of sludge taken from 2nd stage nitrification chamber of the municipal wastewater treatment plant in Rzeszów.
2.2 Calculation of the biomass carriers surface area
 (2)
(2)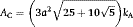 (3)
(3)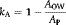 (4)
(4)
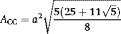 (5)
(5)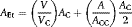 (6)
(6)2.3 Analyses and measurement methods
In order to determine the efficiency of the leachate treatment process, parameters listed in Tab. 2 were monitored both in SBR influents and effluents; whereas, Tab. 3 contains data characterizing the composition of the leachates used in the study.
| Analysis | Measurement metod | References |
|---|---|---|
| pH | Potentiometric Method (Elmetron CPC-401) | 25 |
| Conductivity | Conductivity Method (Elmetron CPC-401) | 26 |
| DO | Membrane Electrode Method (Elmetron CX 105) | |
| COD | Colorimetric Method. Oxidation of organic substances with K2Cr2O7 and H2SO4 in the presence of Ag2SO4 as a catalyst, at 172°C | 27 |
| BOD | Respirometric measurement (OxiTop®) of negative pressure at constant temperature of 20°C and dark (Venticell) in the presence of N-Allythiourea as nitrification inhibitor | 28 |
| NH4+ | Colorimetric Method. Distillation (VELP) of sample in weakly alkaline conditions and titration with Tashiro's indicator | 29 |
| NO2− | Spectrophotometric Method (λ = 520 nm) with sulfanilic acid and N-(1-naphthyl)ethylenediamine dihydrochloride | 30 |
| NO3− | Spectrophotometric Method (λ = 410 nm) with phenol disulfonic acid | 31 |
| Total Kjeldahl nitrogen | Colorimetric Method. Oxidation of nitrogen containing compounds in concentrated H2SO4 in the presence of K2SO4/HgO catalyst | 32 |
| TSS | Weighing method (Radwag). Residuals after 2 h at 103 − 105°C | |
| VSS | Weighing method (Radwag). Residuals after ignition for 2 h at 550°C |
| Parameter | Mean | Min. | Max. | SD |
|---|---|---|---|---|
| pH | 8.16 | 8.1 | 8.8 | ±0.5 |
| Conductivity (mS/cm) | 11.2 | 9.5 | 13.5 | ±1.2 |
| COD (mg/L) | 4125 | 3887 | 4582 | ±98 |
| BOD (mg/L) | 636 | 600 | 658 | ±32 |
| NH4+ (mg/L) | 775 | 770 | 785 | ±12 |
| NO2− (mg/L) | 0.5 | 0.3 | 0.7 | ±0.09 |
| NO3− (mg/L) | 1 | 0.7 | 1.5 | ±0.09 |
| Total Kjeldahl nitrogen (mg/L) | 850 | 800 | 897 | ±20 |
| TSS (mg/L) | 7140 | 6985 | 7589 | ±152 |
| VSS (mg/L) | 1860 | 1587 | 2058 | ±85 |
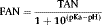 (7)
(7)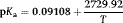 (8)
(8)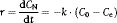 (9)
(9)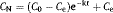 (10)
(10)The inflow of the organic loading rate (OLR) was 2 kg/m3 day, and the nitrogen loading rate (NLR) was 0.4 kg/m3 per day.
The statistical analysis of differences between the concentrations of N-NH4+ in the outflow from SBRs (after their stabilization) were determined by the Kruskal-Wallis test (Statistica 10).
3 Results and discussion
3.1 Calculation of PU foam effective surface area
One of the most important technical parameters characterizing a biomass carrier, and thus, influencing efficiency of the biological treatment process is the material's total (developed) and specific (related to the material unit mass or volume) surface area. Relevant calculations are quite simple in case of media with simple and repeatable shapes such as plastic balls, rings, or Kaldnes moldings 35, but porous substances characterized by high surface development coefficients, such as mineral materials with various degrees of fineness, sintered ceramic materials, or polymer foams of the type used in the present study, give rise to some mathematical difficulties.
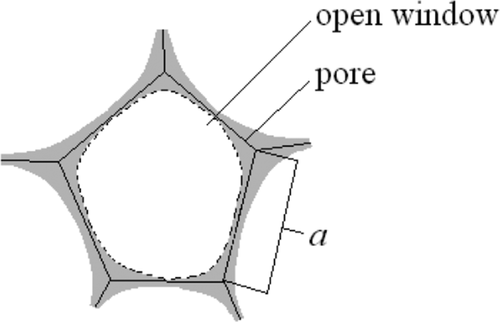
Determining PU foam surface area, Lim et al. 20 have measured foam pores by means of images obtained from scanning microscopy, but in their calculations they have adopted the methodology proposed by Rhodes 36 assuming that all pores have the same dimension and are spherical inside and hemispherical on the carrier outer surfaces. However, in the process of expanding PU sponges, a structure in the form of a reticulated foam develops edge struts of which form open, penta-, as well as hexa- and quadrilateral flat pores or windows, composing thus adjoining three-dimensional cells. Currently, the structure which most effectively represents a foam filling the three-dimensional space with cells of the same volume at a minimum surface area (the problem first formulated by Sir William Thomson [Lord Kelvin] in 1887) is the Waeire–Phelan structure 37 consisting of two kinds of solids: Irregular dodecahedrons (pyritohedrons) and tetrakaidecahedrons with two hexagonal and twelve pentagonal faces. Similar structures have been found in materials such as metal, ceramic, or polymer foams, including PU, and may be adopted as a base for modeling their properties 38. In the present paper, pentagonal regular dodecahedron model was adopted for calculations 39 with each cell having identical volume; however, it has been assumed also that all pores are open, so the actual surface area is much less than this obtained from theoretical calculations.
3.2 Leachate treatment efficiency
The average concentration of organic substances in raw leachate measured by means of chemical oxidation demand (COD) was 4125 mg/L, and expressed as biochemical oxidation demand (BOD5) with 636 mg/L. As a result, the BOD5/COD ratio was 0.15. The raw leachates were dominated by N-NH4+, with an average concentration of 775 mg/L. The N/COD ratio was low and equaled 0.18 (Tab. 2). It follows from the review of available literature that this is the composition typical for a landfill in its stabilized phase 40-42.
3.3 Removal of organics from leachates
The experiment proved that neither fill presence in the SBR nor its fraction had effect on the process of organics removal from leachates. In SBR1, the average COD was 3820.24 mg/L, in SBR2 3726.94 mg/L, and in SBR3 3955.39 mg/L. The BOD5 removal efficiency exceeded 95% and, as a result, the BOD5/COD ratio in the effluent ranged from 0.008 to 0.01 (Fig. 2).
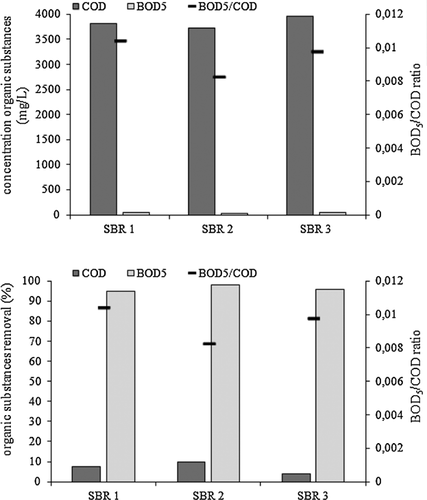
3.4 Removal of ammonium nitrogen from leachates
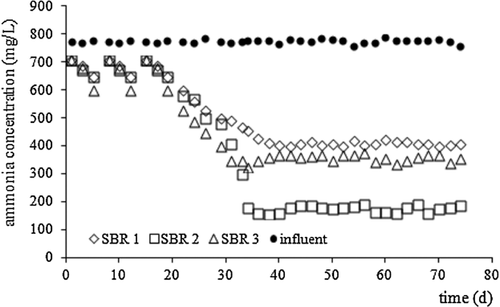
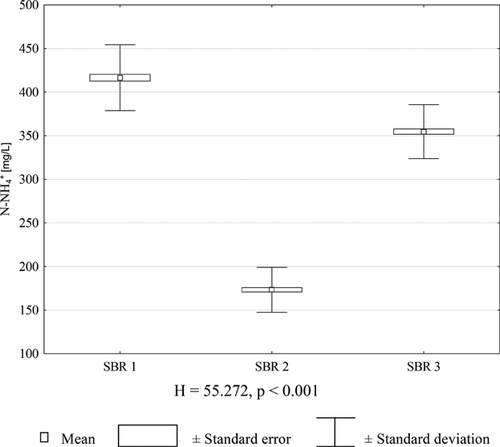
In all reactors, regardless of fill presence and/or fraction, stabilization of effluents occurred after 33 days of the experiment. Ammonium nitrogen dominated both in the SBR inflow and outflow (Fig. 3), whereas, N-NO2− and N-NO3− was not found. Statistically significant differences in N-NH4+ concentration between effluents from all the SBRs were found (Fig. 4).
The pH value has an effect on the production of FAN which shows inhibitory action on ammonium oxidizing bacteria (AOB). Akgul et al. 43 report that at pH > 8.7, FAN was about 550 mg/L. In turn, Yusof et al. 3 inform that at an initial ammonium concentration of 1452 mg/L, pH 8.45, and 25°C, FAN was 243 mg/L. The pH value of raw leachates used in this study was 8.16. After treatment in SBRs, the pH increased up to >9.5 regardless of the presence and fraction of filling. As a result, the average FAN calculated according to Eq. 7 was 347.98 mg/L in SBR1, 140.18 mg/L in SBR2, and 302.2 mg/L in SBR3. It follows from data available in the literature that FAN has an inhibiting effect on AOB when its concentration is within the range of 10–150 mg/L, whereas, its inhibits the development of nitrite-oxidizing bacteria (NOB) at 0.1–1 mg/L 44, 45. According to Eq. 7, FAN depends on the pH and temperature more strongly than on N-NH4+ concentration 34.
Akgul et al. 43 reported that when NLR in the influent was at the level of 0.47 kg/m3 per day, the N-NH4+ removal efficiency was 74%, with 65% of the removed N-NH4+ being transformed into N-NO2−. According to the present authors’ research, with influent NLR at the level 0.4 kg/m3 per day, and DO of 2 mg/L, no presence of either N-NO2− or N-NO3− was found in treated leachate, whereas the N-NH4+ removal efficiency was highest (77.6%) in the reactor filled with foam blocks with a porosity of 30 ppi (SBR2), and the lowest (46.3%) was observed in SBR1 operating without any filling. In SBR3, the N-NH4+ removal efficiency was 54.2%. On the other hand, van Dongen et al. 46 reported that at NLR of 1.2 kg/m3 per day, >80% of the N-NH4+ was transformed into molecular nitrogen.
In the process of N-NH4+ removal from leachates, temperature plays an important role. At temperatures exceeding 30°C, the specific AOB growth rate is twice as high as this of NOB, namely 1 day−1 and 0.5 day−1, respectively 47, which means that high temperature leads to fast cultivation of AOB 48, 49. Guo et al. 9 reported that with increasing temperature, the N-NH4+ removal effectiveness also increased. Moreover, higher temperature raises enzymatic activity of microorganisms 50 which improves not only the efficiency of N-NH4+ removal from the leachates but also the reaction rate. The temperature increase from 22.5 to 32.5°C resulted in 1.36 times higher N-NH4+ oxidation rate and 1.26 times higher N-NO2− oxidation rate 9.
The temperature higher than 37°C practically suppresses the second step of nitrification, which guide the process with bypassing of N-NO3− generation. This support the autotrophic process of ammonia removal under the deficiency of biodegradable organic compounds. Ambient temperature could be more suitable from the point of view of energy consumption. However, it must be first considered if this is not more economically justified than application of expensive external sources of carbon, necessary to maintain denitrification at lower temperatures.
It also should be mentioned, that on some landfills, including those described in this study, that there is some excess of thermal energy derived from combustion of biogas in cogeneration systems. This energy is sometimes utilized in such spendthrift processes as evaporation or stripping at high temperature.
Temperature has an important effect on ammonia removal, especially that the volatilization process is endothermic. Higher temperature was beneficial to air stripping, but usually this process is conducted at 60–70°C 51.
The pH value is even more crucial for the occurrence of the nitrogen forms in SBRs. At neutral pH, most of ammonia exists as ammonium ion (NH4+). With increasing pH, ammonia can be partly transformed to free ammonia (FAN), thus we assume that some amount of ammonia could be stripped to the air at pH > 8.5, but this process, as the other authors report, is intense when pH value is adjusted at least to 11 52.
In the experiment significant differences were observed between the reactors equipped with biomass carriers and operated with suspended sludge only. If these differences were considered as a result of physical process only, it is difficult to imagine the effective mechanism based on the differences of highly developed surface areas of chemically inert media.
Obviously, it must assumed that a certain amount of nitrogen may be removed by physical process. However, a process of physical nature should run with constant performance in the subsequent days of the experiment. In case of this experiment, the first day after starting the reactor, the ammonia removal efficiency was about 4%, which is at the level of statistical error.
The raw leachate subjected to the treatment had a pH of 8.16, an increase in pH was a result of the processes taking place in the reactor without the involvement of any reagents. This fact could be interesting because usually SND processes are considered to be effective in maintaining optimum pH level 53.
The SND processes can be explained in physical and biological ways. According to the first explanation, DO and other substrates cannot reach the inner part of the biofilm or sludge flocs thus, anoxic or anaerobic zones can develop inside. From the biological point of view, there are aerobic denitrifiers and anaerobic nitrifiers, thus, at least partial nitrification under anaerobic conditions is possible, as well as denitrification in the presence of oxygen 54.
The inner structure of the PU unclear foam served as biomass carrier (surface area and cell space) can influence the structure of biofilm (thickness of the layers and biodiversity of the microbial population).
Based on N-NH4+ concentration profiles determined during the aeration phase in each SBR, a kinetic analysis has been performed. It has been found that within the SBR operation cycle, neither N-NO2− nor N-NO3− were found in the leachates (Fig. 5).
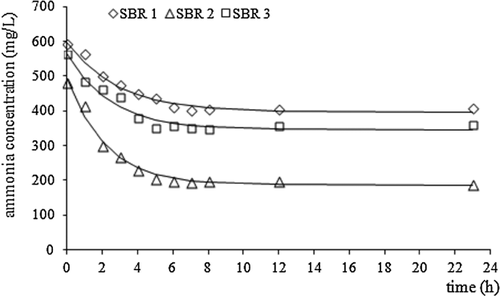
Ammonium nitrogen removal from SBRs occurred according to the first-order reaction. The reaction rate constant k was highest in case of SBR2 (0.45 h−1) and lowest in SBR1 (0.35 h−1). However, the reaction rate calculated according to Eq. 9 was highest in SBR1 and lowest in SBR3 (Tab. 4).
| SBR 1 | SBR 2 | SBR 3 | |
|---|---|---|---|
| C0 [mg/L] | 397 | 309 | 345 |
| Ce [mg/L] | 201 | 186 | 220 |
| k [h−1] | 0.35 | 0.45 | 0.4 |
| r [mg/L h] | 68.6 | 55.3 | 50 |
| Pearson correlation | 0.64 | 0.6 | 0.58 |
According to the information provided by Kulikowska 4 and Guo et al. 9, DO concentration also affects the N-NH4+ removal rate. At DO at the level of 1.2–1.3 mg/L, the observed nitrification rate was 19.4 mg/L·h. Reduction of DO to 0.8–0.9 mg/L resulted in an increase of the nitrification rate up to 21.1 mg/L·h 4. A downward trend in DO necessary to obtain the highest possible efficiency of SND was observed with increasing temperature. Guo et al. 9 proved that the decrease of N-NH4+ concentration was accompanied by an increase of DO concentration. The process occurs faster at lower temperatures. On the other hand, increased DO hinders denitrification which occurs under anaerobic conditions. One of the possible solutions to improve the growth of specific microbial populations can be the use of cellular structured filtration media (like PU foam). Inside the foam cells overgrown by biofilm, anaerobic conditions prevail, even when the DO concentration is high (2 mg/L) in the remaning volume of the reactor. Then, even in a relatively small volume of SBR, anaerobic and aerobic conditions can be maintained simultaneously.
The reaction rate observed in the experiment decreased with decreasing foam porosity. Lim et al. 20 observed a decrease in the reaction rate (r) with increasing SBR fill fraction, from 20 mg/L min where the volume of carriers comprised 20% of the working volume of the reactor to 15.7 mg/L min in the reactor filled with 40%. The decrease of r could be explained by a decrease of the specific external surface area (calculated on reactor volume). It seems that the main role in N-NH4+ removal is rather the suspended biomass than biomass attached to carriers. In addition, higher amount of carriers creates larger zones with anoxic conditions, thus, the remaining working volume of the reactor, with conditions appropriate for AOB organisms decreases. In the present study, the cause was smaller porosity (SBR3 with 15 ppi) of the fill placed in the SBR, and thus, also smaller inner surface area available to biomass for colonization.
The NLR value in the present study was 0.4 kg/m3 per day and the achieved N-NH4+ removal efficiency was at the level of 77.6%. PU foam cuboids used as the reactor fill created conditions favorable for occurrence of SND which was observed in the experiment. Occurrence of SND without any additional carbon source may be an indication of accumulation of carbon in deeper regions of the fill 20. Autotrophic nitrificants grow slower than heterotrophic bacteria, therefore, SND requires a degraded source of carbon in order to reduce the rate of heterotrophic nitrification and denitrification processes 55. Competition between autotrophic and heterotrophic microorganisms can occur also at high organic load rates. In SBRs, competition for oxygen between heterotrophic and autotrophic bacteria occurs at OLR > 2 kg/m3 per day 9. At 30°C, the COD/N ratio should be close to ten. Lower COD/N proportion results in an abrupt deficit of available carbon and unstable SND process 56. However, introducing biomass carriers into SBR results in development of microbial community structure and the population of nitrifiers, as well as a reversed COD/N ratio 57. For this reason, COD/N should not be considered a key parameter in SND.
4 Concluding remarks
The study concerned on the effect of porosity of PU foam used as a biomass carrier, on the efficiency of ammonium nitrogen removal. In SBRs equipped with that kind of carriers the SND process occurred, which could be evidenced by the N-NH4+ removal efficiency at the level of 46–77.6% and absence of N-NO2− and N-NO3− in the effluents. The lower efficiency of N-NH4+ removal, observed in SBR3 operated with 15 ppi carriers could be a result of the lower, than in case of 30 ppi (SBR2), inner surface area of filter media. The application of PU foam cubes could create also anaerobic zones, suitable for denitrification processes, at relatively high DO in SBRs. Further analysis of microbial populations dynamics would provide more information about the nature of this process.
5 Nomenclature
-
- a
-
- edge of the pentagonal pore
-
- A
-
- surface area of the foam cubes
-
- AC
-
- theoretical surface area of a single foam cell cm2
-
- ACC
-
- the largest cross-section area of theoretic cell cm2
-
- AEt
-
- estimated total surface of the biomass carrier cm2
-
- AOW
-
- average surface area of the pore's open window
-
- AP
-
- average pore surface area
-
- BOD/COD
-
- biochemical oxidation demand/chemical oxidation demand ratio
-
- C0
-
- ammonium concentration at the beginning of the SBR cycle mg/L
-
- Ce
-
- ammonium concentration at the end of the SBR cycle mg/L
-
- CN
-
- nitrogen concentration mg/L
-
- k
-
- constant of the nitrogen removal rate h−1
-
- kA
-
- pore opening coefficient
-
- r
-
- nitrogen removal rate mg/L h
-
- T
-
- temperature K
-
- t
-
- time h
-
- V
-
- volume of the foam cubes
-
- VC
-
- theoretical volume of a single foam cell
The authors have declared no conflict of interest.




