What can we learn from other clinical settings on the influence of the gut microbiome on the brain?
Potential conflict of interest: Nothing to report.
Abbreviations
-
- HE
-
- hepatic encephalopathy
-
- IBS
-
- irritable bowel syndrome
-
- PD
-
- Parkinson's disease
-
- SIBO
-
- small-intestinal bacterial overgrowth
From the Gut-Brain Axis to the Microbiota-Gut-Brain-Axis
The concept of the brain-gut axis, a bidirectional channel of communication between the “big brain” in the cranium and the “little brain” (i.e., the enteric nervous system) in the abdomen linked by neurons of the sympathetic and parasympathetic nervous systems, as well as by circulating hormones and other neuromodulatory molecules, is far from new and has long been visualized as the mediator of stress-related gastrointestinal symptoms. Interactions between brain and gut extend well beyond the bounds of the stressed, anxious, or depressed gut and should also encompass situations where the brain, the gut, and their linkage, through the autonomic nervous system, are affected by the same pathological process, as in Parkinson disease (PD); those instances where neurological symptoms are a consequence of a primarily gastrointestinal pathology, as in the malabsorption syndromes; and, finally, to a host of common gastrointestinal symptoms (or syndromes) that reflect dysfunction somewhere along the gut-brain axis, such as irritable bowel syndrome (IBS).1 Most recently, this axis has been extended to include a new member; the microbiota (the microbiota-gut-brain axis) and tantalizing evidence to suggest that bacteria resident in the gut could impact on the “big brain” and even contribute to neuropsychiatric disease has emerged. Accordingly, the microbiome has emerged as a potential therapeutic target in disorders as diverse as IBS, PD, and depression.2 Although many other neuropsychiatric disorders including Alzheimer disease, amyotrophic lateral sclerosis, autism, stroke, and drug addiction have been linked, in one way or another, to the microbiome,2 this review will briefly focus on lessons learned from IBS, PD, and depression.
Clinical and Experimental Evidence for a Role of the Microbiota in Disorders of the Gut-Brain Axis
Irritable Bowel Syndrome
That the microbiota might be a factor in IBS was first suggested by the observation that IBS could develop de novo in the aftermath of acute enteric bacterial, viral, and parasitic infections.3 More recently, modern sequencing technology has been applied to the study of the fecal and colonic microbiota in IBS in general, and relationships between a variety of clinical and demographic parameters and the microbiota have been investigated. Although data remain limited and not always consistent, it is evident that IBS patients have an altered fecal microbiota relative to healthy individuals.3, 4 Small intestinal bacteria might also play a role: Although its precise prevalence and role in IBS remain uncertain, evidence has been presented to associate small-intestinal bacterial overgrowth (SIBO) with IBS.3 Finally, clinical trials have demonstrated that two interventions that modulate the microbiome, an antibiotic and a probiotic, can alleviate IBS symptoms.3, 4 Quite how either intervention achieves this effect is unclear; effects on gut-brain signaling, motility, visceral sensation, the gut barrier, and the mucosal and systemic inflammatory responses3, 4 have been demonstrated, and any one or a combination of these effects could be relevant.
Parkinson Disease
Although the role of the microbiota in IBS may relate to its ability to influence, somewhere along the gut-brain axis, central perception and modulation of gut events, a much more fundamental involvement of gut bacteria has been invoked in PD. There is growing evidence, for example, that the pathological process that eventually emerges as classical PD originates in the gut and that an abnormal microbiome may participate through its stimulation of the innate immune system, thereby leading to misfolding of α-synuclein.5, 6 Roles for Helicobacter pylori infection and SIBO have also been proposed.5, 6 That the microbiota could exert such a profound impact on the development, morphology, and function of various parts of the central nervous system has been amply demonstrated in experiments in germ-free animals.7, 8
Depression
Experimental models have revealed the ability of modifications of the microbiota to influence behavior, cognition, mood, and related neurotransmitter function in experimental animals.7-10 Given the proinflammatory phenotype associated with depression, the aforementioned proinflammatory effects of a disrupted microbiome and the anti-inflammatory actions of some probiotics may be relevant here also; experimental evidence supports this concept.11 Other routes of influence, including the vagus nerve and microbial metabolites, have also been invoked.1, 9
Implications for Chronic Liver Disease
Having recognized the clinical relevance of the microbiota-gut-liver-brain axis in the pathogenesis of hepatic encephalopathy (HE) more than five decades ago, hepatologists will smile knowingly as they witness the excitement of their gastroenterological colleagues over their recent “discovery” of this same axis. Indeed, many of the phenomena invoked in the pathophysiology of HE; SIBO, an abnormal microbiota, impaired gut barrier function, a proinflammatory state, and the appearance in the systemic circulation of neuroactive molecules generated by bacterial metabolism will all strike a very familiar note (Fig. 1). This commonality of mechanisms should hasten a more thorough examination of how precisely gut bacteria, whether commensal or unwelcome, can influence cerebral function. From the nonhepatic literature some generic messages can be gleaned. First, microbiota-gut-brain communications are bidirectional, and one must always consider the possibility that changes in the microbiota are secondary. Second, the microbiota may communicate with the brain through several routes: through neuroactive peptides synthesized by bacteria, via other bacterial metabolites (e.g., ammonia) that can impact on the blood-brain barrier or brain function, through inflammatory mediators released locally or in the liver that can mediate neuroinflammation, or along the vagus. Each needs to be considered.
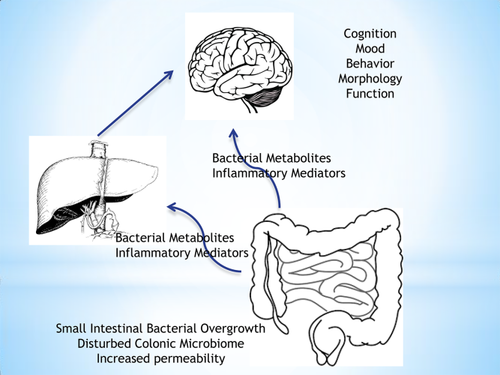
Microbiome-gut-liver-brain interactions. In the gut, a disordered microbiome in the colon or small intestinal bacterial overgrowth in the small intestine leads to the generation of potentially neuroactive metabolites and impairs gut barrier function. Activation of the vagus or the arrival of bacterial metabolites or bacterial components in the gut wall and circulation generates an inflammatory respose with the release of mediators such as cytokines. All of these impact on the liver and brain, and can impact on cognition, mood, and behavior. In the longer term, these bacterial influences can fundamentally alter brain morphology and neurochemistry.
With the interesting exception of HE and IBS, where the very same antibiotic, rifaximin, has proven effective and probiotics show promise,3 revelations on the microbiota-gut-brain axis in the laboratory have not, as yet, translated into new diagnostic tools or novel therapeutic approaches. When we reach that stage, the development of clinical trial endpoints will be a challenge; psychosocial and behavioral endpoints are symptom driven, and thus prone to considerable placebo responses; objective endpoints are relatively few, and those that have been studied, such as brain imaging, are not widely available and are expensive.
Conclusions
It should come as no surprise that studies of the microbiota-gut-brain axis in a number of neuropsychiatric disorders have revealed roles for factors that are known to be relevant to HE; a clinical entity that is still the most well-defined clinical illustration of a disordered microbiota-gut-liver-brain axis.