Novel treatments targeting metabolic and signaling mechanisms in primary biliary cholangitis
Dr. Trauner consults for, is on the speakers' bureau for, and received grants from Falk and MSD. He consults for and received grants from Albireo and Intercept. He is on the speakers' bureau for and received travel support from Roche. He consults for Novartis, Phenex and Bristol-Myers Squibb. He received grants from Takeda and travel support from Gilead. Dr. Halilbasic consults for Intercept and Novartis. She received travel support from Falk. Dr Fuchs received travel support from Boehringer Ingelheim, Falk, Gilead, Merck, Roche and Vifor.
Abstract
Watch a video presentation of this article
Abbreviations
-
- ALP
-
- alkaline phosphatase
-
- ASBT
-
- apical sodium-dependent bile acid transporter
-
- BA
-
- bile acid
-
- BSEP
-
- bile salt export pump FGF
-
- FXR
-
- farnesoid X receptor
-
- HNF4
-
- hepatocyte nuclear factor 4
-
- MDR3
-
- multidrug resistance protein 3
-
- NF-κB
-
- nuclear factor “kappa-light-chain-enhancer” of activated B cells
-
- norUDCA
-
- nor-ursodeoxycholic acid
-
- NTCP
-
- Na+-taurocholate cotransporting polypeptide
-
- OCA
-
- obeticholic acid
-
- PBC
-
- primary biliary cholangitis
-
- PL
-
- phospholipid
-
- PPARα
-
- peroxisome proliferator-activated receptor alpha
-
- UDCA
-
- ursodeoxycholic acid
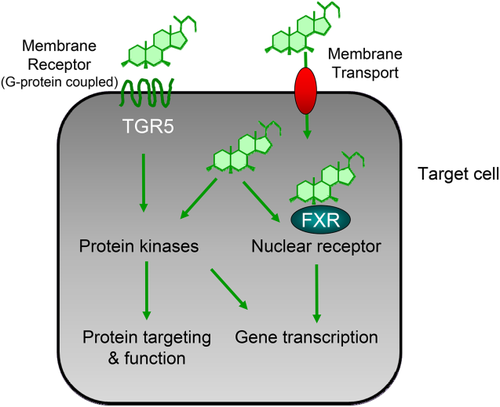
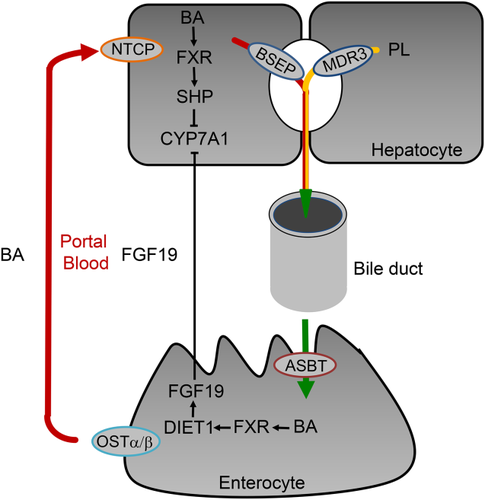
Primary biliary cholangitis (PBC) is characterized by immune-mediated damage of biliary epithelial cells resulting in destruction of small intrahepatic bile ducts with progressive cholestasis, associated with risk for progression to biliary fibrosis, cirrhosis, and hepatocellular cancer.1 As a result of considerable limitations in our current understanding of the etiology and pathogenesis of PBC, the therapeutic options are still limited. Ursodeoxycholic acid (UDCA) attenuates disease progression and improves survival, but more than 30% of patients do not appropriately respond to UDCA treatment and continue to be at risk for liver-related death or liver transplantation.1, 2 Thus, new therapeutic approaches are urgently needed. Importantly, bile acids (BAs) have hormonal signaling function via dedicated receptors such as the nuclear receptor farnesoid X receptor (FXR) and G protein-coupled receptor TGR5 (Fig. 1), which regulate BA homeostasis and BA-induced injury and/or inflammation. These receptors are important pharmacological targets in cholestatic disorders such as PBC.2 In addition, pharmacological inhibition of enterohepatic BA transporters (e.g., apical sodium-dependent bile acid transporter [ASBT] and Na+-taurocholate cotransporting polypeptide [NTCP]) (Fig. 2) may also modulate BA signaling.
Novel Therapeutic Targets
FXR and FGF15/19
FXR (predominately expressed in the liver, intestine, kidney, and adrenal glands) regulates hepatic BA homeostasis via the induction of small heterodimer partner in the liver and induction of fibroblast growth factor (FGF) 19 in the intestine, both inhibiting the rate-limiting enzyme cholesterol 7α-hydroxylase resulting in reduced hepatic BA synthesis (Fig. 2). FXR also regulates BA uptake (NTCP) and efflux (bile salt export pump [BSEP]) systems, thereby limiting hepatic BA overload.3 In addition, FXR exerts anti-inflammatory effects by attenuating nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB)-mediated inflammation and may have immunomodulatory effects.4 Furthermore, activation of FXR may improve intestinal inflammation and barrier function under cholestatic conditions.5 These immunometabolic FXR actions may have important implications for treatment of immune-mediated cholestatic disorders such as PBC. The steroidal FXR agonist, obeticholic acid (OCA), has already been successfully tested in clinical phase II2 and III trials (POISE),6 and has recently been approved for treatment of PBC by the US Food and Drug Administration. Both UDCA naive PBC patients and UDCA nonresponders showed improvement of serum levels of alkaline phosphatase (ALP) and other liver enzymes under OCA. Although there was no change in transient elastography or in advanced fibrosis score, and no improvement in overall quality of life as measured by PBC-40 questionnaire was observed after 12 months of OCA treatment, improvement of immunological (inflammation) parameters such as C-reactive protein, IgM, IgA, IgG, interleukin 12, and TNFα is suggestive of some disease-modifying effects.6 Whether these beneficial effects on disease surrogate parameters lead to amelioration of clinical outcomes in patients with PBC who have more advanced liver disease is going to be answered in the COBALT study that is currently enrolling patients (NCT02308111).
Several nonsteroidal FXR agonists are also tested in phase II studies in PBC. Some of these compounds seem to have gut-selective FXR ligand function, stimulating intestinal FGF19 and improving gut integrity, intestinal inflammation, and dysbiosis related to cholestatic disorders.7 Although FXR ligands also operate, at least in part, via induction of FGF19, novel FGF19 mimetics, with more selective effects on bile and energy metabolism, are promising for treatment of PBC, even if the reported mitogenic potential of FGF198 caused concerns and directed the drug development toward FGF19 variants with fully retained BA regulatory activity, but without protumoral activity.9 Such FGF19 mimetic NGM282 (M70) was tested in patients with PBC not responding to UDCA. In those patients it robustly decreased C4 and slightly decreased total BA levels, along with showing a significant reduction of ALP levels (NCT02135536).10
TGR5
TGR5 is highly expressed in macrophages (including Kupffer cells), cholangiocytes (including gall bladder epithelium), and intestinal L cells.11 As such, TGR5 inhibits macrophage activation with secretion of proinflammatory cytokines11 and stimulates biliary bicarbonate secretion, as well as production of glucagon-like-peptide 1 by L cells.12 Interestingly, a single-nucleotide polymorphism of TGR5 was associated with PSC,12 suggesting that this receptor could be a potential therapeutic target. However, a pure TGR5 agonist was not able to improve cholestatic liver injury in the Mdr2−/− mouse model of sclerosing cholangitis.13 Additional concerns include the potential role of TGR5 in pruritus,14 its effects on gallbladder relaxation,15 and potential proproliferative and antiapoptotic effects in cholangiocytes/cholangiocellular carcinoma cells.16 Moreover, TGR5 has also been linked to carcinogenesis via its interaction with EGFR.11 For this reason, TGR5 ligands do not appear to be developed clinically for cholestatic disorders.
Peroxisome Proliferator-Activated Receptor alpha
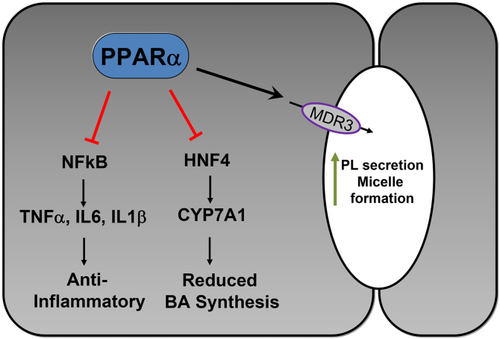
Fibrates that activate peroxisome proliferator-activated receptor alpha (PPARα) have been proposed to be beneficial as second-line therapy for PBC.17 Potential mechanisms of action comprise the upregulation of multidrug resistance protein 3 (MDR3), leading to increased biliary phospholipid (PL) concentration protecting cholangiocytes from potentially toxic BAs, repression of BA synthesis, and direct anti-inflammatory effects18 (Fig. 3). Despite nearly two decades of clinical experience with fibrates in PBC with small, mostly uncontrolled studies,17 their clinical benefit is still controversial. A recent retrospective study reported potential beneficial effects of fenofibrate on transplant-free survival of patients with PBC not responding to UDCA.19 However, potential concerns include increases in serum creatinine20 and bilirubin in patients with cirrhosis.19 The results of an ongoing prospective phase III study BEZURSO (NCT01654731) exploring bezafibrate effects in patients with PBC with incomplete biochemical response to UDCA are eagerly awaited.
ASBT Inhibitors
Ileal ASBT critically determines hepatocellular BA reuptake and subsequently biliary BA concentrations (Fig. 2). Therefore, inhibition of ASBT may counteract BA-driven toxicity to the liver and biliary system. Pharmacological ASBT inhibition reduced cholestatic liver injury and fibrosis by increasing fecal BA excretion, lowering total and especially hydrophobic biliary BA concentrations, whereas preserving biliary bicarbonate and PL secretion in Mdr2−/− mice (as model of sclerosing cholangitis).21 A phase I clinical study with the ASBT inhibitor (A4250) showed dose-dependent reduction of serum BA and FGF19 levels, whereas plasma C4 and fecal BAs increased in all A4250 treatment groups. Major adverse events were abdominal discomfort such as diarrhea and nausea.22 Currently, a phase II clinical study with A4250 in patients with PBC is being conducted (NCT02360852). However, another selective ASBT inhibitor lopixibat (formerly LUM001), in combination with UDCA in patients with PBC with pruritus (CLARITY, NCT01904058), had rather disappointing effects on ALP and itching, although serum BA reductions and C4 increases suggested pharmacological activity.23
Nor-ursodeoxycholic Acid
Nor-ursodeoxycholic acid (norUDCA), a side-chain-shortened, conjugation-resistant, and more hydrophilic homologue of UDCA, is passively absorbed by cholangiocytes and undergoes cholehepatic shunting, which allows ductular targeting.2 Furthermore, it promotes formation of biliary bicarbonate secretion that renders bile duct epithelial cells more resistant toward toxic bile. norUDCA also induces phase I and II detoxification enzymes and alternative basolateral efflux transporters, resulting in increased renal BA excretion.24 In addition, norUDCA has anti-inflammatory, antiproliferative, antiapoptotic, and antifibrotic effects. In line with this, norUDCA (but not UDCA)24 attenuated sclerosing cholangitis in the Mdr2−/− mice. A phase II clinical study in the treatment of patients with PSC demonstrated that norUDCA ameliorated serum levels of AP in a dose-dependent manner.25
Conclusions
Several novel therapeutic options beyond UDCA now allow targeting of BA homeostasis and signaling. Although UDCA acts mainly via post-transcriptional mechanisms, the new therapeutic options target mainly transcription factors such as FXR and PPARα. In addition, FGF19 mimetics, the UDCA homologue norUDCA, and ASBT inhibitors open novel therapeutic opportunities.