Palladium-Catalyzed Carbonylation of ortho-Alkenyl Iodobenzenes for the Construction of 3-Arylindenones
Minxiu Qian
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorLingyun Yao
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorZhi Li
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorXusheng Shao
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Quannan Wang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMinxiu Qian
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorLingyun Yao
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorZhi Li
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorXusheng Shao
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Quannan Wang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
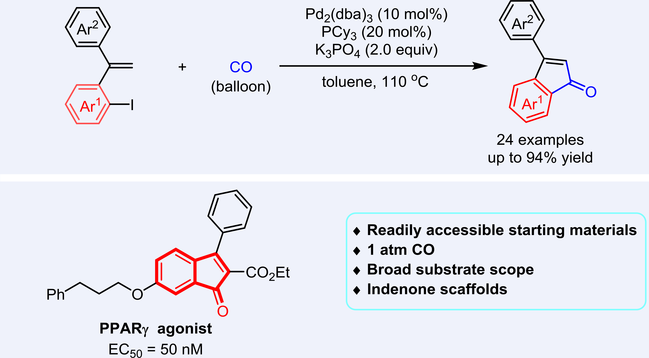
We developed a novel Pd-catalyzed carbonylation of ortho-alkenyl iodobenzenes with CO, affording a diverse array of 3-arylindenones in good to excellent yields (up to 94% yield). This methodology exhibits broad substrate scope and good functional group compatibility. The synthetic utility was demonstrated by a gram-scale reaction, diverse product derivatizations, and the preparation of an intermediate for a PPARγ agonist.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401269-sup-0001-supinfo.pdfPDF document, 6.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Ouyang, D.-W.; Ni, X.; Xu, H.-Y.; Chen, J.; Yang, P.-M.; Kong, D.-Y. Pterosins from Pteris multifida. Planta Med. 2010, 76, 1896–1900; (b) Harrowven, D. C.; Newman, N. A.; Knight, C. A. On the Identity of a Neo-lignan from the Fruits of Virola Sebifera. Tetrahedron Lett. 1998, 39, 6757–6760; (c) Ernst-Russell, M. A.; Chai, C. L. L.; Wardlaw, J. H.; Elix, J. A. Euplectin and Coneuplectin, New Naphthopyrones from the Lichen Flavoparmelia Euplecta. J. Nat. Prod. 2000, 63, 129–131; (d) Park, C. H.; Siomboing, X.; Yous, S.; Gressier, B.; Luyckx, M.; Chavatte, P. Investigations of New Lead Structures for the Design of Novel Cyclooxygenase-2 Inhibitors. Eur. J. Med. Chem. 2002, 37, 461–468; (e) Ahn, J. H.; Shin, M. S.; Jung, S. H.; Kang, S. K.; Kim, K. R.; Rhee, S. D.; Jung, W. H.; Yang, S. D.; Kim, S. J.; Woo, J. R.; Lee, J. H.; Cheon, H. G.; Kim, S. S. Indenone Derivatives: A Novel Template for Peroxisome Proliferator-Activated Receptor γ (PPARγ) Agonists. J. Med. Chem. 2006, 49, 4781–4784.
- 2(a) Glass, A. C.; Morris, B. B.; Zakharov, L. N.; Liu, S.-Y. Synthesis of Substituted Naphthalenes via a Catalytic Ring-Expansion Rearrangement. Org. Lett. 2008, 10, 4855–4857; (b) Jeffrey, J. L.; Sarpong, R. Concise Synthesis of Pauciflorol F Using a Larock Annulation. Org. Lett. 2009, 11, 5450–5453; (c) Prasher, P.; Sharma, M. Medicinal Chemistry of Indane and Its Analogues: A Mini Review. ChemistrySelect 2021, 6, 2658–2677.
- 3(a) Larock, R. C.; Doty, M. J.; Cacchi, S. Synthesis of Indenones via Palladium-Catalyzed Annulation of Internal Alkynes. J. Org. Chem. 1993, 58, 4579–4583; (b) Tsukamoto, H.; Kondo, Y. Palladium(II)-Catalyzed Annulation of Alkynes with ortho-Ester-Containing Phenylboronic Acids. Org. Lett. 2007, 9, 4227–4230; (c) Pletnev, A. A.; Tian, Q.; Larock, R. C. Carbopalladation of Nitriles: Synthesis of 2,3-Diarylindenones and Polycyclic Aromatic Ketones by the Pd-Catalyzed Annulation of Alkynes and Bicyclic Alkenes by 2-Iodoarenenitriles. J. Org. Chem. 2002, 67, 9276–9287; (d) Miura, T.; Murakami, M. Rhodium- Catalyzed Annulation Reactions of 2-Cyanophenylboronic Acid with Alkynes and Strained Alkenes. Org. Lett. 2005, 7, 3339–3341; (e) Morimoto, T.; Yamasaki, K.; Hirano, A.; Tsutsumi, K.; Kagawa, N.; Kakiuchi, K.; Harada, Y.; Fukumoto, Y.; Chatani, N.; Nishioka, T. Rh(I)-Catalyzed CO Gas-Free Carbonylative Cyclization Reactions of Alkynes with 2-Bromophenylboronic Acids Using Formaldehyde. Org. Lett. 2009, 11, 1777–1780; (f) Feng, J.; Lu, G.; Lv, M.; Cai, C. Palladium-Catalyzed Annulation of Alkynes with ortho-Halide-Containing Benzyl Alcohols in Aqueous Medium. J. Org. Chem. 2014, 79, 10561–10567; (g) Liu, F.; Li, Y.; Wang, X.; Qiang, Q.; Yan, Z.; Zhang, Y.; Rong, Z.-Q. Regioselective Synthesis of Indenones via Nickel-Catalyzed Larock Annulations. Org. Chem. Front. 2021, 8, 3847–3852.
- 4(a) Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Transition- Metal-Catalyzed C−H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333−9403; (b) Gensch, T.; James, M. J.; Dalton, T.; Glorius, F. Increasing Catalyst Efficiency in C−H Activation Catalysis. Angew. Chem. Int. Ed. 2018, 57, 2296−2306; (c) Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192−2452; (d) Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C-H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025−14040; (e) Rago, A. J.; Dong, G. Synthesis of Indoles, Indolines, and Carbazoles via Palladium-Catalyzed C–H Activation. Green Synth. Catal. 2021, 2, 216−227; (f) Sinha, S. K.; Guin, S.; Maiti, S.; Biswas, J. P.; Porey, S.; Maiti, D. Toolbox for Distal C–H Bond Functionalizations in Organic Molecules. Chem. Rev. 2022, 122, 5682−5841; (g) Lu, M.-Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.-S.; Loh, T.-P. Recent Advances in Alkenyl sp2 C–H and C–F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479−17646; (h) Zhang, F.; Zhao, R.; Zhu, L.; Yu, Y.; Liao, S.; Wang, Z.-X.; Huang, X. Divergent Isoindolinone Synthesis Through Palladium-Catalyzed Isocyanide Bridging C–H Activation. Cell Rep. Phys. Sci. 2022, 3, 100776; (i) Zhang, J.; Yao, L.; Su, J.-Y.; Liu, Y.-Z.; Wang, Q.; Deng, W.-P. Transition-Metal-Catalyzed Aromatic C–H Functionalization Assisted by the Phosphorus-Containing Directing Groups. Green Synth. Catal. 2023, 4, 206−225.
- 5(a) Li, B.-J.; Wang, H.-Y.; Zhu, Q.-L.; Shi, Z.-J. Rhodium/Copper-Catalyzed Annulation of Benzimides with Internal Alkynes: Indenone Synthesis through Sequential C–H and C–N Cleavage. Angew. Chem. Int. Ed. 2012, 51, 3948−3952; (b) Qi, Z.; Wang, M.; Li, X. Access to Indenones by Rhodium(III)-Catalyzed C–H Annulation of Arylnitrones with Internal Alkynes. Org. Lett. 2013, 15, 5440−5443; (c) Chen, S.; Yu, J.; Jiang, Y.; Chen, F.; Cheng, J. Rhodium-Catalyzed Direct Annulation of Aldehydes with Alkynes Leading to Indenones: Proceeding through in Situ Directing Group Formation and Removal. Org. Lett. 2013, 15, 4754−4757; (d) Yu, W.; Zhang, W.; Liu, Z.; Zhang, Y. Cobalt(III)-Catalyzed Annulation of Esters and Alkynes: A Facile Route to Indenones. Chem. Commun. 2016, 52, 6837−6840; (e) Kong, L.; Yang, X.; Zhou, X.; Yu, S.; Li, X. Cobalt(III)-Catalyzed Efficient Synthesis of Indenones Through Carboannulation of Benzoates and Alkynes. Org. Chem. Front. 2016, 3, 813−816.
- 6(a) Zhu, X.-T.; Zhao, Q.; Liu, F.; Wang, A.-F.; Cai, P.-J.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Silver-Mediated Radical 5-exo-dig Cyclization of 2-Alkynylbenzonitriles: Synthesis of Phosphinylated 1-Pndenones. Chem. Commun. 2017, 53, 6828−6831; (b) Yao, T.; Zhao, S.; Liu, T.; Wu, Y.; Ma, Y.; Li, T.; Qin, X. Transition-Metal-Free Approaches to 2,3-Diarylated Indenones from 2-Alkynylbenzaldehydes and Phenols with Tunable Selectivity. Chem. Commun. 2022, 58, 4592–4595; (c) Mondal, S.; Biswas, K.; Ganesh, V. In Situ Triflation-Tandem Cross- Coupling Enables Synthesis and Photophysical Studies of Indenones and Benzofulvenes. Adv. Synth. Catal. 2024, 366, 3277–3282; (d) Popek, L.; Cihan, M.; Blanchard, N.; Bizet, V. Palladium-Catalyzed Regioselective Synthesis of 2-SF5-Indenols and Further Derivatizations. Angew. Chem. Int. Ed. 2024, 63, e202315909.
- 7 Uemura, N.; Ishikawa, H.; Tamura, N.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Stereoselective Photodimerization of 3-Arylindenones in Solution and in the Solid State. J. Org. Chem. 2018, 83, 2256–2262.
- 8(a) Ma, S.; Gu, Z. 1,4-Migration of Rhodium and Palladium in Catalytic Organometallic Reactions. Angew. Chem. Int. Ed. 2005, 44, 7512−7517; (b) Rahim, A.; Feng, J.; Gu, Z. 1,4-Migration of Transition Metals in Organic Synthesis. Chin. J. Chem. 2019, 37, 929−945; (c) Li, M.-Y.; Wei, D.; Feng, C.-G.; Lin, G.-Q. Tandem Reactions Involving 1,4-Palladium Migrations. Chem. Asian J. 2022, 17, e202200456; (d) Zhang, Y. Intermolecular Difunctionalization of C,C-Palladacycles Obtained by Pd(0)-Catalyzed C–H Activation. Acc. Chem. Res. 2022, 55, 3507–3518; (e) Wei, D.; Lin, G.-Q. Functionalization of C,C-Palladacycles: Application in the Synthesis of Functional Molecules. Sci. China Chem. 2023, 66, 2721−2733; (f) Fang, Y.; Ding, M.; Huang, X. Taming the Selective C−H Bond Activation through 1,5-Palladium Migration. ChemCatChem 2024, 16, e202301320.
- 9 Hu, T.-J.; Zhang, G.; Chen, Y.-H.; Feng, C.-G.; Lin, G.-Q. Borylation of Olefin C−H Bond via Aryl to Vinyl Palladium 1,4-Migration. J. Am. Chem. Soc. 2016, 138, 2897−2900.
- 10(a) Hu, T.-J.; Li, M.-Y.; Zhao, Q.; Feng, C.-G.; Lin, G.-Q. Highly Stereoselective Synthesis of 1,3-Dienes through An Aryl to Vinyl 1,4-Palladium Migration/Heck Sequence. Angew. Chem. Int. Ed. 2018, 57, 5871−5875; (b) Xue, Z.-J.; Li, M.-Y.; Zhu, B.-B.; He, Z.-T.; Feng, C.-G.; Lin, G.-Q. Stereoselective Synthesis of Conjugated Trienes via 1,4-Palladium Migration/Heck Sequence. Chem. Commun. 2020, 56, 14420−14422; (c) Xue, Z.-J.; Li, M.-Y.; Zhu, B.-B.; He, Z.-T.; Feng, C.-G.; Lin, G.-Q. A 1,4-Palladium Migration/Heck Sequence with Unactivated Alkenes: Stereoselective Synthesis of Trisubstituted 1,3-Dienes. Adv. Synth. Catal. 2021, 363, 2089−2092; (d) Lin, J.; Huang, Z.; Ma, J.; Xu, B.-H.; Zhou, Y.-G.; Yu, Z. Tunable Construction of Multisubstituted 1,3-Dienes and Allenes via a 1,4-Palladium Migration/Carbene Insertion Cascade. J. Org. Chem. 2022, 87, 12019–12035.
- 11(a) Chen, Z.-Y.; Ji, X.-M.; Wang, X.; Asgari, M.; Fu, J.-G.; Zhang, S.-S.; Feng, C.-G. Highly Stereoselective Synthesis of 1,3-Enynes via Aryl to Vinyl 1,4-Palladium Migration/Sonogashira Coupling Sequence. ChemCatChem 2024, 16, e202400478; (b) Lin, J.; Ma, J.; Wang, L.; Wu, P.; Wu, K.; Zhou, Y.-G.; Yu, Z. Stereoselective Construction of Trisubstituted 1,3-Enynes via Aryl to Vinyl 1,4-Palladium Migration. Adv. Synth. Catal. 2024, 366, 4509−4514.
- 12(a) Rocaboy, R.; Baudoin, O. 1,4-Palladium Shift/C(sp3)−H Activation Strategy for the Remote Construction of Five-Membered Rings. Org. Lett. 2019, 21, 1434−1437; (b) Zhang, S.-S.; Hu, T.-J.; Li, M.-Y.; Song, Y.-K.; Yang, X.-D.; Feng, C.-G.; Lin, G.-Q. Asymmetric Alkenylation of Enones and Imines Enabled by A Highly Efficient Aryl to Vinyl 1,4-Rhodium Migration. Angew. Chem. Int. Ed. 2019, 58, 3387−3391; (c) Wang, Q.; Chen, R.; Lou, J.; Zhang, D. H.; Zhou, Y.-G.; Yu, Z. Highly Regioselective C–H Alkylation of Alkenes through An Aryl to Vinyl 1,4-Palladium Migration/C–C Cleavage Cascade. ACS Catal. 2019, 9, 11669−11675.
- 13(a) Li, M.-Y.; Han, P.; Hu, T.-J.; Wei, D.; Zhang, G.; Qin, A.; Feng, C.-G.; Tang, B. Z.; Lin, G.-Q. Suzuki-Miyaura Coupling Enabled by Aryl to Vinyl 1,4-Palladium Migration. iScience 2020, 23, 100966; (b) Lin, J.; Ma, J.; Wang, L.; Wu, K.; Zhou, Y.-G.; Yu, Z. Regioselective Polyfluoroarylation of Alkenyl C–H Bonds via Aryl to Vinyl 1,4-Palladium Migration. Org. Chem. Front. 2023, 10, 5144−5150; (c) Tan, Q.; Li, C.; Yang, L.; Wang, Z.; Huang, Y.; Wang, C.; Liu, L.; Chen, W.-H.; Chen, T. Pd/Cu Cooperative Catalysis for Heteroarylation of Vinyl C−H Bond Forming Polyaryl Ethylenes via C−O/Dual C−H Cleavage. ACS Catal. 2024, 14, 9151−9165.
- 14 Chen, Y.-Z.; Bao, G.-Y.; Zhan, X.-C.; Fu, J.-G.; Ji, X.-M.; Zhang, S.-S.; Feng, C.-G. Highly Stereoselective Synthesis of 2,2-Disubstituted Vinylphosphonates via Aryl to Vinyl 1,4-Palladium Migration. Chin. J. Chem. 2022, 40, 2188−2192.
- 15(a) Xu, S.; Chen, R.; Fu, Z.; Zhou, Q.; Zhang, Y.; Wang, J. Palladium-Catalyzed Formal [4 + 1] Annulation via Metal Carbene Migratory Insertion and C(sp2)–H Bond Functionalization. ACS Catal. 2017, 7, 1993–1997; (b) Wei, D.; Hu, T.-J.; Feng, C.-G.; Lin, G.-Q. Synthesis of Substituted Naphthalenes by 1,4-Palladium Migration Involved Annulation with Internal Alkynes. Chin. J. Chem. 2018, 36, 743−748; (c) Wei, D.; Li, M.-Y.; Zhu, B. B.; Yang, X.-D.; Zhang, F.; Feng, C.-G.; Lin, G.-Q. Asymmetric Alkenylation of Enones and Imines Enabled by a Highly Efficient Aryl to Vinyl 1,4-Rhodium Migration. Angew. Chem. Int. Ed. 2019, 58, 16543−16547; (d) Zhang, H.; Yu, Y.; Huang, X. Facile access to 2,2-DiAryl 2H-Chromenes through a Palladium-Catalyzed Cascade Reaction of ortho-Vinyl Bromobenzenes with N-Tosylhydrazones. Org. Biomol. Chem. 2020, 18, 5115–5119; (e) Feng, X.-J.; Fu, J.-G.; Zhao, Q.; Lu, Y.-H.; Zhang, S. S.; Feng, C.-G. Synthesis of 1,1-Disubstituted Indenes via Palladium-Catalyzed Cross-Coupling of ortho-Alkenyl Bromobezenes and α-Aryl-α-diazoesters. Synthesis 2023, 55, 1922–1928; (f) Zhan, X.-C.; Lin, G.-Q.; Fu, J.-G.; Ji, X.-M.; Zhang, S.-S.; Feng, C.-G. Synthesis of Dibenzo[b,f]azepines via Palladium-Catalyzed Cascade [4 + 3] Annulation of o-Alkenyl Bromoarenes and o-Bromoaniline Derivatives. Adv. Synth. Catal. 2023, 365, 4127–4131; (g) Gagnier, S. V.; Larock, R. C. Palladium-Catalyzed Carbonylative Cyclization of Unsaturated Aryl Iodides and Dienyl Triflates, Iodides, and Bromides to Indanones and 2-Cyclopentenones. J. Am. Chem. Soc. 2003, 125, 4804–4807.
- 16(a) Liu, Q.; Zhang, H.; Lei, A. Oxidative Carbonylation Reactions: Organometallic Compounds (R–M) or Hydrocarbons (R–H) as Nucleophiles. Angew. Chem. Int. Ed. 2011, 50, 10788–10799; (b) Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of Alkenes with CO Surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310−6320; (c) Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2019, 5, 526−552; (d) Zhang, S.; Neumann, H.; Beller, M. Synthesis of α,β-Unsaturated Carbonyl Compounds by Carbonylation Reactions. Chem. Soc. Rev. 2020, 49, 3187−3210; (d) Wu, Z.; Cheng, C.; Zhang, Y.; Transition Metal-Catalyzed Reactions of C–H Bonds with Carbon Monoxide. Chin. J. Org. Chem. 2021, 41, 2155−2174.
- 17 Ye, T.; Cheng, F.; Zhang, J.; Liu, Y.-Z.; Wang, Q.; Deng, W.-P. Highly Regioselective C–H Carbonylation of Alkenes with Phenyl Formate via Aryl to Vinyl 1,4-Palladium Migration. Org. Chem. Front. 2023, 10, 1537−1543.
- 18 Li, M.; Li, S.-X.; Chen, D.-p.; Gao, F.; Qiu, Y.-F.; Wang, X.-C.; Quan, Z.-J.; Liang, Y. M. Regioselective C–H Active Carbonylation via 1,4-Palladium Migration. Org. Lett. 2023, 25, 2761–2766.
- 19 Yum, E. K.; Park, W. S.; Kim, S. H.; Kang, S. K.; Kim, S. S.; Ahn, J. H. Facile Synthesis of Alkyl 1-Oxo-3-phenyl-1H-indene-2-carboxylate through Palladium-Catalyzed Carboalkoxylation from 2-Bromo-3- phenylinden-1-ones. Chem. Lett. 2008, 37, 1068–1069.
- 20 Cen, M.; Ma, X.; Yang, X.; Zhang, S.; Liu, L.; Szostak, M.; Chen, T. Site-Selective Decarbonylative [4+2] Annulation of Carboxylic Acids with Terminal Alkynes by C–C/C–H Activation Strategy and Cluster Catalysis. Chem. Sci. 2024, 15, 20346–20354.
- 21(a) Lafrance, M.; Gorelsky, S. I.; Fagnou, K. High-yielding Palladium- Catalyzed Intramolecular Alkane Arylation: Reaction Development and Mechanistic Studies. J. Am. Chem. Soc. 2007, 129, 14570−14571; (b) Chaumontet, M.; Piccardi, R.; Audic, N.; Hitce, J.; Peglion, J.-L.; Clot, E.; Baudoin, O. Synthesis of Benzocyclobutenes by Palladium- Catalyzed C−H Activation of Methyl Groups: Method and Mechanistic Study. J. Am. Chem. Soc. 2008, 130, 15157−15166.




