Catalytic Difunctionalization of Alkenes under Visible Light Irradiation: Synthesis of Iminofuranones
Boyu Liu
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorGuangxian Chen
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorLele Zhang
Key Laboratory of Chemical Genomics of Guangdong Province, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorFeiwei Yan
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorFeiming Li
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorZhixiong Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorMingqiang Huang
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorLina Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorCorresponding Author
Shunyou Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Key Laboratory of Chemical Genomics of Guangdong Province, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorBoyu Liu
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorGuangxian Chen
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorLele Zhang
Key Laboratory of Chemical Genomics of Guangdong Province, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorFeiwei Yan
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorFeiming Li
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorZhixiong Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorMingqiang Huang
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorLina Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Search for more papers by this authorCorresponding Author
Shunyou Cai
Key Laboratory of Modern Analytical Science and Separation Technology of Fujian Province, School of Chemistry Chemical Engineering, and Environment, Minnan Normal University, Zhangzhou, Fujian, 363000 China
Key Laboratory of Chemical Genomics of Guangdong Province, School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
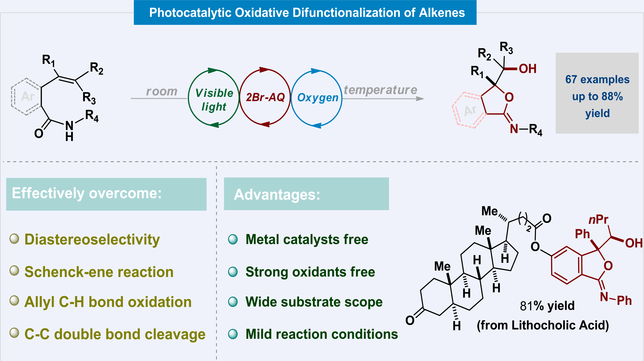
Here, we show a cost-effective and environmentally friendly method for synthesizing iminofuranones using visible light and the photocatalyst 2-bromoanthraquinone. Our approach uses only oxygen as the oxidant, avoiding the need for additional transition metals and strong oxidizing agents. By employing a mixed solvent system of DMF and CHCl3 under ambient conditions, we have achieved highly diastereoselective conversions of various 2-vinyl benzamides and alkenyl amides into functionalized iminoisobenzofuranones and iminofuranones. This versatile process is broadly applicable and enables late-stage structural modifications of complex substrates with bioactive moieties.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401116-sup-0001-supinfo.pdfPDF document, 19.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Pratley, C.; Fenner, S.; Murphy, J. Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev. 2022, 122, 8181–8260; (b) Kennemur, J. L.; Maji, R.; Scharf, M. J.; List, B. Catalytic Asymmetric Hydroalkoxylation of C-C Multiple Bonds. Chem. Rev. 2021, 121, 14649–14681; (c) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C-C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561.
- 2(a) Li, Y.; Wu, D.; Cheng, H.-G.; Yin, G. Difunctionalization of Alkenes Involving Metal Migration. Angew. Chem. Int. Ed. 2020, 59, 7990–8003; (b) Lin, J.; Song, R.-J.; Hu, M.; Li, J.-H. Recent Advances in the Intermolecular Oxidative Difunctionalization of Alkenes. Chem. Rec. 2019, 19, 440–451; (c) Sauer, G. S.; Lin, S. An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes. ACS Catal. 2018, 8, 5175–5187; (d) Li, Z. L.; Fang, G. C.; Gu, Q. S.; Liu, X. Y. Recent Advances in Copper-catalysed Radical-involved Asymmetric 1,2-Difunctionalization of Alkenes. Chem. Soc. Rev. 2020, 49, 32–48.
- 3For selected reviews, see: (a) Ghogare, A. A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034; (b) Adam, W.; Kazakov, D. V.; Kazakov, V. P. Singlet-Oxygen Chemiluminescence in Peroxide Reactions. Chem. Rev. 2005, 105, 3371–3387.
- 4(a) Ding, G.; Liu, S.; Guo, L.; Zhou, Y.; Che, Y. Antifungal Metabolites from the Plant Endophytic Fungus Pestalotiopsis Foedan. J. Nat. Prod. 2008, 71, 615–618; (b) Naganuma, M.; Nishida, M.; Kuramochi, K.; Sugawara, F.; Yoshida, H.; Mizushina, Y. 1-Deoxyrubralactone, a Novel Specific Inhibitor of Families X and Y of Eukaryotic DNA Polymerases from a Fungal Strain Derived from Sea Algae. Bioorg. Med. Chem. 2008, 16, 2939–2944; (c) Wang, Y.; Zhang, Z.; Yang, F.; Sun, Q.; He, H.; Di, Y.; Mu, S.; Lu, Y.; Chang, Y.; Zheng, Q.; Ding, M.; Dong, J.; Hao, X. Benzylphenethylamine Alkaloids from Hosta Plantaginea with Inhibitory Activity Against Tobacco Mosaic Virus and Acetylcholinesterase. J. Nat. Prod. 2007, 70, 1458–1461; (d) Kohler, D.; Podlech, J. A New Secondary Metabolite from Alternaria Alternata: Structure Elucidation and Total Synthesis of Altenuic Acid IV. Eur. J. Org. Chem. 2019, 1748–1753.
- 5For selected reviews, see: (a) Bellotti, P.; Huang, H.; Faber, T. Glorius F. Photocatalytic Late-Stage C-H Functionalization. Chem. Rev. 2023, 123, 4237–4352; (b) Holmberg-Douglas, N. Nicewicz, D. A. Photoredox-Catalyzed C-H Functionalization Reactions. Chem. Rev. 2022, 122, 1925–2016; (c) Corbin, D. A.; Miyake, G. M. Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): Precision Polymer Synthesis Using Organic Photoredox Catalysis. Chem. Rev. 2022, 122, 1830–1874; (d) Kwon, K.; Simons, R.; Nandakumar, M.; Roizen, J. Strategies to Generate Nitrogen-Centered Radicals that May Rely on Photoredox Catalysis: Development in Reaction Methodology and Applications in Organic Synthesis. Chem. Rev. 2022, 122, 2353–2428; (e) Yu, X.; Zhao, Q.; Chen, J.; Xiao, W.; Chen, J. When Light Meets Nitrogen-Centered Radicals: from Reagents to Catalysts. Acc. Chem. Res. 2020, 53, 1066–1083; (f) Allen, A. R.; Noten, E. A.; Stephenson, C. R. J. Aryl Transfer Strategies Mediated by Photoinduced Electron Transfer. Chem. Rev. 2022, 122, 2695–2751.
- 6For selected examples, see: (a) Campbell, M. W.; Compton, J. S.; Kelly, C. B.; Molander, G. A. Three-Component Olefin Dicarbofunctionalization Enabled by Nickel/Photoredox Dual Catalysis. J. Am. Chem. Soc. 2019, 141, 20069–20078; (b) Griffin, J. D.; Cavanaugh, C. L.; Nicewicz, D. A. Reversing the Regioselectivity of Halofunctionalization Reactions through Cooperative Photoredox and Copper catalysis. Angew. Chem. Int. Ed. 2017, 56, 2097–2100; (c) Nguyen, S. T.; Zhu, Q.; Knowles, R. R. PCET-enabled Olefin Hydroamidation Reactions with N-alkyl Amides. ACS Catal. 2019, 9, 4502–4507; (d) Wang, H.; Toh, R.; Shi, X.; Wang, T.; Cong, X.; Wu, J. Photo-Mediated Selective Deconstructive Geminal Dihalogenation of Trisubstituted Alkenes. Nat. Commun. 2020, 11, 4462; (e) Cai, S.; Xu, Y.; Chen, D.; Li, L.; Chen, Q.; Huang, M.; Weng, W. Visible-Light-Enabled Decarboxylative Sulfonylation of Cinnamic Acids with Sulfonylhydrazides under Transition-Metal-Free Conditions. Org. Lett. 2016, 18, 2990–2993; (f) Hamilton, D. S.; Nicewicz, D. A. Direct Catalytic Antimarkovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc. 2012, 134, 18577–18580; (g) Sahoo, B.; Hopkinson, M. N.; Glorius, F. Combining Gold and Photoredox Catalysis: Visible Light-Mediated Oxy- and Aminoarylation of Alkenes. J. Am. Chem. Soc. 2013, 135, 5505–5508; (h) Miller, D. C.; Ganley, J. M.; Musacchio, A. J.; Sherwood, T. C.; Ewing, W. R.; Knowles, R. R. Anti-Markovnikov Hydroamination of Unactivated Alkenens with Primary Alkyl Amines. J. Am. Chem. Soc. 2019, 141, 16590–16594; (i) Zhou, X.; Zhang, Z.; Qu, W.; Liu, X.; Xiao, W.; Jiang, M.; Chen, J. Asymmetric [3+2] Photocycloaddition of β-keto Esters and Vinyl Azides by Dual Photoredox/Nickel Catalysis. J. Am. Chem. Soc. 2023, 145, 12233–12243; (j) Zeller, M. A.; Riener, M.; Nicewicz, D. A. Butyrolactone Synthesis via Polar Radical Crossover Cycloaddition Reactions: Diastereoselective Syntheses of Methylenolactocin and Protolichesterinic Acid. Org. Lett. 2014, 16, 4810–4813; (k) Morse, P. D.; Nicewicz, D. A. Divergent Regioselectivity in Photoredox-Catalyzed Hydrofunctionalization Reactions of Unsaturated Amides and Thioamides. Chem. Sci. 2015, 6, 270–274; (l) Griesbeck, A. G.; Hundertmark, T.; Steinwascher, J. Regio- and Diastereoselective Formation of 1,2-Azidohydroperoxides by Photooxygenation of Alkenes in the Presence of Azide Anions. Tetrahedron Lett. 1996, 37, 8367–8370; (m) Zhu, Q.; Graff, D. E.; Knowles, R. R. Intermolecular Antimarkovnikov Hydroamination of Unactivated Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2018, 140, 741–747; (n) Patra, T.; Das, M.; Daniliuc, C. G.; Glorius, F. Metal-Free Photosensitized Oxyimination of Unactivated Alkenes with Bifunctional Oxime Carbonates. Nat. Catal. 2021, 4, 54–61; (o) Wang, S.; Luo, X.; Wang, Y.; Liu, Z.; Yu, Y.; Wang, X.; Ren, D.; Wang, P.; Chen, Y.; Qi, X.; Yi, H.; Lei, A. Radical-Triggered Translocation of C-C Double Bond and Functional Group. Nat. Chem. 2024, 16, 1621–1629; (p) Qi, X.; Zheng, M. J.; Yang, C.; Zhao, Y.; Guo, L.; Xia, W. Metal-Free Amino (hetero) Arylation and Aminosulfonylation of Alkenes Enabled by Photoinduced Energy Transfer. J. Am. Chem. Soc. 2023, 145, 16630–16641; (q) Zhong, P.; Tu, J.; Zhao, Y.; Zhong, N.; Yang, C.; Guo, L.; Xia, W. Photoelectrochemical Oxidative C(sp3)-H Borylation of Unactivated Hydrocarbons. Nat. Commun. 2023, 14, 6530.
- 7Our previous studies on photoredox catalysis, see: (a) Liu, C.; Liu, H.; Zheng, X.; Chen, S.; Lai, Q.; Zheng, C.; Huang, M.; Cai, K.; Cai, Z.; Cai, S. Visible-Light-Enabled Allylic C–H Oxidation: Metal-Free Photocatalytic Generation of Enones. ACS Catal. 2022, 12, 1375–1381; (b) Chen, S.; Lai, Q.; Liu, C.; Liu, H.; Huang, M.; Cai, S. Oxidative Lactonization of C(sp3)-H Bond in Methyl Aromatic Alcohols Enabled by Proton- Coupled Electron Transfer. Sci. China Chem. 2022, 65, 1526–1531; (c) Liu, H.; Liu, C.; Chen, S.; Lai, Q.; Lin, Y.; Cai, Z.; Huang, M.; Cai, S. Phthalide Synthesis through Dehydrogenated Lactonization of the C(sp3)-H Bond by Photoredox Catalysis. Green Chem. 2021, 23, 8212–8216; (d) Liang, Z.; Liu, C.; Fan, J.; Wang, M.; Yan, X.; Huang, M.; Cai, S. Photocatalytic Dehydrogenated Etherification of 2-Aryl Benzylic Alcohols. Green Chem. 2022, 24, 7442–7447; (e) Liang, Z.; Yu, Y.; Zhang, L.; Xue, G.; Liu, M.; Zhang, Y.; Huang, M.; Cai, L.; Cai, S. Visible-Light-Enabled Catalytic Approach to N,O-spirocycles through Amidyl Radical Addition/Cyclization. Org. Lett. 2024, 26, 298–303.
- 8For detailed information, see the Supporting Information.
- 9For detailed test method for the excited state oxidation potential of 2-bromoanthraquinone, see the Supporting Information.
- 10(a) Matuszak, Z.; Reszka, K.; Chignell, C. Reaction of Melatonin and Related Indoles with Hydroxyl Radicals: EPR and Spin Trap Investigations. Free Radic. Biol. Med. 1997, 23, 367–372; (b) Villamena, F.; Merle, J.; Hadad, C.; Zweier, J. Superoxide Radical Anion Adduct of 5,5-Dimethyl-1-pyrroline N-oxide (DMPO). 2. The Thermodynamics of Decay and EPR Spectral Properties. J. Phys. Chem. A 2005, 109, 6089–6098.
- 11 Cismesia, M. A.; Yoon, T. P. Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci. 2015, 6, 5426–5434.
- 12(a) Romero, N. A.; Nicewicz, D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166; (b) Mangion, D.; Kendall, J.; Arnold, D. R. Photosensitized (Electron-Transfer) Deconjugation of 1-Arylcyclohexenes. Org. Lett. 2001, 3, 45–48; (c) Yasuda, M.; Yamashita, T.; Shima, K.; Pac, C. Photochemical Reactions of Aromatic Compounds. 43. Direct Photoamination of Arenes with Ammonia and Primary Amines in the Presence of Electron Acceptors. J. Org. Chem. 1987, 52, 753–759.
- 13(a) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547; (b) Griffith, J. C.; Jones, K. M.; Picon, S.; Rawling, M. J.; Kariuki, B. M.; Campbell, M.; Tomkinson, N. C. Alkene Syn Dihydroxylation with Malonoyl Peroxides. J. Am. Chem. Soc. 2010, 132, 14409–14411; (c) Southgate, E. H.; Pospech, J.; Fu, J.; Holycross, D. R.; Sarlah, D. Dearomative Dihydroxylation with Arenophiles. Nat. Chem. 2016, 8, 922–928; (d) Voronov, A.; Casnati, A.; Hoch, M.; Pancrazzi, F.; Mazzeo, P.; Merli, D.; Maestri, G.; Fehér, P.; Stirling, A.; Capaldo, L.; Della Ca', N. Photocatalyzed Aerobic Dearomatization of Naphthylamines under Visible-Light Irradiation. Adv. Synth. Catal. 2024, 366, 4187–4193.




