Total Synthesis of Laurane and Guaiane Sesquiterpenoids via Oxidative Nazarov Reaction
Yuye Chen
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
These authors contributed equally.
Search for more papers by this authorWenqing Chen
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
These authors contributed equally.
Search for more papers by this authorZhiting Zhang
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jing Xu
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorYuye Chen
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
These authors contributed equally.
Search for more papers by this authorWenqing Chen
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
These authors contributed equally.
Search for more papers by this authorZhiting Zhang
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jing Xu
Shenzhen Grubbs Institute and Department of Chemistry and Guangming Advanced Research Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
As one of the most common structural motifs in natural products, cyclopentenones usually can be fabricated by Nazarov cyclization using divinyl ketones or functionalized tertiary divinyl carbinols (TDCs) as substrates. However, straightforward method for transforming unfunctionalized TDCs to their corresponding cyclopentenones is currently lacking. Herein, we wish to report the total syntheses of four structurally distinct terpenoids, namely laurane-type marine sesquiterpenoids isolaurene, debromoaplysin and aplysin, and guaiane sesquiterpenoid guaiadienone A, all using a novel synthetic method, named oxidative Nazarov cyclization, as the key step. This work demonstrated our robust method is suitable for synthesizing various highly substituted cyclopentenones.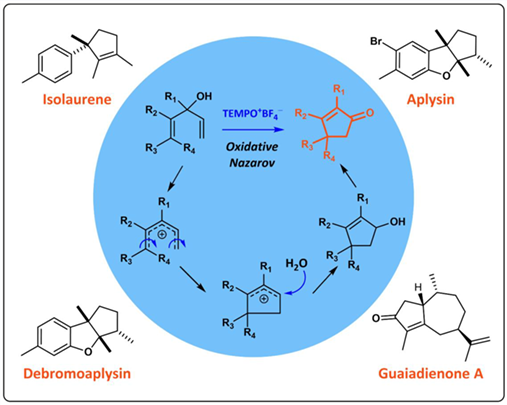
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400014-sup-0001-supinfo.pdfPDF document, 3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews in Nazarov reaction, see: (a) Denmark, S. E. The Nazarov and Related Cationic Cyclizations. Comprehensive Organic Synthesis, Vol. 5, Combining C-C π Bonds, Ed.: Paquette, L. A., Pergamon Press, Oxford, 1991, pp. 751–784;
10.1016/B978-0-08-052349-1.00138-4 Google Scholar(b) West, F. G.; Scadeng, O.; Wu, Y.-K.; Fradette, R. J.; Joy, S. The Nazarov Cyclization. Comprehensive Organic Synthesis, 2nd ed., Vol. 5, Combining C-C π Bonds, Ed.: P. Knochel, Elsevier Ltd, 2014, pp. 827–866; (c) Pellissier, H. Recent developments in the Nazarov process. Tetrahedron 2005, 61, 6479–6517; (d) Frontier, A. J.; Collison, C. The Nazarov cyclization in organic synthesis. Recent advances. Tetrahedron 2005, 61, 7577–7606; (e) Vaidya, T.; Eisenberg, R.; Frontier, A. J. Catalytic Nazarov cyclization: The state of the art. ChemCatChem 2011, 3, 1531–1548; (f) Shimada, N.; Stewart, C.; Tius, M. A. Asymmetric Nazarov cyclizations. Tetrahedron 2011, 67, 5851–5870; (g) Spencer III, W. T.; Vaidya, T.; Frontier, A. J. Beyond the divinyl ketone: innovations in the generation and Nazarov cyclization of pentadienyl cation intermediates. Eur. J. Org. Chem. 2013, 3621–3633; (h) Wenz, D. R.; Read de Alaniz, J. The Nazarov cyclization: A valuable method to synthesize fully substituted carbon stereocenters. Eur. J. Org. Chem. 2015, 23–37; (i) Frontier, A. J.; Hernandez, J. J. New twists in Nazarov cyclization chemistry. Acc. Chem. Res. 2020, 53, 1822−1832.
- 2For a recent review in the natural product synthesis using Nazarov reactions, see: (a) Vinogradov, M. G.; Turova, O. V.; Zlotin, S. G. Org. Biomol. Chem. 2017, 15, 8245−8269. For selected recent synthesis not covered in review 2a, see: (b) Shvartsbart, A.; Smith, A. B., III. Total synthesis of (−)-calyciphylline N. J. Am. Chem. Soc. 2014, 136, 870−873; (c) Shvartsbart, A.; Smith, A. B., III. The Daphniphyllum alkaloids: Total synthesis of (−)-calyciphylline N. J. Am. Chem. Soc. 2015, 137, 3510−3519; (d) Chen, Y.; Hu, J.; Guo, L.-D.; Zhong, W.; Ning, C.; Xu, J. A concise total synthesis of (−)-himalensine A. Angew. Chem. Int. Ed. 2019, 58, 7390−7394; (e) Zhong, J.; Chen, K.; Qiu, Y.; He, H.; Gao, S. A unified strategy to construct the tetracyclic ring of calyciphylline A alkaloids: Total synthesis of himalensine A. Org. Lett. 2019, 21, 3741−3745; (f) Que, Y.; Shao, H.; He, H.; Gao, S. Total Synthesis of Farnesin through an Excited-State Nazarov Reaction. Angew. Chem. Int. Ed. 2020, 59, 7444−7449; (g) Gao, J.; Rao, P.; Xu, K.; Wang, S.; Wu, Y.; He, C.; Ding, H. Total Synthesis of (−)-Rhodomollanol A. J. Am. Chem. Soc. 2020, 142, 4592−4597; (h) Trost, B. M.; Zhang, G.; Gholami, H.; Zell, D. Total Synthesis of Kadcoccinic Acid A Trimethyl Ester. J. Am. Chem. Soc. 2021, 143, 12286−12293; (i) Amberg, W. M.; Carreira, E. M. Enantioselective Total Synthesis of (+)-Aberrarone. J. Am. Chem. Soc. 2022, 144, 15475−15479; (j) Kong, L.; Su, F.; Yu, H.; Jiang, Z.; Lu, Y.; Luo, T. Total Synthesis of (−)-Oridonin: An Interrupted Nazarov Approach. J. Am. Chem. Soc. 2019, 141, 20048−20052; (k) Brandstätter, M.; Freis, M.; Huwyler, N.; Carreira, E. M. Total Synthesis of (−)-Merochlorin A. Angew. Chem. Int. Ed. 2019, 58, 2490–2494; (l) Zhou, W.; Voituriez, A. Synthesis of Cyclopentenones with C4-Quaternary Stereocenters via Stereospecific [3,3]-Sigmatropic Rearrangement and Applications in Total Synthesis of Sesquiterpenoids. J. Am. Chem. Soc. 2021, 143, 17348−17353; (m) Wang, Y.-P.; Fang, K.; Tu, Y.-Q.; Yin, J.-J.; Zhao, Q.; Ke, T. An efficient approach to angular tricyclic molecular architecture via Nazarov-like cyclization and double ring-expansion cascade. Nat. Commun. 2022, 13, 2335; (n) Shao, H.; Liu, W.; Liu, M.; He, H.; Zhou, Q.-L.; Zhu, S.-F.; Gao, S. Asymmetric synthesis of cyclopamine, a hedgehog(hh) signaling pathway inhibitor. J. Am. Chem. Soc. 2023, 145, 25086–25092; (o) Jin, Y.; Hok, S.; Bacsa, J.; Dai, M. Convergent and Efficient Total Synthesis of (+)-Heilonine Enabled by C−H Functionalizations. J. Am. Chem. Soc. 2024, 146, 1825–1831.
- 3For asymmetric Nazarov cyclizations, see: (a) Yang, B.-M.; Cai, P.-J.; Tu, Y.-Q.; Yu, Z.-X.; Chen, Z.-M.; Wang, S.-H.; Wang, S.-H.; Zhang, F.-M. Organocatalytic asymmetric tandem Nazarov cyclization/semipinacol rearrangement: rapid construction of chiral spiro[4.4]nonane-1,6-diones. J. Am. Chem. Soc. 2015, 137, 8344–8347; (b) Jolit, A., Walleser, P. M., Yap, G. P. A.; Tius, M. A. Catalytic enantioselective Nazarov cyclization: construction of vicinal all-carbon-atom quaternary stereocenters. Angew. Chem. Int. Ed. 2014, 53, 6180–6183; (c) Cao, P.; Deng, C.; Zhou, Y.-Y.; Sun, X.-L.; Zheng, J.-C.; Xie, Z.-W.; Tang, Y. Asymmetric Nazarov reaction catalyzed by chiral tris(oxazoline)/copper(II). Angew. Chem. Int. Ed. 2010, 49, 4463–4466; (d) Tang, S.; Zhang, P.; Shao, Y.; Sun, J. Enyne diketones as substrate in asymmetric Nazarov cyclization for construction of chiral allene cyclopentenones. Nat. Commun. 2022, 13, 3146; (e) Cao, J.; Hu, M.-J.; Liu, S.-Y.; Zhang, X.-Y.; Zhu, S.-F.; Zhou, Q.-L. Enantioselective Silicon-Directed Nazarov Cyclization. J. Am. Chem. Soc. 2021, 143, 6962−6968; (f) Hutson, G. E.; Türkmen, Y. E.; Rawal, V. H. Salen promoted enantioselective Nazarov cyclizations of activated and unactivated dienones. J. Am. Chem. Soc. 2013, 135, 4988−4991; (g) Raja, S.; Nakajima, M.; Rueping, M. Experimental and computational study of the catalytic asymmetric 4π-electrocyclization of N-heterocycles. Angew. Chem. Int. Ed. 2015, 54, 2762−2765; (h) Takeda, T.; Harada, S.; Nishida, A. Catalytic asymmetric Nazarov cyclization of heteroaryl vinyl ketones through a crystallographically defined chiral dinuclear nickel complex. Org. Lett. 2015, 17, 5184−5187; (i) Wang, G.-P.; Chen, M.-Q.; Zhu, S.-F.; Zhou, Q.-L. Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids. Chem. Sci. 2017, 8, 7197−7202; (j) Hong, Y.; Jarrige, L.; Harms, K.; Meggers, E. Chiral-at-iron catalyst: expanding the chemical space for asymmetric earth-abundant metal catalysis. J. Am. Chem. Soc. 2019, 141, 4569−4572; (k) Aggarwal, V. K.; Belfield, A. J. Catalytic asymmetric Nazarov reactions promoted by chiral Lewis acid complexes. Org. Lett. 2003, 5, 5075−5078; (l) Liang, G.; Trauner, D. Enantioselective Nazarov reactions through catalytic asymmetric proton transfer. J. Am. Chem. Soc. 2004, 126, 9544−9545; (m) Rueping, M.; Ieawsuwan, W.; Antonchick, A. P.; Nachtsheim, B. J. Chiral Brønsted acids in the catalytic asymmetric Nazarov cyclization-the first enantioselective organocatalytic electrocyclic reaction. Angew. Chem. Int. Ed. 2007, 46, 2097−2100; (n) Walz, I.; Togni, A. Ni(II)-catalyzed enantioselective Nazarov cyclizations. Chem. Commun. 2008, 4315−4317; (o) Rueping, M.; Ieawsuwan, W. A catalytic asymmetric electrocyclization-protonation reaction. Adv. Synth. Catal. 2009, 351, 78−84; (p) Kawatsura, M.; Kajita, K.; Hayase, S.; Itoh, T. Iron- or cobalt-catalyzed Nazarov cyclization: asymmetric reaction and tandem cyclization-fluorination reaction. Synlett 2010, 8, 1243−1246; (q) Xu, Z.; Ren, H.; Wang, L.; Tang, Y. Efficient catalytic enantioselective Nazarov cyclizations of divinyl ketoesters. Org. Chem. Front. 2015, 2, 811−814; (r) Zhang, H.; Lu, Z. Nickel-catalyzed enantioselective sequential Nazarov cyclization/decarboxylation. Org. Chem. Front. 2018, 5, 1763−1767; (s) Zhang, H.; Cheng, B.; Lu, Z. Enantioselective cobalt-catalyzed sequential Nazarov cyclization/electrophilic fluorination: access to chiral α-fluorocyclopentenones. Org. Lett. 2018, 20, 4028−4031; (t) Mietke, T.; Cruchter, T.; Larionov, V. A.; Faber, T.; Harms, K.; Meggers, E. Asymmetric Nazarov cyclizations catalyzed by chiral-atmetal complexes. Adv. Synth. Catal. 2018, 360, 2093−2100; (u) Süsse, L.; Vogler, M.; Mewald, M.; Kemper, B.; Irran, E.; Oestreich, M. Enantioselective Nazarov cyclizations catalyzed by an axial chiral C6F5-substituted boron Lewis acid. Angew. Chem. Int. Ed. 2018, 57, 11441−11444; (v) Ouyang, J.; Kennemur, J. L.; De, C. K.; Farés, C.; List, B. Strong and confined acids enable a catalytic asymmetric Nazarov cyclization of simple divinyl ketones. J. Am. Chem. Soc. 2019, 141, 3414−3418; (w) Metternich, J. B.; Reiterer, M.; Jacobsen, E. N. Asymmetric Nazarov cyclizations of unactivated dienones by hydrogen-bonddonor/Lewis acid co-catalyzed, enantioselective proton-transfer. Adv. Synth. Catal. 2020, 362, 4092−4097; (x) Basak, A. K.; Shimada, N.; Bow, W. F.; Vicic, D. A.; Tius, M. A. An organocatalytic asymmetric Nazarov cyclization. J. Am. Chem. Soc. 2010, 132, 8266−8267.
- 4For selected interrupted Nazarov cyclization reviews, see: (a) Grant, T. N.; Rieder, C. J.; West, F. G. Interrupting the Nazarov reaction: domino and cascade processes utilizing cyclopentenyl cations. Chem. Commun. 2009, 5676–5688; (b) Yadykova A. V.; Shirinian, V. Z. Recent advances in the interrupted Nazarov reaction. Adv. Synth. Catal. 2020, 362, 702–723, and ref. 1.
- 5For selected dehydrative Nazarov cyclizations, see: (a) Threlkel, R. S.; Bercaw, J. E.; Seidler, P. F.; Stryker, J. M.; Bergman, R. G. 1,2,3,4,5-Pentamethylcyclopentadiene. Org. Synth. 1987, 65, 42; (b) Halterman, R. L.; Tretyakov, A. Synthesis of camphor-derived chiral cyclopentadienes via the Nazarov cyclization: Preparation of chiral bis(cyclopentadienyl)zirconium and -titanium dichlorides. Tetrahedron 1995, 51, 4371–4382; (c) Hastings, C. J.; Pluth, M. D.; Bergman, R. G.; Raymond, K. N. Enzymelike catalysis of the Nazarov cyclization by supramolecular encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940; (d) Rieder, C. J.; Winberg, K. J.; West, F. G. Olefination of a,a’-divinyl ketones through catalytic Meyer-Schuster Rearrangement. J. Org. Chem. 2011, 76, 50–56; (e) Zheng, H.; Lejkowski, M.; Hall, D. G. Mild boronic acid catalyzed Nazarov cyclization of divinyl alcohols in tandem with Diels–Alder cycloaddition. Tetrahedron Lett. 2013, 54, 91–94; (f) Jin, J.; Zhao, Y.; Gouranourimi, A.; Ariafard, A.; Chan, P. W. H. Chiral Brønsted acid catalyzed enantioselective dehydrative Nazarov-type electrocyclization of aryl and 2-thienyl vinyl alcohols. J. Am. Chem. Soc. 2018, 140, 5834−5841.
- 6For Nazarov reactions involving epoxide opening, see: (a) Malona, J. A.; Cariou, K.; Frontier, A. J. Nazarov Cyclization Initiated by Peracid Oxidation: The Total Synthesis of (±)-Rocaglamide. J. Am. Chem. Soc. 2009, 131, 7560–7561; (b) Spencer, W. T. III; Levin, M. D.; Frontier, A. J. Oxidation-Initiated Nazarov Cyclization of Vinyl Alkoxyallenes. Org. Lett. 2011, 13, 414–417; (c) Malona, J. A.; Cariou, K.; Spencer, W. T., III; Frontier, A. J. Total Synthesis of (±)-Rocaglamide via Oxidation−Initiated Nazarov Cyclization. J. Org. Chem. 2012, 77, 1891−2908; (d) Frontier, A. J.; Sinclair, P. P. Merging Strategy, Improvisation, and Conversation to Solve Problems in Target Synthesis. Acc. Chem. Res. 2021, 54, 1817−1829; (e) Sudhakar, G.; Satish, K. Nazarov cyclization of divinyl and arylvinyl epoxides: Application in the synthesis of resveratrol-based natural products. Chem. Eur. J. 2015, 21, 6475–6480; (f) Satish, N.; Raju, S. K.; Nanubolu, J. B.; Sudhakar, G. The synthesis of indeno[de]isochromene derivatives from arylvinyl epoxides and carbonyl compounds via tandem Nazarov and oxa-Pictet–Spengler cyclizations. New J. Chem. 2021, 45, 16248–16253; (g) Satish, N.; Sudhakar, G. Scandium triflate catalyzed Nazarov cyclization of arylvinyl epoxides derived from alkoxides and chloro(aryl)carbenes: A facile access to resveratrol-derived natural products. Synlett 2021, 32, 605–610.
- 7For selected, related work, see: (a) Alachouzos, G.; Frontier, A. J. Diastereoselective Construction of Densely Functionalized 1-Halocyclopentenes Using an Alkynyl Halo-Prins/Halo-Nazarov Cyclization Strategy. Angew. Chem. Int. Ed. 2017, 56, 15030–15034; (b) Alachouzos, G.; Frontier, A. J. Cationic Cascade for Building Complex Polycyclic Molecules from Simple Precursors: Diastereoselective Installation of Three Contiguous Stereogenic Centers in a One-Pot Process. J. Am. Chem. Soc. 2019, 141, 118–122; (c) Holt, C.; Alachouzos, G.; Frontier, A. J. Leveraging the Halo-Nazarov Cyclization for the Chemodivergent Assembly of Functionalized Haloindenes and Indanones. J. Am. Chem. Soc. 2019, 141, 5461–5469; (d) Alachouzos, G.; Holt, C.; Frontier, A. J. Stereochemical Relay through a Cationic Intermediate: Helical Preorganization Dictates Direction of Conrotation in the halo-Nazarov Cyclization. Org. Lett. 2020, 22, 4010–4015; (e) Abdul-Rashed, S.; Alachouzos, G.; Brennessel, W. W.; Frontier, A. J. One-Pot Double-Annulation Strategy for the Synthesis of Unusual Fused Bis-Heterocycles. Org. Lett. 2020, 22, 4350–4354; (f) Hernandez, J. J.; Frontier, A. J. Synthesis of Spirocyclic Isoindolones Using an Alkynyl aza-Prins/Oxidative halo-Nazarov Cyclization Sequence. Org. Lett. 2021, 23, 1782−1786; (g) Nair, V.; Bindu, S.; Sreekumar, V.; Chiaroni, A. A Novel Approach to the Synthesis of Bicyclic Lactones via an Interrupted Nazarov Reaction of gem-Divinyl Dihydrofurans. Org. Lett. 2002, 4, 2821–2823; (h) Huang, J.; Frontier, A. J. Development of a Nazarov cyclization/Wagner−Meerwein rearrangement sequence for the stereoselective synthesis of spirocycles. J. Am. Chem. Soc. 2007, 129, 8060–8061; (i) Leboeuf, D., Gandon, V., Ciesielski, J.; Frontier, A. J. Experimental and theoretical studies on the Nazarov cyclization/Wagner–Meerwein rearrangement sequence. J. Am. Chem. Soc. 2012, 134, 6296–6308; (j) Leboeuf, D., Huang, J., Gandon, V.; Frontier, A. J. Using Nazarov electrocyclization to stage chemoselective [1,2]-migrations: stereoselective synthesis of functionalized cyclopentenones. Angew. Chem. Int. Ed. 2011, 50, 10981–10985; (k) He, W.; Sun, X.; Frontier, A. J. Polarizing the Nazarov cyclization: efficient catalysis under mild conditions. J. Am. Chem. Soc. 2003, 125, 14278–14279.
- 8(a) Cai, S.; Xiao, Z.; Shi, Y.; Gao, S. The Photo−Nazarov Reaction: Scope and Application. Chem. Eur. J. 2014, 20, 8677−8681; (b) Shi, Y.; Yang, B.; Cai, S.; Gao, S. Total Synthesis of Gracilamine. Angew. Chem. Int. Ed. 2014, 53, 9539−9543; (c) Xue, D.; Que, Y.; Shao, H.; He, H.; Zhao, X.; Gao, S. Stereoselective Synthesis of the Core Structures of Pyrrocidines and Wortmannines through the Excited−State Nazarov Reactions. Org. Lett. 2021, 23, 2736−2741, and Refs 2f and 2n.
- 9(a) Yang, B.; Gao, S. The application of photochemical rearrangement of santonin in total synthesis of complex natural terpenoids. Acta Chim. Sinica 2018, 76, 161−167; (b) Wang, Y.; Tian, H.; Gui, J. Gram-Scale Synthesis of Bufospirostenin A by a Biomimetic Skeletal Rearrangement Approach. J. Am. Chem. Soc. 2021, 143, 19576–19586; (c) Huang, J.; Cao, T.; Zhang, Z.; Yang, Z. Semisynthesis of (-)-Bufospirostenin A Enabled by Photosantonin Rearrangement Reaction. J. Am. Chem. Soc. 2022, 144, 2479–2483.
- 10(a) Lempenauer, L.; Duñach, E.; Lemière, G. Tuning the Reactivity of Functionalized Diallylic Alcohols: Brønsted versus Lewis Acid Catalysis. Chem. Eur. J. 2017, 23, 10285–10288;
(b) Komatsuki, K.; Sadamitsu, Y.; Sekine, K.; Saito, K.; Yamada, T. Stereospecific Decarboxylative Nazarov Cyclization Mediated by Carbon Dioxide for the Preparation of Highly Substituted 2-Cyclopentenones. Angew. Chem. Int. Ed. 2017, 56, 11594–11598;
(c) Kozuma, A.; Komatsuki, K.; Saito, K.; Yamada, T. Thermal Decarboxylative Nazarov Cyclization of Cyclic Enol Carbonates Involving Chirality Transfer. Chem. Lett. 2020, 49, 60–63;
(d) Tius, M. A.; Astrab, D. P. A cationic cyclopentannelation. Tetrahedron Lett. 1984, 25, 1539–1542;
(e) Bee, C.; Tius, M. A. Convergent Cyclopentannelation Process. Org. Lett. 2003, 10, 1681–1684;
10.1021/ol034309+ Google Scholar(f) Tius, M. A.; Kwok, C. K.; Gu, X.-Q.; Zhao, C. Cyclopentannelation Reaction for Rapid Assembly of Multifunctional Products. (d,l)-Desepoxymethylenomycin a Methyl Ester. Synth. Commun. 1994, 24, 871–885; (g) Tius, M. A.; Zhou, X.-M. Densely functionalized cyclopentenones. Tetrahedron Lett. 1989, 30, 4629–4632; (h) Komatsuki, K.; Kozuma, A.; Saito, K.; Yamada, T. Decarboxylative Nazarov Cyclization-Based Chirality Transfer for Asymmetric Synthesis of 2-Cyclopentenones. Org. Lett. 2019, 21, 6628–6632; (i) Lempenauer, L.; Duñach, E.; Lemière, G. Catalytic Rearrangement of 2-Alkoxy Diallyl Alcohols: Access to Polysubstituted Cyclopentenones. Org. Lett. 2016, 18, 1326–1329; (j) Schultz-Fademrecht, C.; Tius, M. A.; Grimme, S.; Wibbeling, B., Hoppe, D. Synthesis of Enantioenriched 5-Alkylidene-2-cyclopentenones from Chiral Allenyl Carbamates: Generation of a Chiral Lithium Allenolate and Allylic Activation for a Conrotatory 4π-Electrocyclization. Angew. Chem. Int. Ed. 2002, 41, 1532–1535;10.1002/1521-3773(20020503)41:9<1532::AID-ANIE1532>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar(k) Tius, M. A. Cationic cyclopentannelation of allene ethers. Acc. Chem. Res. 2003, 36, 284–290; (l) Tius, M. A. Allene ether Nazarov cyclization. Chem. Soc. Rev. 2014, 43, 2979–3002.
- 11For synthetic application of Nazarov cyclizations using functionalized tertiary divinyl carbinols, see: (a) Tius, M. A.; Astrab, D. P.; Fauq, A. H.; Ousset, J. B.; Trehan, S. Cationic cyclopentaannelation: an efficient methylenomycin synthesis. J. Am. Chem. Soc. 1986, 108, 3438–3442; (b) Tius, M. A.; Trehan, S. A concise synthesis of d,l-methylenomycin A and d,l-epi-methylenomycin A. J. Org. Chem. 1989, 54, 46–51; (c) Tius, M. A.; Drake, D. J. Synthesis of (±)-xanthocidin. Tetrahedron 1996, 52, 14651–14660; (d) Tius, M. A.; Hu, H.; Kawakami, J. K.; Busch-Petersen, J. Synthesis of 15-Deoxy-12-hydroxy-10-(trifluoromethyl)-Δ7-PGA1 Methyl Ester. J. Org. Chem. 1998, 63, 5971–5976; (e) Nakajima, D.; Yokoshima, S. Construction of the [7−5−5] Tricyclic Core of Daphniphyllum Alkaloids via a Cationic Cascade Reaction. Org. Lett. 2022, 24, 9520−9524.
- 12 Zhang, Y.; Chen, Y.; Song, M.; Tan, B.; Jiang, Y.; Yan, C.; Jiang, Y.; Hu, X.; Zhang, C.; Chen, W.; Xu, J. Total syntheses of calyciphylline a-type alkaloids (−)-10-deoxydaphnipaxianine A, (+)-daphlongamine E, and (+)-calyciphylline R via late-stage divinyl carbinol rearrangements. J. Am. Chem. Soc. 2022, 144, 16042–16051.
- 13
Irie, T.; Yasunari, Y.; Suzuki, T.; Imai, N.; Kurosawa, E.; Masamune, T. A new sesquiterpene hydrocarbon from Laurencia glandulifera. Tetrahedron Lett. 1965, 6, 3619–3624.
10.1016/S0040-4039(01)99550-4 Google Scholar
- 14 Yamamura, S.; Hirata, Y. Structures of aplysin and aplysinol, naturally occurring bromo-compounds. Tetrahedron 1963, 19, 1485–1496.
- 15For total synthesis of isolaurene see: (a) Tonari, K.; Ichimoto, I.; Ueda, H. Synthesis of Isolaurene and Its Related Compounds from Cyclotene. Agric. Biol. Chem. 1980, 44, 625–629; (b) Tomari, K.; Machiya, K.; Ichimoto, I.; Ueda, H. Synthesis of 2-Hydroxy-3-p-tolyl-2-cyclopentenone and Its Application to Isolaurene. Agric. Biol. Chem. 1980, 44, 2135–2138; (c) Hitoshi, T.; Akira, M.; Satoshi, N. Synthetic Photochemistry. XXVIII. A Photochemical C5-Homologation of 4-Isopropenyltoluene with Methyl 2,4-Dioxopentanoate to Isolaurene and a Formal Synthesis of Cuparene. Bull. Chem. Soc. Jpn. 1984, 57, 3152–3155; (d) Fadel, A.; Canet, J.-L.; Salaün, J. Asymmetric construction of quaternary carbons from chiral malonates : Total syntheses of (+)-epilaurene and (−)-Isolaurene. Tetrahedron Asymmetry 1993, 4, 27–30; (e) Niyogi, S.; Khatua, A.; Bisai, V. Unified approach to the sesquiterpenoids, lauranes and cyclolauranes: Total synthesis of (±)-isolaurene. Tetrahedron Lett. 2019, 60, 150941.
- 16For total synthesis of debromoaplysin and aplysin, see: (a) Ghosh, A.; Biswas, S.; Venkateswaran, R. V. Stereocontrolled synthesis of (±)-debromoaplysin and (±)-aplysin. J. Chem. Soc., Chem. Commun. 1988, 1421–1421; (b) Laronze, J.-Y.; El Boukili, R.; Cartier, D.; Laronze, J.; Levy, J. The Sheradsky rearrangement of α,α’-disubstituted cyclopentanone aryloximes: a synthesis of the sesquiterpenes (±)-aplysin and (±)-filiformin. Tetrahedron Lett. 1989, 30, 2229–2232; (c) Biswas, S.; Ghosh, A.; Venkateswaran, R. V. Stereocontrolled synthesis of (±)-debromoaplysin, (±)-aplysin, (±)-debromoaplysinol, (±)-aplysinol, and (±)-isoaplysin. J. Org. Chem. 1990, 55, 3498–3502; (d) Laronze, J.-Y.; El Boukili, R.; Patigny, D.; Dridi, S.; Cartier, D.; Levy, J. The rearrangement of some cyclopentanone-aryloximes: synthesis of (±)-aplysin, (±)-filiformin and of their debromo analogs. Tetrahedron 1991, 47, 10003–10014; (e) Nath, A.; Ghosh, A.; Venkateswaran, R. V. Rapid, high-yield synthesis of the marine sesquiterpenes debromoaplysin and aplysin via the acid-catalyzed rearrangement of a cyclobutachromanol. J. Org. Chem. 1992, 57, 1467–1472; (f) Takano, S.; Moriya, M.; Ogasawara, K. Tetrahedron Lett. 1992, 33, 329e332; (g) Nemoto, H.; Nagamochi, M.; Ishibashi, H.; Fukumoto, K. J. Org. Chem. 1994, 59, 74–79; (h) Harrowven, D. C.; Lucas, M. C.; Howes, P. D. A diastereoselective radical to polar crossover sequence for the synthesis of the isolaurinterols and aplysins. Tetrahedron Lett. 1999, 40, 8271–8272; (i) Harrowven, D. C.; Lucas, M. C.; Howes, P. D. The synthesis of a natural product family: from debromoisolaurinterol to the aplysins. Tetrahedron 2001, 57, 791–804; (j) Srikrishna, A.; Babu, N. C. An enantiospecific formal total synthesis of (–)-aplysin and (–)-debromoaplysin. Tetrahedron Lett. 2001, 42, 4913–4914; (k) Yoshida, M.; Shoji, Y.; Shishido, K. Enantioselective formal total synthesis of aplysin utilizing a palladium-catalyzed addition of an arylboronic acid to an allenic alcohol–Eschenmoser/Claisen rearrangement. Tetrahedron 2010, 66, 5053–5058; (l) Fletcher, C. J.; Blair, D. J.; Wheelhouse, K. M. P.; Aggarwal, V. K. The total synthesis of (–)-aplysin via a lithiation–borylation–propenylation sequence. Tetrahedron 2012, 68, 7598–7604.
- 17 Alarif, W. M.; Al-Lihaibi, S. S.; Ayyad, S.-E. N.; Abdel-Rhman, M. H.; Badria, F. A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtuse as potential antitumor–antimicrobial agents. Eur. J. Med. Chem. 2012, 55, 462–466.
- 18 Zdero, C.; Bohlmann, F. Isocedrene and Guaiane Derivatives from Pleocarphus revolutus. J. Nat. Prod. 1988, 51, 509–512.
- 19For its synthesis, see: (a) Büchi, G.; Kauffman, J. M.; Loewenthal, H. J. E. Synthesis of 1-Epicyclocolorenone and Stereochemistry of Cyclocolorenone. J. Am. Chem. Soc. 1966, 88, 3403–3408; (b) Blay, G.; Garcia, B.; Molina, E.; Pedro, J. R. Synthesis of all 7αH-guaia-4,11- dien-3-one diastereomers from (+)-dihydrocarvone. Tetrahedron 2005, 61, 11156–11162.
- 20 Brocksom, T. J.; Brocksom, U.; de Sousa, D. P.; Frederico, D. Enantiopure cycloheptenones from (R)-(−)-carvone: intermediates for perhydroazulene terpenoids. Tetrahedron: Asymmetry 2005, 16, 3628–3632.




