Photoinduced Dehalocyclization to Access Oxindoles Using Formate as a Reductant
Wei Ge
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorJia-Xin Wang
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Ming-Chen Fu
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Anhui Province Key Laboratory of Value-Added Catalytic Conversion and Reaction Engineering, Hefei University of Technology, Hefei, Anhui, 230009 China
E-mail: [email protected]; [email protected].Search for more papers by this authorCorresponding Author
Yao Fu
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected].Search for more papers by this authorWei Ge
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorJia-Xin Wang
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Ming-Chen Fu
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
Anhui Province Key Laboratory of Value-Added Catalytic Conversion and Reaction Engineering, Hefei University of Technology, Hefei, Anhui, 230009 China
E-mail: [email protected]; [email protected].Search for more papers by this authorCorresponding Author
Yao Fu
Hefei National Research Center for Physical Sciences at the Microscale, iChEM, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Province Key Laboratory of Biomass Clean Energy, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]; [email protected].Search for more papers by this authorComprehensive Summary
Herein, we report an efficient and practical protocol for the photoinduced dehalocyclization of ortho-halophenylacrylamides with formate by the engagement of a CO2 radical anion to access substituted oxindoles. This method proceeds smoothly under mild conditions and exhibits a wide range of substrate as well as remarkable functional group compatibility.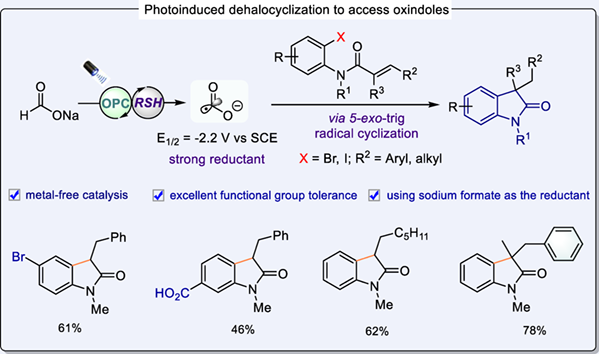
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300740-sup-0001-supinfo.pdfPDF document, 9.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Sumpter, W. C. The chemistry of Oxindole. Chem. Rev. 1945, 37, 443–479.
- 2 Yu, Q.-S.; Zhu, X.; Holloway, H. W.; Whittaker, N. F.; Brossi, A.; Greig, N. H. Anticholinesterase Activity of Compounds Related to Geneserine Tautomers. N-Oxides and 1,2-Oxazines. J. Med. Chem. 2002, 45, 3684–3691.
- 3 Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Shangary, S.; Gao, W.; Qin, D.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Wang, S. Structure-Based Design of Spiro-oxindoles as Potent, Specific Small- Molecule Inhibitors of the MDM2-p53 Interaction. J. Med. Chem. 2006, 49, 3432–3435.
- 4 Peddibhotla, S. 3-Substituted-3-hydroxy-2-oxindole, an Emerging New Scaffold for Drug Discovery with Potential Anti-Cancer and other Biological Activities. Curr. Bioact. Compd. 2009, 5, 20–38.
- 5 Ding, Z.; Zhou, M.; Zeng, C. Recent advances in isatin hybrids as potential anticancer agents. Arch. Pharm. 2020, 353, 1900367.
- 6 Kaur, J.; Kaur, B. P.; Chimni, S. S. Recent advances in the catalytic synthesis of 3-aminooxindoles: an update. Org. Biomol. Chem. 2020, 18, 4692–4708.
- 7 Singh, J.; Sharma, A. Visible Light Mediated Synthesis of Oxindoles. Adv. Synth. Catal. 2021, 363, 4284–4308.
- 8 Dalpozzo, R. Catalytic asymmetric synthesis of hetero-substituted oxindoles. Org. Chem. Front. 2017, 4, 2063–2078.
- 9 Luo, W.; Yu, Q.-S.; Kulkarni, S. S.; Parrish, D. A.; Holloway, H. W.; Tweedie, D.; Shafferman, A.; Lahiri, D. K.; Brossi, A.; Greig, N. H. Inhibition of Human Acetyl- and Butyrylcholinesterase by Novel Carbamates of (−)- and (+)-Tetrahydrofurobenzofuran and Methanobenzodioxepine. J. Med. Chem. 2006, 49, 2174–2185.
- 10 Abel-Snape, X.; Johnson, C. E.; Imbriaco, B.; Lautens, M. Synthesis of spirooxindoles via formal acetylene insertion into a common palladacycle intermediate. Chem. Sci. 2023, 14, 5650–5655.
- 11 Wu, T.; Mu, X.; Liu, G. Palladium-catalyzed oxidative arylalkylation of activated alkenes: dual C–H bond cleavage of an arene and acetonitrile. Angew. Chem. Int. Ed. 2011, 50, 12578–12581.
- 12 Jang, Y.; Larin, E. M.; Lautens, M. Rhodium-Catalyzed Enantioselective Reductive Arylation: Convenient Access to 3,3-Disubstituted Oxindoles. Angew. Chem. Int. Ed. 2017, 56, 11927–11930.
- 13 Yoon, H.; Marchese, A. D.; Lautens, M. Carboiodination Catalyzed by Nickel. J. Am. Chem. Soc. 2018, 140, 10950–10954.
- 14 Marchese, A. D.; Larin, E. M.; Mirabi, B.; Lautens, M. Metal-Catalyzed Approaches toward the Oxindole Core. Acc. Chem. Res. 2020, 53, 1605–1619.
- 15 Chen, X.-W.; Yue, J.-P.; Wang, K.; Gui, Y.-Y.; Niu, Y.-N.; Liu, J.; Ran, C.-K.; Kong, W.; Zhou, W.-J.; Yu, D.-G. Nickel-Catalyzed Asymmetric Reductive Carbo-Carboxylation of Alkenes with CO2. Angew. Chem. Int. Ed. 2021, 60, 14068–14075.
- 16 Xu, P.; Xu, H.; Wang, S.; Hao, T.-Z.; Yan, S.-Y.; Guo, D.; Zhu, X. Transition-metal free oxidative carbo-carboxylation of alkenes with formate in air. Org. Chem. Front. 2023, 10, 2013–2017.
- 17 Larin, E. M.; Masson-Makdissi, J.; Jang, Y.; Lautens, M. Rhodium(I)-Catalyzed Formate-Mediated Domino Heck/1,4-Hydride Addition toward Oxindoles. ACS Catal. 2022, 12, 12744–12749.
- 18 Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926.
- 19 Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor-Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476.
- 20 Melchiorre, P. Introduction: Photochemical Catalytic Processes. Chem. Rev. 2022, 122, 1483–1484.
- 21 Candish, L.; Collins, K. D.; Cook, G. C.; Douglas, J. J.; Suárez, A.; Jolit, A.; Keess, S. Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122, 2907–2980.
- 22 Dong, W.; Liu, Y.; Hu, B.; Ren, K.; Li, Y.; Xie, X.; Jiang, Y.; Zhang, Z. Visible light induced radical cyclization of o-iodophenylacrylamides: a concise synthesis of indolin-2-one. Chem. Commun. 2015, 51, 4587–4590.
- 23 Seo, H.; Katcher, M. H.; Jamison, T. F. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem. 2017, 9, 453–456.
- 24 Yan, S.-S.; Liu, S.-H.; Chen, L.; Bo, Z.-Y.; Jing, K.; Gao, T.-Y.; Yu, B.; Lan, Y.; Luo, S.-P.; Yu, D.-G. Visible-light photoredox-catalyzed selective carboxylation of C(sp3)–F bonds with CO2. Chem 2021, 7, 3099–3113.
- 25 Huang, Y.; Hou, J.; Zhan, L.-W.; Zhang, Q.; Tang, W.-Y.; Li, B.-D. Photoredox Activation of Formate Salts: Hydrocarboxylation of Alkenes via Carboxyl Group Transfer. ACS Catal. 2021, 11, 15004–15012.
- 26 Song, L.; Wang, W.; Yue, J.-P.; Jiang, Y.-X.; Wei, M.-K.; Zhang, H.-P.; Yan, S.-S.; Liao, L.-L.; Yu, D.-G. Visible-light photocatalytic di- and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022, 5, 832–838.
- 27 Majhi, J.; Molander, G. A. Recent Discovery, Development, and Synthetic Applications of Formic Acid Salts in Photochemistry. Angew. Chem. Int. Ed. 2023, e202311853.
- 28 Xiao, W.; Zhang, J.; Wu, J. Recent Advances in Reactions Involving Carbon Dioxide Radical Anion. ACS Catal. 2023, 13, 15991–16011.
- 29 Gui, Y.-Y.; Yan, S.-S.; Wang, W.; Chen, L.; Zhang, W.; Ye, J.-H.; Yu, D.-G. Exploring the applications of carbon dioxide radical anion in organic synthesis. Sci. Bull. 2023, https://doi.org/10.1016/j.scib.2023.11.018.
- 30 Min, S.-Y.; Song, H.-X.; Yan, S.-S.; Yuan, R.; Ye, J.-H.; Wang, B.-Q.; Gui, Y.-Y.; Yu, D.-G. Photocatalytic defluorocarboxylation using formate salts as both a reductant and a carbon dioxide source. Green Chem. 2023, 25, 6194–6199.
- 31 Malandain, A.; Molins, M.; Hauwelle, A.; Talbot, A.; Loreau, O.; D’Anfray, T.; Goutal, S.; Tournier, N.; Taran, F.; Caillé, F.; Audisio, D. Carbon Dioxide Radical Anion by Photoinduced Equilibration between Formate Salts and [11C, 13C, 14C]CO2: Application to Carbon Isotope Radiolabeling. J. Am. Chem. Soc. 2023, 145, 16760–16770.
- 32 Alektiar, S. N.; Han, J.; Dang, Y; Rubel, C. Z.; Wickens, Z. K. Radical Hydrocarboxylation of Unactivated Alkenes via Photocatalytic Formate Activation. J. Am. Chem. Soc. 2023, 145, 10991–10997.
- 33 Alektiar, S. N.; Wickens, Z. K. Photoinduced Hydrocarboxylation via Thiol-Catalyzed Delivery of Formate Across Activated Alkenes. J. Am. Chem. Soc. 2021, 143, 13022–13028.
- 34 Campbell, M. W.; Polites, V. C.; Patel, S.; Lipson, J. E.; Majhi, J.; Molander, G. A. Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and -Acetamides. J. Am. Chem. Soc. 2021, 143, 19648–19654.
- 35 Du, F.-M.; Yan, L.-Y.; Fu, M.-C. Metal-Free Photoinduced Defluorinative Carboxylation of Trifluoromethylalkenes with Formate. Eur. J. Org. Chem. 2023, 26, e202300883.
- 36
Wang, X.-Y.; Xu, P.; Liu, W.-W.; Jiang, H.-Q.; Zhu, S.-L.; Guo, D.; Zhu, X. Divergent defluorocarboxylation of α-CF3 alkenes with formate via photocatalyzed selective mono- or triple C–F bond cleavage. Sci. China Chem. 2023, 66, https://doi.org/10.1007/s11426-023-1731-x.
10.1007/s11426-023-1731-x Google Scholar
- 37 Wang, H.; Gao, Y.; Zhou, C.; Li, G. Visible-Light-Driven Reductive Carboarylation of Styrenes with CO2 and Aryl Halides. J. Am. Chem. Soc. 2020, 142, 8122–8129.
- 38 Hendy, C. M.; Smith, G. C.; Xu, Z.; Lian, T.; Jui, N. T. Radical Chain Reduction via Carbon Dioxide Radical Anion (CO2•–). J. Am. Chem. Soc. 2021, 143, 8987–8992.
- 39 Chmiel, A. F.; Williams, O. P.; Chernowsky, C. P.; Yeung, C. S.; Wickens, Z. K. Non-innocent Radical Ion Intermediates in Photoredox Catalysis: Parallel Reduction Modes Enable Coupling of Diverse Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 10882–10889.
- 40 Liu, C.; Shen, N.; Shang, R. Photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls using o-phosphinophenolate. Nat. Commun. 2022, 13, 354.
- 41 Ye, J.-H.; Bellotti, P.; Heusel, C.; Glorius, F. Photoredox-Catalyzed Defluorinative Functionalizations of Polyfluorinated Aliphatic Amides and Esters. Angew. Chem. Int. Ed. 2022, 61, e202115456.
- 42 Xu, P.; Wang, X.-Y.; Wang, Z.; Zhao, J.; Cao, X.-D.; Xiong, X.-C.; Yuan, Y.-C.; Zhu, S.; Guo, D.; Zhu, X. Defluorinative Alkylation of Trifluoromethylbenzimidazoles Enabled by Spin-Center Shift: A Synergistic Photocatalysis/Thiol Catalysis Process with CO2•–. Org. Lett. 2022, 24, 4075–4080.
- 43 Hendy, C. M.; Pratt, C. J.; Jui, N. T.; Blakey, S. B. Defluoroalkylation of Trifluoromethylarenes with Hydrazones: Rapid Access to Benzylic Difluoroarylethylamines. Org. Lett. 2023, 25, 1397–1402.
- 44 Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 2019, 363, 1429–1434.
- 45 Wang, J.-X.; Ge, W.; Fu, M.-C.; Fu, Y. Photoredox-Catalyzed Allylic Defluorinative Alkoxycarbonylation of Trifluoromethyl Alkenes through Intermolecular Alkoxycarbonyl Radical Addition. Org. Lett. 2022, 24, 1471–1475.
- 46 Fu, M.-C.; Wang, J.-X.; Ge, W.; Du, F.-M.; Fu, Y. Dual nickel/photoredox catalyzed carboxylation of C(sp2)-halides with formate. Org. Chem. Front. 2023, 10, 35–41.
- 47 Yan, L.-Y.; Zhang, T.-Z.; Lv, Z.-Z.; Fu, M.-C. Photoinduced defluorinative alkylation of trifluoromethyl alkenes with carbonyl derivatives by C–C bond scission. Org. Chem. Front. 2023, 10, 6205–6211.
- 48 Bordwell, F. G.; Zhang, X.-M.; Satish, A. V.; Cheng, J.-P. Assessment of the Importance of Changes in Ground-State Energies on the Bond Dissociation Enthalpies of the O–H Bonds in Phenols and the S–H Bonds in Thiophenols. J. Am. Chem. Soc. 1994, 116, 6605–6610.
- 49 Liu, C.; Li, K.; Shang, R. Arenethiolate as a Dual Function Catalyst for Photocatalytic Defluoroalkylation and Hydrodefluorination of Trifluoromethyls. ACS Catal. 2022, 12, 4103–4109.
- 50 Flynn, A. R.; McDaniel, K. A.; Hughes, M. E.; Vogt, D. B.; Jui, N. T. Hydroarylation of Arenes via Reductive Radical-Polar Crossover. J. Am. Chem. Soc. 2020, 142, 9163–9168.
- 51 Xu, J.; Cao, J.; Wu, X.; Wang, H.; Yang, X.; Tang, X.; Toh, R. W.; Zhou, R.; Yeow, E. K. L.; Wu, J. Unveiling Extreme Photoreduction Potentials of Donor-Acceptor Cyanoarenes to Access Aryl Radicals from Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 13266–13273.
- 52 Chen, S.; Pillitteri, S.; Fron, E.; Meervelt, L. V.; Eycken, E. V.; Sharma, U. K. Visible-Light-Induced Cascade Difunctionalization of Indoles Enabled by the Synergy of Photoredox and Photoexcited Ketones: Direct Access to Alkylated Pyrrolophenanthridones. Org. Lett. 2022, 24, 9386–9391.
- 53 Zhang, L.; Hu, F.; Shen, L.; Gao, L.; Yang, Y.; Pan, Z.; Xia, C. Redox-Neutral Intramolecular Dearomative Spirocyclization of Phenols Induced by Visible Light. Org. Lett. 2023, 25, 3168–3172.
- 54 Yatham, V. R.; Shen, Y.; Martin, R. Catalytic Intermolecular Dicarbofunctionalization of Styrenes with CO2 and Radical Precursors. Angew. Chem. Int. Ed. 2017, 56, 10915–10919.
- 55 Zhou, W.-J.; Wang, Z.-H.; Liao, L.-L.; Jiang, Y.-X.; Gao, K.-G.; Ju, T.; Li, Y.; Cao, G.-M.; Yu, D.-G. Reductive dearomative arylcarboxylation of indoles with CO2 via visible-light photoredox catalysis. Nat. Commun. 2020, 11, 3263.
- 56 Ju, T.; Zhou, Y.-Q.; Cao, K.-G.; Fu, Q.; Ye, J.-H.; Sun, G.-Q.; Liu, X.-F.; Chen, L.; Liao, L.-L.; Yu, D.-G. Dicarboxylation of alkenes, allenes and (hetero)arenes with CO2 via visible-light photoredox catalysis. Nat. Catal. 2021, 4, 304–311.
- 57 Xu, P.; Wang, S.; Xu, H.; Liu, Y.-Q.; Li, R.-B.; Liu, W.-W.; Wang, X.-Y.; Zou, M.-L.; Zhou, Y.; Guo, D.; Zhu, X. Dicarboxylation of Alkenes with CO2 and Formate via Photoredox Catalysis. ACS Catal. 2023, 13, 2149–2155.




