Oxidative Dehydrogenation of Propane over Supported Nickel Single-Atom Catalyst†
Qian Zhang
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXunzhu Jiang
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYangyang Li
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYuanlong Tan
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorQike Jiang
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorXiaoyan Liu
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorCorresponding Author
Botao Qiao
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]Search for more papers by this authorQian Zhang
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorXunzhu Jiang
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYangyang Li
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorYuanlong Tan
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorQike Jiang
Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorXiaoyan Liu
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorCorresponding Author
Botao Qiao
CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Single-Atom Catalysis.
Comprehensive Summary
Oxidative dehydrogenation of propane has been an ever-growing field for propylene production due to its exothermic properties, of which overoxidation is the major drawback, with CO and even CO2 as undesired by-products. For the purpose of getting higher propylene selectivity as well as yield, herein, we report Ni single atoms supported on calcium aluminate as an efficient catalyst candidate for propane oxidative dehydrogenation. Beneficial from higher valence states of Ni1 species, it shows 2—3 times as much propylene selectivity as that of Ni nanoparticles. About 14.2% C3H6 yield with 47.3% propylene selectivity has been achieved on Ni single atom catalyst and a good stability during 20 h test can be obtained as well. 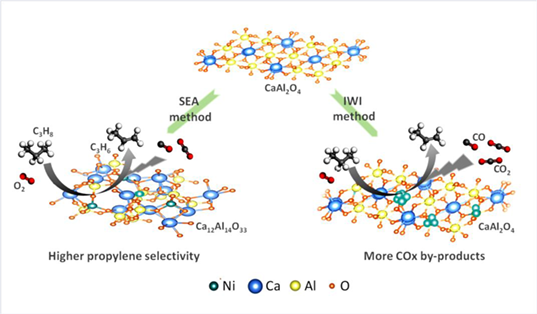
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300504-sup-0001-Supinfo.pdfPDF document, 1,012.6 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131; (b) Ayandiran, A. A.; Bakare, I. A.; Binous, H.; Al-Ghamdi, S.; Razzak, S. A.; Hossain, M. M. Oxidative dehydrogenation of propane to propylene over VOx/CaO–γ-Al2O3 using lattice oxygen. Catal. Sci. Technol. 2016, 6, 5154–5167.
- 2(a) Sheng, J.; Yan, B.; Lu, W. D.; Qiu, B.; Gao, X. Q.; Wang, D.; Lu, A. H. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts. Chem. Soc. Rev. 2021, 50, 1438–1468; (b) Otroshchenko, T.; Jiang, G.; Kondratenko, V. A.; Rodemerck, U.; Kondratenko, E. V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527.
- 3(a) Alexopoulos, K.; Reyniers, M.-F.; Marin, G. B. Reaction path analysis of propane selective oxidation over V2O5 and V2O5/TiO2. J. Catal. 2012, 289, 127–139; (b) Barman, S.; Maity, N.; Bhatte, K.; Ould-Chikh, S.; Dachwald, O.; Haeßner, C.; Saih, Y.; Abou-Hamad, E.; Llorens, I.; Hazemann, J.-L.; Köhler, K.; D’ Elia, V.; Basset, J.-M. Single-Site Vox Moieties Generated on Silica by Surface Organometallic Chemistry: A Way to Enhance the Catalytic Activity in the Oxidative Dehydrogenation of Propane. ACS Catal. 2016, 6, 5908–5921; (c) Carrero, C. A.; Schloegl, R.; Wachs, I. E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380.
- 4Grasselli, R. K.; Burrington, J. D.; Buttrey, D. J.; DeSanto, P.; Lugmair, C. G.; Volpe, A. F.; Weingand, T. Multifunctionality of active centers in (amm)oxidation catalysts: from Bi-Mo-O-x to Mo-V-Nb-(Te, Sb)-O-x. Top. Catal. 2003, 23, 5–22.
- 5(a) Kumar, C. P.; Gaab, S.; Müller, T. E.; Lercher, J. A. Oxidative Dehydrogenation of Light Alkanes on Supported Molten Alkali Metal Chloride Catalysts. Top. Catal. 2008, 50, 156–167; (b) Fu, H.; Qian, W.; Zhang, H.; Ma, H.; Ying, W. Different alkali metals promoted Cr/Al2O3 catalysts for propane dehydrogenation. Fuel 2023, 342, 127774.
- 6(a) Grant, J. T.; Goeltl, F.; Venegas, J.; Mueller, P.; Burt, S. P.; Specht, S. E.; McDermott, W. P.; Chieregato, A.; Hermans, I. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts. Science 2016, 354, 1050–1573; (b) Grant, J. T.; McDermott, W. P.; Venegas, J. M.; Burt, S. P.; Micka, J.; Phivilay, S. P.; Carrero, C. A.; Hermans, I. Boron and Boron-Containing Catalysts for the Oxidative Dehydrogenation of Propane. ChemCatChem 2017, 9, 3623–3626; (c) Tian, J.; Tan, J.; Xu, M.; Zhang, Z.; Wan, S.; Wang, S.; Lin, J.; Wang, Y. Propane oxidative dehydrogenation over highly selective hexagonal boron nitride catalysts:The role of oxidative coupling of methyl. Sci. Adv. 2019, 5, eaav8063.
- 7(a) Lu, W.-D.; Wang, D.; Zhao, Z.; Song, W.; Li, W.-C.; Lu, A.-H. Supported Boron Oxide Catalysts for Selective and Low-Temperature Oxidative Dehydrogenation of Propane. ACS Catal. 2019, 9, 8263–8270; (b) Altvater, N. R.; Dorn, R. W.; Cendejas, M. C.; McDermott, W. P.; Thomas, B.; Rossini, A. J.; Hermans, I. B-MWW Zeolite: The Case Against Single-Site Catalysis. Angew. Chem. Int. Ed. 2020, 59, 6546–6550; (c) Zhou, H.; Yi, X.; Hu, Y.; Wang, L.; Chen, W.; Qin, Y.; Wang, M.; Ma, J.; Chu, X.; Wang, Y.; Hong, X.; Chen, Z.; Meng, X.; Wang, H.; Zhu, Q.; Song, L.; Zheng, A.; Xiao, F.-S. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 2021, 372, 6; (d) Lu, W.-D.; Gao, X.-Q.; Wang, Q.-G.; Li, W.-C.; Zhao, Z.-C.; Wang, D.-Q.; Lu, A.-H. Ordered macroporous boron phosphate crystals as metal-free catalysts for the oxidative dehydrogenation of propane. Chin. J. Catal. 2020, 41, 1837–1845.
- 8(a) Sautel, M.; Kaddouri, A.; Mazzocchia, C.; Anouchinsky, R. Kinetics of oxidative dehydrogenation of propane on the/3 phase of nickel molybdate. Appl. Catal. A: Gen. 1997, 155, 12; (b) Zhaorigetu, B.; Li, W. Z.; Kieffer, R.; Xu, H. Y. Synergetic effect between NiO and Ni3V2O8 in propane oxidative dehydrogenation. React. Kinet. Catal. Lett. 2002, 75, 13; (c) He, Y.; Wu, Y.; Chen, T.; Weng, W.; Wan, H. Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)–Ni–O prepared by modified sol–gel method. Catal. Commun. 2006, 7, 268–271; (d) Zhang, Q.; Cao, C.; Xu, T.; Sun, M.; Zhang, J.; Wang, Y.; Wan, H. NiO-polyoxometalate nanocomposites as efficient catalysts for the oxidative dehydrogenation of propane and isobutane. Chem. Commun. 2009, 2376–2378.
- 9(a) Chen, M.; Wu, J.-L.; Liu, Y.-M.; Cao, Y.; Guo, L.; He, H.-Y.; Fan, K.-N. A practical grinding-assisted dry synthesis of nanocrystalline NiMoO4 polymorphs for oxidative dehydrogenation of propane. J. Solid State Chem. 2011, 184, 3357–3363; (b) Farin, B.; Swalus, C.; Devillers, M.; Gaigneaux, E. M. NiMoO4 preparation from polyampholytic hybrid precursors: Benefiting of the memory effect in the oxidative dehydrogenation of propane. Catal. Today 2013, 203, 24–31; (c) Siahvashi, A.; Chesterfield, D.; Adesina, A. A. Nonoxidative and Oxidative Propane Dehydrogenation over Bimetallic Mo-Ni/Al2O3 Catalyst. Ind. Eng. Chem. Res. 2013, 52, 4017–4026; (d) Fan, X.; Li, J.; Zhao, Z.; Wei, Y.; Liu, J.; Duan, A.; Jiang, G. Synthesis of a new ordered mesoporous NiMoO4 complex oxide and its efficient catalytic performance for oxidative dehydrogenation of propane. J. Energy Chem. 2014, 23, 171–178; (e) Farin, B.; Devillers, M.; Gaigneaux, E. M. Nanostructured hybrid materials as precursors of mesoporous NiMo-based catalysts for the propane oxidative dehydrogenation. Microporous Mesoporous Mater. 2017, 242, 200–207.
- 10Gao, X.; Zhu, L.; Yang, F.; Zhang, L.; Xu, W.; Zhou, X.; Huang, Y.; Song, H.; Lin, L.; Wen, X.; Ma, D.; Yao, S. Subsurface nickel boosts the low-temperature performance of a boron oxide overlayer in propane oxidative dehydrogenation. Nat. Commun. 2023, 14, 1478.
- 11(a) Yu, T.; Li, Z.; Zheng, H.; Chen, L.; Song, W.; Zhao, Z.; Li, J.; Liu, J. The nature of Ni-O pairs for ethane activation on NiO(100) and NiO(110) surfaces. Mol. Catal. 2019, 474, 110417;
(b) Fan, X.; Liu, D.; Zhao, Z.; Li, J.; Liu, J. Influence of Ni/Mo ratio on the structure-performance of ordered mesoporous Ni-Mo-O catalysts for oxidative dehydrogenation of propane. Catal. Today 2020, 339, 67–78;
(c) Li, L.; Wang, H.; Han, J.; Zhu, X.; Ge, Q. Balancing the Activity and Selectivity of Propane Oxidative Dehydrogenation on NiOOH (001) and (010). Trans. Tianjin Univ. 2020, 26, 341–351.
10.1007/s12209-020-00267-3 Google Scholar
- 12(a) Solsona, B.; Concepción, P.; Demicol, B.; Hernández, S.; Delgado, J. J.; Calvino, J. J.; López Nieto, J. M. Selective oxidative dehydrogenation of ethane over SnO2-promoted NiO catalysts. J. Catal. 2012, 295, 104–114; (b) Xie, Q.; Zhang, H.; Kang, J.; Cheng, J.; Zhang, Q.; Wang, Y. Oxidative Dehydrogenation of Propane to Propylene in the Presence of HCl Catalyzed by CeO2 and NiO-Modified CeO2 Nanocrystals. ACS Catal. 2018, 8, 4902–4916.
- 13(a) Qiao, B.; Wang, A.; Yang, X.; Allard, L. F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641; (b) Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81; (c) Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-Atom Catalysts Based on the Metal-Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043; (d) Shi, H.; Li, Y.; Lu, P.; Wu, Z.-S. Single-Atom Cobalt Coordinated to Oxygen Sites on Graphene for Stable Lithium Metal Anodes. Acta Phys.-Chim. Sin. 2020, 37, 2008030; (e) Zhang, H.; Tian, W.; Duan, X.; Sun, H.; Huang, Y.; Fang, Y.; Wang, S. Single-atom catalysts on metal-based supports for solar photoreduction catalysis. Chin. J. Catal. 2022, 43, 2301–2315; (f) Zhu, C.; Liang, J.; Wang, Y.; Li, J. Non-noble metal single-atom catalyst with MXene support: Fe1/Ti2CO2 for CO oxidation. Chin. J. Catal. 2022, 43, 1830–1841.
- 14Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A. F.; Isaacs, M. A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; Cui, Y. T.; Li; L.; Su, Y.; Pan, X.; Wen, W.; Pan, Y.; Wilson, K.; Li, L.; Qiao, B.; Ishii, H.; Liao, Y. F.; Wang, A.; Wang, X.; Zhang, T. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181.
- 15Zhou, Y.; Wei, F.; Qi, H.; Chai, Y.; Cao, L.; Lin, J.; Wan, Q.; Liu, X.; Xing, Y.; Lin, S.; Wang, A.; Wang, X.; Zhang, T. Peripheral-nitrogen effects on the Ru1 centre for highly efficient propane dehydrogenation. Nat. Catal. 2022, 5, 1145–1156.
- 16Ma, R.; Gao, J.; Kou, J.; Dean, D. P.; Breckner, C. J.; Liang, K.; Zhou, B.; Miller, J. T.; Zou, G. Insights into the Nature of Selective Nickel Sites on Ni/Al2O3 Catalysts for Propane Dehydrogenation. ACS Catal. 2022, 12, 12607–12616.
- 17(a) Yang, H.; Li, G.; Jiang, G.; Zhang, Z.; Hao, Z. Heterogeneous selective oxidation over supported metal catalysts: From nanoparticles to single atoms. Appl. Catal. B Environ. 2023, 325, 122384; (b) Vo, N. T.; Mekmouche, Y.; Tron, T.; Guillot, R.; Banse, F.; Halime, Z.; Sircoglou, M.; Leibl, W.; Aukauloo, A. A Reversible Electron Relay to Exclude Sacrificial Electron Donors in the Photocatalytic Oxygen Atom Transfer Reaction with O2 in Water. Angew. Chem. Int. Ed. 2019, 58, 16023–16027.
- 18Shang, Q.; Tang, N.; Qi, H.; Chen, S.; Xu, G.; Wu, C.; Pan, X.; Wang, X.; Cong, Y. A palladium single-atom catalyst toward efficient activation of molecular oxygen for cinnamyl alcohol oxidation. Chin. J. Catal. 2020, 41, 1812–1817.
- 19Li, T.; Liu, F.; Tang, Y.; Li, L.; Miao, S.; Su, Y.; Zhang, J.; Huang, J.; Sun, H.; Haruta, M.; Wang, A.; Qiao, B.; Li, J.; Zhang, T. Maximizing the Number of Interfacial Sites in Single-Atom Catalysts for the Highly Selective, Solvent–Free Oxidation of Primary Alcohols. Angew. Chem. Int. Ed. 2018, 57, 7795–7799.
- 20Ye, T. N.; Xiao, Z.; Li, J.; Gong, Y.; Abe, H.; Niwa, Y.; Sasase, M.; Kitano, M.; Hosono, H. Stable single platinum atoms trapped in sub-nanometer cavities in 12CaO·7Al2O3 for chemoselective hydrogenation of nitroarenes. Nat. Commun. 2020, 11, 1020.
- 21(a) Lu, J.; Lei, Y.; Wan, G.; Mei, Z.; Yu, J.; Zhao, Y.; He, S.; Luo, Y. Weakening the metal-support strong interaction to enhance catalytic performances of alumina supported Ni-based catalysts for producing hydrogen. Appl. Catal. B Environ. 2020, 263, 118177; (b) Yang, R.; Li, X.; Wu, J.; Zhang, X.; Zhang, Z.; Cheng, Y.; Guo, J. Hydrotreating of crude 2-ethylhexanol over Ni/Al2O3 catalysts: Surface Ni species-catalytic activity correlation. Appl. Catal. A: Gen. 2009, 368, 105–112.
- 22Medrano, J. A.; Hamers, H. P.; Williams, G.; van Sint Annaland, M.; Gallucci, F. NiO/CaAl2O4 as active oxygen carrier for low temperature chemical looping applications. Appl. Energy 2015, 158, 86–96.
- 23Wan, W.; Zhao, Y.; Wei, S.; Triana, C. A.; Li, J.; Arcifa, A.; Allen, C. S.; Cao, R.; Patzke, G. R. Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts. Nat. Commun. 2021, 12, 5589.
- 24(a) Sun, G.; Zhao, Z. J.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S. C.; Kropf, A. J.; Miller, J. T.; Zeng, L.; Gong, J. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454; (b) Chen, S.; Zhao, Z.-J.; Mu, R.; Chang, X.; Luo, J.; Purdy, S. C.; Kropf, A. J.; Sun, G.; Pei, C.; Miller, J. T.; Zhou, X.; Vovk, E.; Yang, Y.; Gong, J. Propane Dehydrogenation on Single-Site [PtZn4] Intermetallic Catalysts. Chem 2020, 7, 1–19.
- 25Du, K.; Hao, M.; Li, Z.; Hong, W.; Liu, J.; Xiao, L.; Zou, S.; Kobayashi, H.; Fan, J. Tuning catalytic selectivity of propane oxidative dehydrogenation via surface polymeric phosphate modification on nickel oxide nanoparticles. Chin. J. Catal. 2019, 40, 1057–1062.
- 26Fukudome, K.; Kanno, A.; Ikenaga, N.-O.; Miyake, T.; Suzuki, T. The Oxidative Dehydrogenation of Propane over NiO-ZrO2 Catalyst. Catal. Lett. 2010, 141, 68–77.
- 27Marco, J. F.; Gancedo, J. R.; Gracia, M.; Gautier, J. L.; Ríos, E.; Berry, F. J. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. J. Solid State Chem. 2000, 153, 74–81.
- 28(a) Ojagh, H.; Creaser, D.; Tamm, S.; Hu, C.; Olsson, L. Effect of Thermal Treatment on Hydrogen Uptake and Characteristics of Ni-, Co-, and Mo-Containing Catalysts. Ind. Eng. Chem. Res. 2015, 54, 11511–11524; (b) Farahani, M. D.; Dasireddy, V. D. B. C.; Friedrich, H. B. Oxidative Dehydrogenation of n-Octane over Niobium-Doped NiAl2O4: An Example of Beneficial Coking in Catalysis over Spinel. ChemCatChem 2018, 10, 2059–2069.




