Decarboxylative Amination with Nitroarenes via Synergistic Catalysis†
Meiling Ding
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorSitian Zhou
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShunruo Yao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorChengjian Zhu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Weipeng Li
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMeiling Ding
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorSitian Zhou
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorShunruo Yao
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorChengjian Zhu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Weipeng Li
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jin Xie
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]Search for more papers by this author†Dedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
In this paper, we have developed a decarboxylative amination of carboxylic acids with nitroarenes for the synthesis of secondary amines. The protocol is performed at mild conditions without the use of noble metals as catalysts. A wide range of structurally diverse secondary amines could be obtained in good yields (up to 94%) with good functional group tolerance. This transformation shows good to excellent selectivity, avoiding the generation of over alkylated byproducts.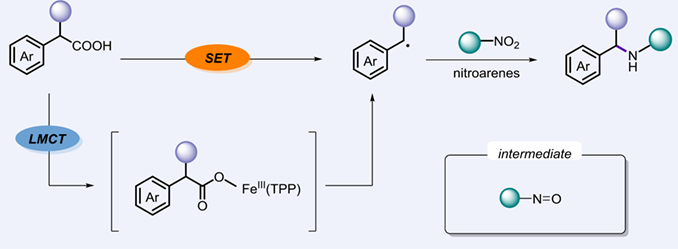
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300493-sup-0001-Supinfo.pdfPDF document, 6.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Trowbridge, A.; Walton, S. M.; Gaunt, M. J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692; (b) Bariwal, J.; Van der Eycken, E. C–N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2013, 42, 9283–9303.
- 2(a) Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P. K.; Bhutani, H.; Paul, A. T.; Kumar, R. U.S. FDA Approved Drugs from 2015–June 2020: A Perspective. J. Med. Chem. 2021, 64, 2339–2381; (b) Salvatore, R. N.; Yoon, C.H.; Jung, K. W. Synthesis of secondary amines. Tetrahedron 2001, 57, 7785–7811.
- 3 Ruiz-Castillo, P.; Buchwald, S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649.
- 4(a) Afanasyev, O. I.; Kuchuk, E.; Usanov, D. L.; Chusov, D. Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev. 2019, 119, 11857–11911; (b) Ramsden, J. I.; Heath, R. S.; Derrington, S. R.; Montgomery, S. L.; Mangas-Sanchez, J.; Mulholland, K. R.; Turner, N. J. Biocatalytic N-Alkylation of Amines Using Either Primary Alcohols or Carboxylic Acids via Reductive Aminase Cascades. J. Am. Chem. Soc. 2019, 141, 1201–1206.
- 5(a) Hartwig, J. F. Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism. Angew. Chem. Int. Ed. 1998, 37, 2046–2067;
10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar(b) Surry, D. S.; Buchwald, S. L. Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide. Chem, Sci. 2011, 2, 27–50; (c) Forero-Cortés, P. A.; Haydl, A. M. The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 1478–1483; (d) Song, G.; Yang, L.; Li, J. S.; Tang, W. J.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.; Xue, D. Chiral Arylated Amines via C−N Coupling of Chiral Amines with Aryl Bromides Promoted by Light. Angew. Chem. Int. Ed. 2021, 60, 21536–21542.
- 6(a) Muller, T. E.; Hultzsch, K. C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892; (b) Huang, L.; Arndt, M.; Goossen, K.; Heydt, H.; Goossen, L. J. Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev. 2015, 115, 2596–2697; (c) Ma, S.; Hill, C. K.; Olen, C. L.; Hartwig, J. F. Ruthenium-Catalyzed Hydroamination of Unactivated Terminal Alkenes with Stoichiometric Amounts of Alkene and an Ammonia Surrogate by Sequential Oxidation and Reduction. J. Am. Chem. Soc. 2021, 143, 359–368; (d) Jia, S. M.; Huang, Y. H.; Wang, Z. L.; Fan, F. X.; Fan, B. H.; Sun, H. X.; Wang, H.; Wang, F. Hydroamination of Unactivated Alkenes with Aliphatic Azides. J. Am. Chem. Soc. 2022, 144, 16316–16324; (e) Xi, Y.; Ma, S.; Hartwig, J. F. Catalytic asymmetric addition of an amine N–H bond across internal alkenes. Nature 2020, 588, 254–260.
- 7(a) Liu, T. L.; Li, Z. F.; Tao, J.; Li, Q. H.; Li, W. F.; Li, Q.; Ren, L. Q.; Peng, Y. G. Cyano-borrowing: titanium-catalyzed direct amination of cyanohydrins with amines and enantioselective examples. Chem. Commun. 2020, 56, 651–654; (b) Rosler, S.; Ertl, M.; Irrgang, T.; Kempe, R. Cobalt-Catalyzed Alkylation of Aromatic Amines by Alcohols. Angew. Chem. Int. Ed. 2015, 54, 15046–15050.
- 8(a) Nepali, K.; Lee, H. Y.; Liou, J. P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893; (b) Inoue, F.; Kashihara, M.; Yadav, M. R.; Nakao, Y. Buchwald–Hartwig Amination of Nitroarenes. Angew. Chem. Int. Ed. 2017, 56, 13307–13309; (c) Sukhorukov, A. Y. Catalytic Reductive Amination of Aldehydes and Ketones with Nitro Compounds: New Light on an Old Reaction. Front Chem. 2020, 8, 215; (d) Sui, D.; Mao, F.; Fan, H.; Qi, Z.; Huang, J. General Reductive Amination of Aldehydes and Ketones with Amines and Nitroaromatics under H2 by Recyclable Iridium Catalysts. Chin. J. Chem. 2017, 35, 1371–1377.
- 9(a) Jagadeesh, R. V.; Surkus, A. E.; Junge, H.; Pohl, M. M.; Radnik, J.; Rabeah, J.; Huan, H.; Schunemann, V.; Bruckner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076; (b) Wienhofer, G.; Sorribes, I.; Boddien, A.; Westerhaus, F.; Junge, K.; Junge, H.; Llusar, R.; Beller, M. General and Selective Iron-Catalyzed Transfer Hydrogenation of Nitroarenes without Base. J. Am. Chem. Soc. 2011, 133, 12875–12879; (c) Li, X.; Wang, Z.; Mao, S.; Chen, Y.; Tang, M.; Li, H.; Wang, Y. Insight into the Role of Additives in Catalytic Synthesis of Cyclohexylamine from Nitrobenzene. Chin. J. Chem. 2018, 36, 1191–1196.
- 10 Cheung, C. W.; Hu, X. Amine synthesis via iron-catalysed reductive coupling of nitroarenes with alkyl halides. Nat. Commun. 2016, 7, 12494.
- 11 Li, G.; Yang, L.; Liu, J. J.; Zhang, W.; Cao, R.; Wang, C.; Zhang, Z.; Xiao, J.; Xue, D. Light-Promoted C–N Coupling of Aryl Halides with Nitroarenes. Angew. Chem. Int. Ed. 2021, 60, 5230–5234.
- 12 Hong, S. Y.; Radosevich, A. T. Chemoselective Primary Amination of Aryl Boronic Acids by PIII/PV=O-Catalysis: Synthetic Capture of the Transient Nef Intermediate HNO. J. Am. Chem. Soc. 2022, 144, 8902–8907.
- 13(a) Kitcatt, D. M.; Nicolle, S.; Lee, A. L. Direct decarboxylative Giese reactions. Chem. Soc. Rev. 2022, 51, 1415–1453; (b) Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 2014, 43, 2714–2742.
- 14(a) Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; Doyle, A. G.; MacMillan, D. W. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440; (b) Xuan, J.; Zhang, Z. G.; Xiao, W.-J. Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem. Int. Ed. 2015, 54, 15632–15641; (c) Zhang, M.; Yuan, X. A.; Zhu, C.; Xie, J. Deoxygenative Deuteration of Carboxylic Acids with D2O. Angew. Chem. Int. Ed. 2019, 58, 312–316; (d) Wang, D.; Yuan, Z.; Liu, Q.; Chen, P.; Liu, G. Decarboxylative Fluorination of Arylcarboxylic Acids Promoted by ortho-Hydroxy and Amino Groups. Chin. J. Chem. 2018, 36, 507–514; (e) He, J.; Chen, G.; Zhang, B.; Li, Y.; Chen, J. R.; Xiao, W. J.; Liu, F.; Li, C. Catalytic Decarboxylative Radical Sulfonylation. Chem 2020, 6, 1149–1159; (f) Li, R.; Dong, Y.; Khan, S. N.; Zaman, M. K.; Zhou, J.; Miao, P.; Hu, L.; Sun, Z. Decarboxylative oxidation-enabled consecutive C-C bond cleavage. Nat. Commun. 2022, 13, 7061; (g) Liang, Y.; Zhang, X.; MacMillan, D. W. C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 2018, 559, 83–88; (h) Sun, X.; Chen, J.; Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 2018, 10, 1229–1233; (i) Fu, M. C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 2019, 363, 1429–1434; (j) Wei, Q.; Lee, Y.; Liang, W.; Chen, X.; Mu, B. S.; Cui, X. Y.; Wu, W.; Bai, S.; Liu, Z. Photocatalytic direct borylation of carboxylic acids. Nat. Commun. 2022, 13, 7112; (k) Ruzi, R.; Liu, K., Zhu, C.; Xie, J. Upgrading ketone synthesis direct from carboxylic acids and organohalides. Nat. Commun. 2020, 11, 3312; (l) Zhang, M.; Xie, J.; Zhu, C. A general deoxygenation approach for synthesis of ketones from aromatic carboxylic acids and alkenes. Nat. Commun. 2018, 9, 3517; (m) Shu, X. L.; Xu, R. T.; Liao, S. H. Photocatalytic divergent decarboxylative amination: a metal-free access to aliphatic amines and hydrazines. Sci. China Chem. 2021, 64, 1756; (n) Lu, K.; Ma, Y.; Liu, S.; Guo, S.; Zhang, Y. Highly Stereoselective C-Glycosylation by Photocatalytic Decarboxylative Alkynylation on Anomeric Position: A Facile Access to Alkynyl C-Glycosides. Chin. J. Chem. 2022, 40, 681–686.
- 15 Ning, Y.; Wang, S.; Li, M.; Han, J.; Zhu, C.; Xie, J. Site-specific Umpolung amidation of carboxylic acids via triplet synergistic catalysis. Nat. Commun. 2021, 12, 4637.
- 16 Wang, S.; Li, T.; Gu, C.; Han, J.; Zhao, C. G.; Zhu, C.; Tan, H.; Xie, J. Decarboxylative tandem C-N coupling with nitroarenes via SH2 mechanism. Nat. Commun. 2022, 13, 2432.
- 17(a) Hu, A. H.; Guo, J. J.; Pan, H.; Tang, H. M.; Gao, Z. B.; Zuo, Z. W. δ-Selective Functionalization of Alkanols Enabled by Visible-Light- Induced Ligand-to-Metal Charge Transfer. J. Am. Chem. Soc. 2018, 140, 1612–1616; (b) Hu, A. H.; Guo, J. J.; Pan, H.; Zuo, Z. W. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 2018, 361, 668–672; (c) An, Q.; Xing, Y. Y.; Pu, R. H.; Jia, M. H.; Chen, Y. G.; Hu, A. H.; Zhang, S. Q.; Yu, N.; Du, J. B.; Zhang, Y. X.; Chen, J. Q.; Liu, W. M.; Hong, X.; Zuo, Z. W. Identification of Alkoxy Radicals as Hydrogen Atom Transfer Agents in Ce-Catalyzed C–H Functionalization. J. Am. Chem. Soc. 2023, 145, 359–376; (d) Xia, S.; Hu, K.; Lei, C.; Jin, J. Intramolecular aromatic C-H acyloxylation enabled by iron photocatalysis. Org. Lett. 2020, 22, 1385–1389; (e) Li, Z.; Wang, X.; Xia, S.; Jin, J. Ligand-Accelerated Iron Photocatalysis Enabling Decarboxylative Alkylation of Heteroarenes. Org. Lett. 2019, 21, 4259–4265.
- 18 Chen, Y. Y.; Lu, L. Q.; Yu, D. G.; Zhu, C. J.; Xiao, W. J. Visible light- driven organic photochemical synthesis in China. Sci. China Chem. 2019, 62, 24.
- 19 Zhou, W. J.; Wu, X. D.; Miao, M.; Wang, Z. H.; Chen, L.; Shan, S. Y.; Cao, G. M.; Yu, D. G. Light Runs Across Iron Catalysts in Organic Transformations. Chem. Eur. J. 2020, 26, 15052.




