Recent Advances in Catalytic Carbonylation Reactions in Alternative Reaction Media
Comprehensive Summary
Since the discovery of the hydroformylation (oxo-synthesis or Roelen reaction) and the Reppe-reaction, the transition metal-catalyzed carbonylation reactions, providing versatile, facile, and even atom-economic methods for the selective incorporation of C=O functionality to various skeletons, have gained tremendous importance in synthetic organic chemistry from laboratories to industrial applications. The carbonylation of carbon-carbon multiple bonds, aromatic halides, triflates, etc., in the presence of various nucleophiles has led the way to produce aldehydes, carboxylic acids, esters, amides, etc., in the fine chemical industry. However, these protocols usually proceed in conventional, fossil-based, and usually toxic reaction environments. Thus, several attempts have been directed to developing efficient carbonylation methods in alternative, less harmful, non-fossil-based and even in renewable reaction media. In this review, we overview the recent applications of alternative solvents such as water, biomass-based alcohols, γ-valerolactone (GVL), 2-methyltetrahydrofuran (2-MeTHF), ionic liquids (ILs), deep eutectic solvents (DES), alkyl levulinates, limonene, α-pinene, and dimethyl carbonate as well as fluorous media to improve efficiency, safety and environmentally benign nature of carbonylation protocols.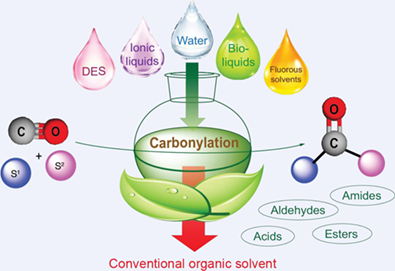
1 Introduction
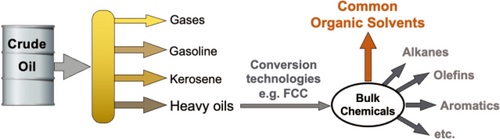
Our modern civilization utilizes an enormous amount of energy and consumer products of the chemical industry, including transportation fuels, fertilizers, polymers and composites, pharmaceuticals, detergents, food additives, electronics, sports equipment, clothes, dyes, and agrochemicals. So far, more than 130 million artificial chemical substances have been produced, which have not existed in nature before.[1] The utilization of fossil-based carbon resources received only occasional applications until the industrial revolution, when coal and later crude oil and natural gas have become the primary sources of energy and carbon-based chemicals. Today, refinery processing of crude oil continuously provides bulk chemicals including C1-building block, carbon monoxide, and common organic solvents for the chemical industry (Figure 1), which produces value-added consumer end-products in million-ton scales establishing our recent living standards.
The dramatically increased utilization of fossil-based resources in association with increased carbon dioxide emission, which reached 36.2 GT in 2021,[2] has directed research attention to develop environmentally friendly innovative approaches for chemical industry.[3] According to the principles of Green Chemistry,[4] the applications of selective catalytic processes could decrease the energy requirements and use of resources via reduced by-product formation.[5] On the other hand, the elimination of auxiliary materials that is primarily realized in large-scale solvent applications could establish more sustainable and economically viable processes. In the last three decades, significant efforts have been made to identify and characterize appropriate reaction media that can fit the industrially important transition metal-catalyzed catalytic processes.[6-10]
1.1 Carbonylation reactions in organic synthesis
One of the key challenges of practical organic chemistry is the efficient and even selective formation of new carbon-carbon and carbon-heteroatom bonds.[11-13] Due to their enormous synthetic potential, versatility, functional group tolerance, and often excellent chemoselectivity, transition metal-assisted coupling reactions have gained incredible popularity in the last few decades. Among these transformations, the d8–d10 metal-catalyzed carbonylation reactions provide extremely useful synthetic methods for the incorporation of one or two carbon monoxide molecule(s) into a complex molecular structure.[14-16]
The first carbonylation reaction, which led to the formation of aldehydes by the reaction of a carbon-carbon double bond with carbon monoxide and dihydrogen in the presence of a transition metal catalyst, was discovered by German chemist Otto Roelen in 1938.[17, 18] The accidental discovery of CO addition to olefinic bond of ethylene[19] was made during the development of the cobalt-catalyzed Fischer-Tropsch reaction, while the formation of propanal was observed from ethylene and syngas (H2 : CO = 1 : 1). The product yield became less and less by increasing the temperature indicating the presence of a temperature-sensitive species [HCo(CO)4].[19] At the same time and in Germany as well, Walter Reppe revealed that methanol could be carbonylated in the presence of metal carbonyls of VIIIB elements as catalysts.[20] However, the reaction requires extremely high pressure (up to 700 bar) and temperature (ca. 300 °C) compared to hydroformylation. The process so-called Reppe-carbonylation was first commercialized in 1960.[21] Since its discovery, the transition metal-catalyzed carbonylation reactions have become one of the most important homogeneous industrial processes,[22] which can be exemplified by the large-scale industrial hydroformylation processes[23] including Ruhrchemie/Rhône-Poulenc process for butanal[24] or by the Cativa process for acetic acid production.[25]
Beyond the functionalization of carbon-carbon multiple bonds, other easily accessible and usually cheap substrates, i.e., alkyl–X and aryl–X (X = OH, OTf, Cl, Br, I, etc.) compounds can be subjected to carbonylation in the presence of various H–Nucleophiles (H–Nu, Nu: OH, OR, NHR, NRR’, SR, SeR, etc.) opening a facile protocol for manufacturing various functionalized building blocks and/or end-products for fine chemical and pharmaceutical industries.[26-31] Depending on the Nu reaction partner, the transformation can undergo the formation of amides, carboxamides, carboxylic acids, O- and S-esters, heterocycles, azides, selenoesters, etc., via an atom-economical manner.[32-38] These structures are important class of starting materials and/or building blocks for organic synthesis of complex molecular structures including biologically active pharmaceuticals, pesticides, fine chemicals, etc.,[39-43] as well as can be found in liquid crystals,[44, 45] and photosensitizers, for examples.[46] It should be noted that the use of heterobifunctional nucleophiles in selective carbonylation protocol could open alternative and easier synthetic routes for the construction of complex molecular structures.[47]
1.2 Solvents in carbonylation processes
Solvents can be considered an intrinsic part of chemical reactions and chemical processes. They fundamentally determine the pivotal properties of systems from the molecular level to large- scale industrial processes and play a key role in their efficient operations. The solvents provide one or more liquid phase(s) for the reactions, stabilize reagents, intermediates, and transition states of the reactions, regulate temperatures via reflux, control exothermic reaction, clean equipment, assist filtration, facilitate mixing, regulate density and viscosity, purify and isolate compounds by extraction, crystallization, generate or destruct azeotrope as well as assist structural and/or analytical characterization of compounds, etc. Consequently, the “solvent-friendly process thinking” has evolved and resulted in the utilization of enormous amounts of solvents, which certainly contribute to the release of millions of tons of organic compounds[48] annually, resulting in serious environmental concerns and causing significant economic issues. For example, ca. 7 million tons of non-methane volatile organic compounds were released in the EU26 in 2020.[49] From the chemical industry, the fine chemical and pharmaceutical sectors have been identified as the major sources,[50] largely due to the multistep production of complex structures, which requires large quantities of auxiliaries including solvent(s).
The carbonylation reactions typically proceed in fossil-based common organic solvents. According to FDA guidelines[51] these media, however, are classified into Class 1 or 2, of which utilization has to be avoided such as benzene and chlorinated hydrocarbons or limited including toluene, hexane, acetonitrile, dimethyl formamide (DMF), dichloromethane (DCM), tetrahydrofuran (THF), etc., just to name a few. It should be noted that eliminating hazardous solvents from carbonylation protocols is fundamentally important for the pharmaceutical industry, where the residual solvent traces could result in serious health issues. Accordingly, the successful introduction of safe reaction media is crucial for this sector of the chemical industry.
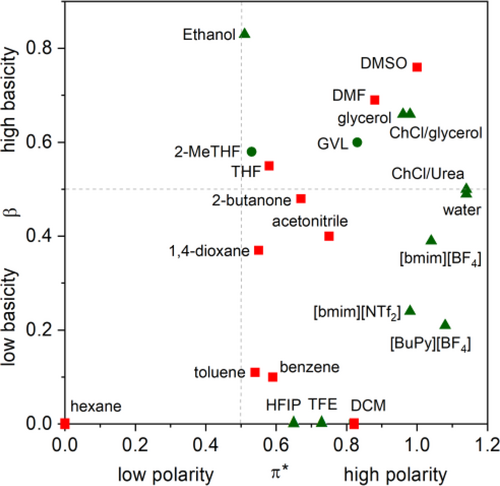
Beyond the roles above, it is well-known that solvents could strongly affect the activity and selectivity of the transition metal- catalyzed reactions. For example, the efficiency of the carbonylation reaction is highly influenced by the ligand substitution processes of catalytically active carbonyl compounds[52] involving the possible coordination of the solvent molecule to the coordinatively unsaturated catalyst species, which could result in a lowered activity. The coordination ability of a solvent can be described by Kamlet-Taft solvatochromic parameters, which refer to acidity/ basicity and polarizability of the corresponding medium. These values for selected conventional (Table 1, entries 1—10) and alternative (Table 1, entries 11—25) media are collected. Figure 2 depicts the solvents used for the carbonylation reactions as a function of their polarity/polarizability (π*) and hydrogen bond accepting ability or basicity (β). It can be easily visualized that common solvents (except hexane, π* = 0) have medium or high polarity and a wide range of basicity. Thus, alternative solvents can be identified within this area. From the recently used surrogates that were evaluated by GSK's solvent selection guide,[65c] GVL, 2-MeTHF, HFIP, TFE, ethanol and glycerol exhibit these characteristics.
| # | Solvent | LD50 oral, rat/(mg·kg–1) | Kamlet–Taft parameters[53] | ||
| αb | βc | π*d | |||
| 1 | 1,4-dioxane | 5200[54] | 0 | 0.37 | 0.55 |
| 2 | 2-butanone | 2900[55] | 0.06 | 0.48 | 0.67 |
| 3 | acetonitrile | 175[56] | 0.19 | 0.4 | 0.75 |
| 4 | benzene | 3306[57] | 0 | 0.1 | 0.59 |
| 5 | DCM | 1600[58] | 0.13 | 0 | 0.82 |
| 6 | DMF | 2800[57] | 0 | 0.69 | 0.88 |
| 7 | DMSO | 14500[57] | 0 | 0.76 | 1 |
| 8 | hexane | 28710[57] | 0 | 0 | –0.04 |
| 9 | THF | 1650[57] | 0 | 0.55 | 0.58 |
| 10 | toluene | 5000[59] | 0 | 0.11 | 0.54 |
| 11 | GVL | 8800[60] | 0 | 0.6 | 0.83 |
| 12 | 2-MeTHF | n.a | 0 | 0.58 | 0.53 |
| 13 | ethanol | 7060[57] | 0.98 | 0.83 | 0.51 |
| 14 | glycerol | 12600[61] | 0.88 | 0.66 | 0.96 |
| 15 | water | — | 1.23 | 0.49 | 1.14 |
| 16 | [bmim][BF4] | n.a | 0.77 | 0.39 | 1.04 |
| 17 | [bmim][NTf2] | n.a | 0.62 | 0.24 | 0.98 |
| 18 | [BuPy][BF4] | n.a | 0.53 | 0.21 | 1.08 |
| 19 | ChCl/Glycerol (1 : 2) | 7733a[62] | 0.94 | 0.66 | 0.98 |
| 20 | ChCl/Urea (1 : 2) | 5640a[63] | 1.42 | 0.5 | 1.14 |
| 21 | HFIP | 600a[57] | 1.96 | 0 | 0.65 |
| 22 | TFE | 240[57] | 1.51 | 0 | 0.73 |
| 23 | TFA | 200[64] | n.a | n.a | n.a |
| 24 | Perfluorotoluene | n.a | n.a | n.a | n.a |
| 25 | Trifluorotoluene | n.a | n.a | n.a | n.a |
- a Mouse instead of rat. b Hydrogen-bond donating ability. c Hydrogen-bond accepting ability (basicity). d Polarity/polarizability. HFIP: hexafluoro isopropanol, TFE: tetrafluoro ethanol, TFA: trifluoroacetic acid.
The toxicity data (LD50 oral, rat) are also collected in Table 1 for selected solvents for comparison. However, these data cannot be used alone for the evaluation of the corresponding reaction media, because other important parameters such as water solubility, biodegradability, ability to form peroxide under air, flammability, open cup flash point, density, etc., have to be taken into account.[65a-b]
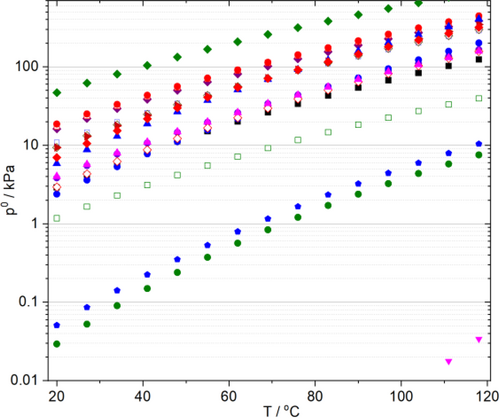
The temperature-dependent vapor pressures, a key solvent property to control VOC emission in general,[66] for selected common organic and recently utilized alternative solvents for carbonylation reactions are compared in Figure 3. It can be clearly shown that except for DMSO, GVL, and glycerol, the vapor pressures exceed 10 kPa over 80 °C, where most of the reactions take place effectively.
Thus, the selection of an appropriate reaction medium is crucial for both the reaction efficiency and environmental impact. One of the elegant approaches is the “co-solvent free” transformation, which could offer viable environmentally friendly solutions. In our case, it can be realized for alkoxycarbonylations, where EtOH acts as solvent and reagent. However, most of carbonylation reactions can only be operated in the presence of fossil-based, common apolar or polar aprotic solvents, depending on the reaction type.
Consequently, the replacement of conventional, fossil-based organic solvents with green and even biomass-based alternatives having low vapor pressure minimizing the emission, low or no toxicity and persistency, low flammability, and limited negative impacts on the environment has to be an intrinsic part of green innovation for advanced greener carbonylation processes (Figure 4). It is important to emphasize that beyond the primarily physical and physical-chemical characteristic, some additional properties have to be taken into account during the searching for alternative or even “green” solvents. Recently, several evaluation studies and guidelines were published[65] to assist chemists and chemical engineers in identifying better and safer reaction media.
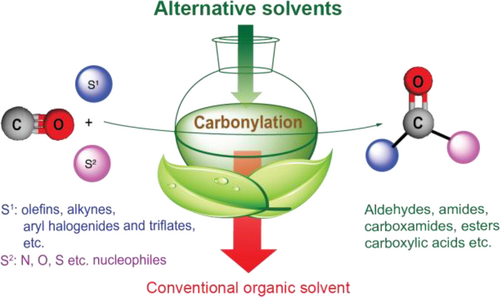
1.3 Scope of the review
The realization of environmentally friendly alternative carbonylation protocols involving the utilization of non-fossil-based and/or less hazardous reaction environments remains challenging even in fine and pharmaceutical synthetic processes. In these sectors of the chemical industry, the desired high activity and selectivity of certain catalytic systems should meet the utilization of solvents that have no or very limited impact on the environment. There are a lot of powerful fundamental research work on catalytic carbonylation involving the introduction of alternative reaction media in the last five years. Previous results for carbonylation in non-conventional media were overviewed by Xiao in 2007,[67] and excellent reviews were published for Pd-based systems involving renewable solvents by Gabriele,[33] Bayer,[68] and Schwab.[69] However, a general review focusing on using non- conventional solvents for transition-metal catalyzed carbonylation reactions has not been published for the last five years.
This review aims to overview the recent achievements and important advances in transition-metal catalyzed carbonylation reaction using alternative reaction media, i.e., water, alcohols, ionic liquids, fluorous and biomass-based solvents. We sincerely hope that it will be useful for guiding academic and industrial researchers toward facts and opportunities concerning the development of greener and sustainable carbonylation methods.
2 Carbonylation in Alternative Solvents
2.1 Water
Water is an excellent solvent for many chemical reactions because it is non-toxic, readily available in “unlimited” quantity, nonflammable, non-toxic, cheap, and could offer the easy separation of reagents or catalysts from many organic products. It could be a good medium for ionic reactions via easy solvating anions and cations. Water could serve both as a solvent and as a ligand, which could stabilize coordinatively unsaturated species, the key issue for long term catalyst stability and is a good σ-donor with very weak back donation capability. Stabilizing structures and effecting reactions by hydrogen bond(s) are also important solvent properties of water. Water can act as an O-nucleophiles in carbonylation reactions that lead to the formation of carboxylic acids.[70]
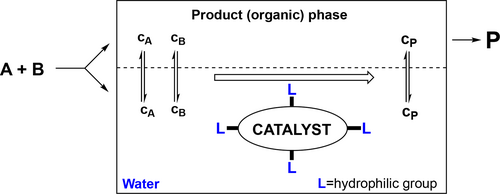
Beyond its many advantageous properties, water can be utilized as a catalyst and/or reagent phase. Thus, the combination of transition metal catalysis and the concept aqueous biphasic chemistry (Figure 5) could lead to the establishment of very active, selective, and robust catalytic processes with facile recycling of the catalysts and separation of the products, important issues for commercial applications.
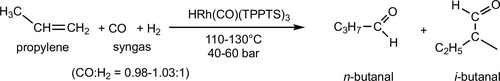
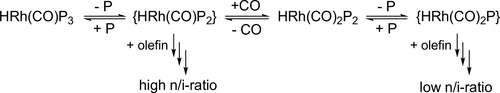
The first large-scale aqueous carbonylation process is the Ruhrchemie Rhône-Poulenc biphasic hydroformylation of propylene to butanal in the presence of the water soluble TPPTS modified rhodium catalyst (Scheme 1). The main product of the process is n-butanal with a ratio n-/i-butanal of ca. 25, surprisingly high in comparison with the conventional PPh3-modified Rh catalyst. It is supposed that the coordination of the olefin to the coordinatively unsaturated {HRh(CO)(TPPTS)2} species results in high n-aldehyde selectivity. On the other hand, the {HRh(CO)2(TPPTS)} containing cycle gives low selectivity (Scheme 2).[71] While, this commercialized process is excellent for propylene conversion, the low solubilities of higher olefins limit the applications for the production of higher aldehydes.[72]
Over Rh-based systems, the TPPTS modified Pt- and Pd-catalysts were successfully introduced into aqueous carbonylation reactions for the preparation of 2-phenyl-propionic acid and substituted derivatives as a class of non-steroidal anti-inflammatory drugs as well as of 2-arylpropionic acids, including the potent anti-inflammatory agent naproxen.[73, 74]
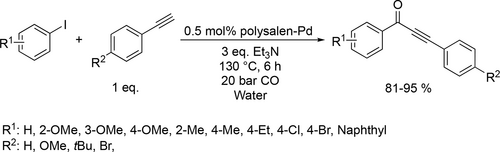
Recent developments were mainly focused on the applications of Pd-based catalyst systems. Efficient Pd-catalyzed carbonylative Sonogashira coupling reaction was carried out in water. For the catalyst system a highly cross-linked polymer matrix polysalen was prepared using acrylamide and N,N’-methylene-bisacrylamide and was successfully applied for immobilizing palladium complex to produce polysalen-Pd catalyst. This phosphine- free carbonylative Sonogashira coupling of various iodobenzene and terminal alkynes was carried out with excellent yields (81%—95%) to form α,β-alkynyl ketones (Scheme 3). Furthermore, the catalyst could be recycled without significant loss of activity over 5 times.[75]
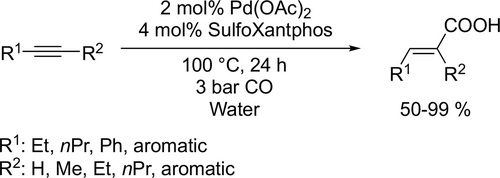
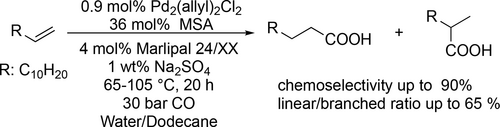
Sulfonated phosphines (TPPTS, Xantphos derivatives) modified palladium-catalyzed hydroxycarbonylation of alkynes with CO and water was developed to obtain α,β-unsaturated carboxylic acids (Scheme 4). Good to excellent yields (50%—99%) were achieved and in the formation of unsymmetrical carboxylic acids the regioselectivity mainly depended on the electronic properties of the aromatic ring.[76] 1-Dodecene was subjected to the palladium- catalyzed hydroxycarbonylation in microemulsion system (Scheme 5). It was found that the phase behavior did not control the reaction performance. Moreover, the type and the concentration of the nonionic surfactants are crucial in microemulsion systems as they provide the interfacial area between the oil and the water and determine the local concentrations of the substrates by their hydrophilicity.[77]
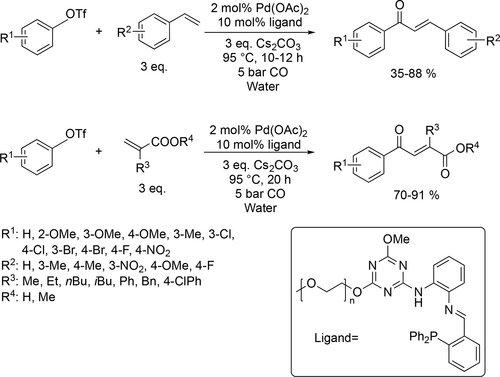
Pd-catalyzed carbonylative Heck coupling reaction was carried out in water to obtain different chalcone derivatives and 1,4-dicarbonyl esters (Scheme 6). Good to excellent yields (35%—91%) were obtained using a hydrophilic and recycable methoxypolyethylene glycol-modulated s-triazine-based multifunctional Schiff base/N,P-ligand.[78]
A porous organic polymer-supported (UPOP) palladium catalyst was successfully developed for the heterogeneous hydroxycarbonylation of aryl iodides in water. Overall excellent yields (84%—91%) were achieved (Scheme 7) at 100 °C under 5 bar CO. The catalyst could be recycled 3 times without significant loss of activity. However, for the 4th run a decrease in the activity was detected.[79]
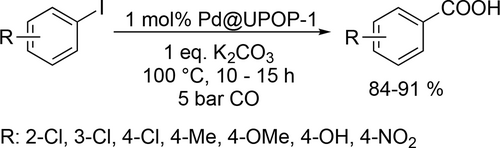
Yin and co-workers investigated the aqueous carbonylation of 5-bromofurfural[80] in Et2O/water biphase system and 5-bromofuroic acid[81] in water. In both cases >95% yield of formyl-2-furancarboxylic acid and 2,5-furandicarboxylic acid (Scheme 8) could be achieved in the gram-scale synthesis under the optimal reaction conditions (respectively 10 bar CO, 70 °C, 48 h and 12.5 bar CO, 80 °C, 16 h). In case of the 5-bromofuroic acid the catalyst could be recycled 10 times without significant loss of the activity.
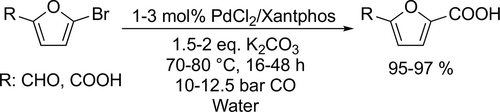
The heterogeneous palladium-catalyzed aminocarbonylation of aryl halogenides with alkyl and aryl amines was investigated (Scheme 9). Trzeciak and co-workers examined Pd/DNA catalyst and achieved good to excellent yields (70%—98%) using triethylamine as base at 80 °C under 1 bar CO after 6 h.[82] Lei and co-workers used an amphiphilic porous organic polymer supported palladium catalyst which showed excellent activity (82%—98%) with various aryl halogenides and amines.[83] In both cases the catalyst systems could be recycled 5 times with a slight decrease of activity.
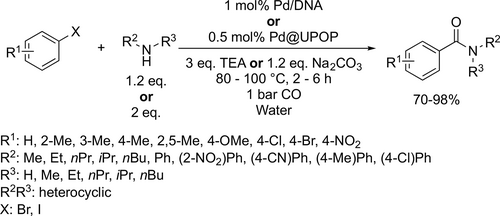
TPPTS-modified rhodium catalyst was reported for the tandem hydroformylation-acetalization of different olefins and polyols (Scheme 10). Under the optimal conditions (40 bar, 80 °C, 5 h) good to excellent yields of acetals (54%—94%) were achieved. Moreover, the catalytic system could be recycled and used without significant loss of activity over 5 runs.[84]
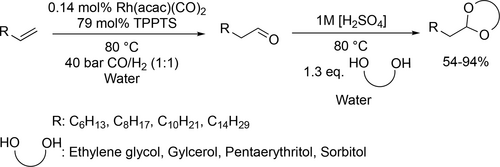
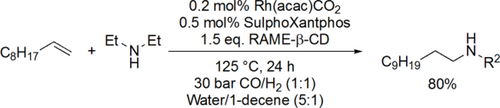
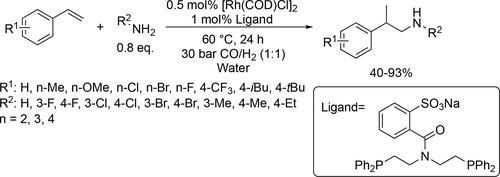
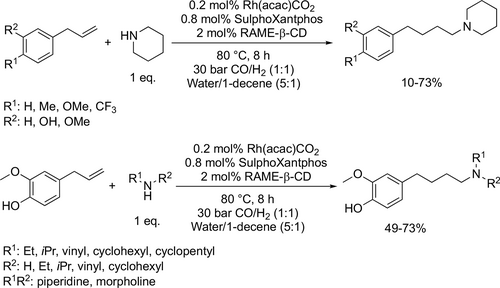
Vogt and co-workers synthesized N,N-diethylundecan-1-amine from 1-decene, CO and diethylamine using randomly methylated β-cyclodextrin (RAME β-CD) as mass transfer agent in the biphasic hydroaminomethylation. Good yield (80%) was achieved under the optimal conditions (125 °C, 40 bar CO/H2 (1 : 1)) after 30 h (Scheme 11).[85] Chen and co-workers carried out the hydroaminomethylation of various vinyl arenes with anilines in water (Scheme 12). Good yields (40%—93%) and high selectivities (90%—99%) of branched amines were achieved at 60 °C under 30 bar CO/H2 (1 : 1) after 24 h.[86] Bhanage and co-workers examined the hydroaminomethylation of various natural olefins such as eugenol, anethole and estragole with CO and amines like piperidine using RAME β-CD as mass transfer agent. Good yields (76%—90%) were achieved to the formation of the total amine (Scheme 13).[87]
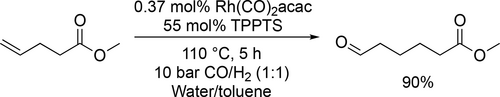
The rhodium catalyzed systems were investigated in detail in either water or water/toluene biphasic system. The latter medium was applied to overcome low solubilities of organic substrates in water. The efficiency of the biphasic system can be exemplified by the hydroformylation of different olefins using water-soluble rhodium catalyst system. Vries and co-workers applied this method for the hydroformyltaion of methyl 4-pentenoate to obtain methyl 5-formyl-valerate (Scheme 14) in the biomass-based synthesis route of ε-caprolactam. Excellent yield (90%) was achieved at 110 °C under 10 bar after 5 h.[88]
Chen and co-workers performed hydroformylation of various substituted vinyl arenes (Scheme 15). Good to excellent yields (65%—99%) were obtained and the branched aldehyde was preferred to the linear aldehyde (3.3 : 1—24 : 1) under optimal conditions (50—60 °C, 30—40 bar).[89, 90]
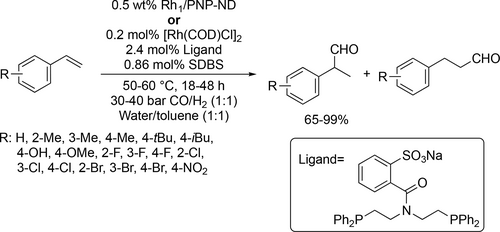
Rhodium-catalyzed hydroformylation of olefins with synthesis gas was examined in aqueous/organic biphasic solvent system (Scheme 16). Smith and co-workers carried out hydroformylation of 1-octene to examine different mono- and multivalent phosphorus-based ligand. Most of the synthesized Rh-complexes (Scheme 16 1) had over 200 h–1 TOF under the optimal conditions (75 °C, 40 bar), but the recyclability studies had poor result as after the first run 90% of each catalyst leached into the organic phase.[91] Sulfonated, salicylaldiminato-aryl ether mono- and trimeric ligands (Scheme 16 2) were examined as well. Their application showed better activity (>500 h–1 TOF) in the hydroformylation of 1-octene at 85 °C under 50 bar.[92]
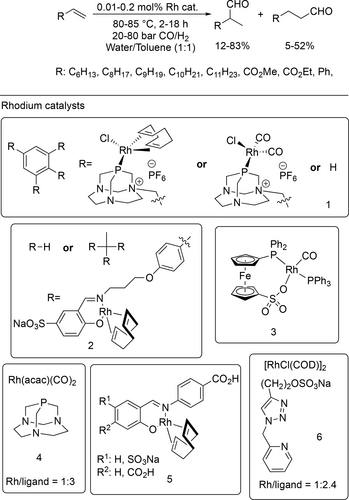
Rhodium(I) complexes with a polar phosphinoferrocene sulfonate ligand (Scheme 16 3) had moderate activity and selectivity for hydroformylation at 80 °C under 10 bar synthesis gas in water or water/toluene biphase aqueous mixture due to the low solubility in the applied solvent.[93]
Rhodium and 1,4,7-triaza-9-phosphatricyclo[5.3.2.14, 9]tridecane complex (Scheme 16 4) showed excellent activity and regioselectivity to branched aldehyde in the hydroformylation of styrene and aliphatic olefins. Due to the application of water/toluene biphasic solvent system the catalyst system could be easily separated from the products and recycled six times without significant loss of activity.[94]
Makhubela and co-workers applied Schiff-base rhodium(I) water-soluble complexes (Scheme 16 5) in the hydroformylation of various olefins. Good to excellent aldehyde chemoselectivity (71%—100%) and good selectivity for the branched aldehyde (60%—100) were achieved under the optimal reaction conditions (85 °C, 40 bar) after 8 h.[95]
Scrivanti and co-workers applied sulfonated pyridyl-triazolyl N,N-bidentate ligand (Scheme 16 6) for the hydroformylation of styrene and 1-hexene in water/toluene solvent system. Excellent yield (>99%) was achieved for both substrates at 80 °C under 80 bar CO/H2 (1 : 1) after 18 h.[96]
Various Rh-based catalyst systems were applied in the hydroformylation of terminal alkenes to obtain different aldehydes. Dreimann and co-workers reported the first-time application of RAME-β-CD as a mass transfer agent in a continuous process. The Rh-catalyzed hydroformylation of 1-decene had an excellent chemoselectivity to the aldehyde (>90%). Moreover, 47% aldehyde yield and >97% linear aldehyde selectivity were achieved in a continuous miniplant over a 200 h steady-state operation time with an extremely low catalyst leaching (Scheme 17).[97]
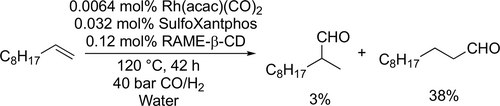
Pogrzeba and co-workers developed a mini-plant system for the continuous Rh-catalyzed hydroformylation of 1-dodecene in microemulsion. More than 50 hour steady-state operation was achieved with 39% product yield and 99% selectivity toward the linear aldehyde (Scheme 18).[98]

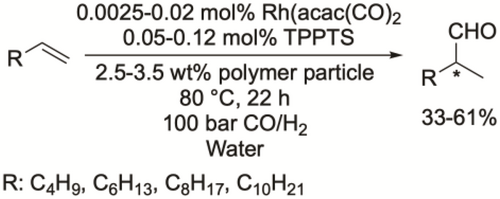
Vogt and co-workers synthesized core-shell polymer particles and applied them as carriers for the rhodium catalyst and as phase transfer agents for the hydroformylation of various terminal alkenes (C6—C12). Moderate conversion (33%—61%) and good selectivity toward the linear aldehyde (>70%) were achieved at 80 °C under 100 bar syngas (Scheme 19).[99, 100] Smith and co-workers reported two water-soluble Rh(I) complexes as catalysts for the hydroformylation of 1-octene. Good aldehyde yield (>83%) was achieved at 75 °C under 40 bar syngas. However, significant drop was observed after every run in the recyclability test (Scheme 20).[101]
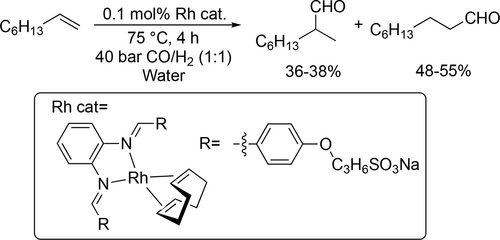
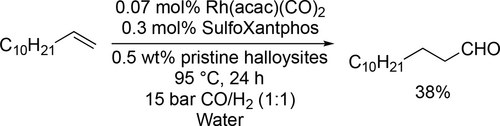
Trzeciak and co-workers synthesized rhodium nanoparticles (Rh NP) stabilized by polyvinylpyrrolidone, polyvinyl alcohol or DNA and applied them as a source of soluble, catalytically active species in the hydroformylation of 1-hexene, styrene and vinyl acetate in water. 88%—91% aldehyde yields were achieved with a 77% selectivity toward the linear aldehyde under mild reaction conditions (80 °C, 10 bar, 3 h). Under the same reaction conditions high selectivity toward the aldehyde was achieved in the hydroformylation of styrene and vinyl acetate (Scheme 21).[102, 103] Hapiot and co-workers synthesized a β-CD-based phosphane and applied it in the rhodium-catalyzed hydroformylation of higher olefins (C10—C18) as a ligand as well as the CD cavity helped to overcome the limitation of the mass transfer. The conversion was moderate and decreased (55%—29%) with the length of the alkyl chain because of the higher hydrophobicity at 80 °C under 50 bar (Scheme 22).[104] Potier and co-workers applied pillar5arenes substituted by carboxylate functions and methyl groups or polyethylene glycol chains to overcome the mass transfer limitation in the aqueous biphasic rhodium catalyzed hydroformylation of 1-decene and 1-hexadecene. The catalyst systems were found to be robust as they could be recycled 4 consecutive times without significant loss of activity (Scheme 23).[105] Lvov and co-workers found halloysites as inexpensive natural nanoparticles to stabilize oil-water emulsions in the aqueous biphasic hydroformylation of 1-dodecene and to give efficient dodecene conversion to tridecanal (Scheme 24).[106]
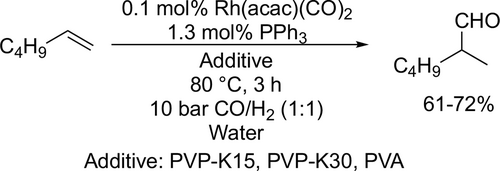
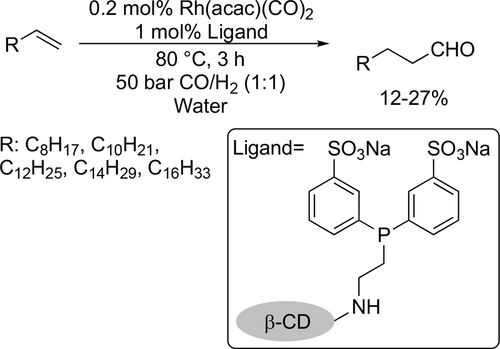
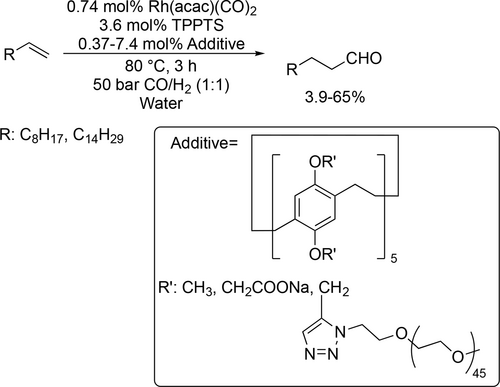
A kinetic study was carried out by Vorholt to investigate the relationship between liquid-liquid (LL) interfacial area and catalytic activity as mass transport limitation plays an important role in biphasic catalysis. According to the photo-optical in-situ measurement of the interfacial area in the hydroformylation of 1-octene, it was found that the product nonanal has a significant influence on the LL interfacial area due to its amphiphilic properties. Moreover, it was found that the relationship between the LL interfacial area and the catalytic activity is not linear as was previously assumed in the literature.[107] Schomäcker and co-workers adapted an existing kinetic model of hydroformylation in microemulsion systems according to experiments of the Rh-catalyzed hydroformylation of 1-octene. It was found that the reaction rate is first order with respect to alkene concentration and fractional order with respect to catalyst and ligand concentration. Moreover, the reaction rate had a maximum at about 10 bar with respect to the syngas pressure.[108]
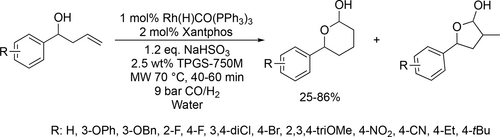
Petricci and co-workers applied micellar catalysis and microwave irradiation in the Rh-catalyzed hydroformylation of terminal olefins. The aldehydes were obtained with good to high yields (37%—95%) and good selectivity for the linear aldehydes (33%—100%) under mild reaction conditions (70 °C, 9 bar, 40 min). This method could be applied to the hydroformylation in tandem with intramolecular hemiacetilization of hydroxy group containing alkenes to obtain 5 or 6 member cyclic hemiacetals (Scheme 25) with good yields (25%—86%) and excellent selectivity for the 6 member cyclic hemiacetals (86%—100%).[109]
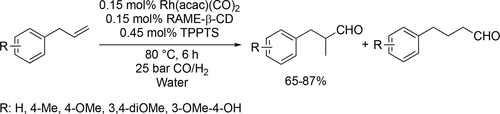
The Rh-catalyzed hydroformylation of eugenol and other allyl benzene derivatives was carried out in an aqueous medium using RAME-β-CD (Scheme 26). Good yields (65%—87%) were achieved toward the formation of the linear aldehyde under the optimal conditions (80 °C, 25 bar, 6 h). Moreover, the catalyst system could be recycled 4 times without the loss of activity and selectivity.[110]
2.2 Biomass-based reaction media
2.2.1 Ethanol
A possible approach to overcome the low solubility of organics in water is the use of alcohols that could provide tunable solvent performance by the effects of the alkyl group(s) and the hydrogen bonding of the hydroxyl group(s). The optimal solvent structure can easily be approached by variations of their numbers and positions. For carbonylation, the short-chain linear aliphatic alcohols are used preferably.
From this series the biomass-originated ethanol is the most preferred as the second most used solvent after water in several various sectors, from petrochemical to cosmetic industries. Ethanol has a dual role in these reactions. It acts as a solvent and O-nucleophile, as well. In comparison with other solvents, it is a relatively low-cost and large-scale produced substance exhibiting several advantages of a solvent, e.g., fully miscible with water assisting biodegradation and relative low-toxicity.
Recently several efficient carbonylation processes were carried out in ethanol mainly focusing on the incorporation of a ethoxylate group into the product molecular structure.
The carbonylative Suzuki-Miyaura cross-coupling of iodobenzene and phenylboronic acid was carried out using rice-husk derived carbon-silica supported palladium nanoparticle catalysts (Scheme 27). The applied solvent had a huge impact on the selectivity of the reaction towards the formation of the benzophenone or 1,1-biphenyl. Applying ethanol as solvent resulted in good conversion (80%) and selectivity to the biaryl ketone (87%).[111]

Ethanol was used as a solvent as well as reagent in many palladium-catalyzed ethoxycarbonylation reactions. The ethoxycarbonylation of gem-difluoroalkene was carried out at 120 °C under 40 bar CO by Beller and co-workers. Excellent yield (92%) and regioselectivity toward the branched aldehyde (>99%) were reached after 20 h (Scheme 28).[112]
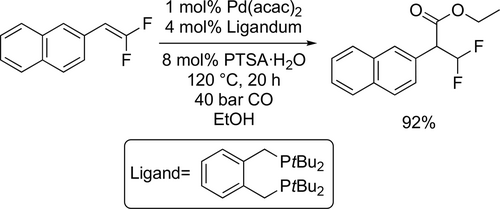
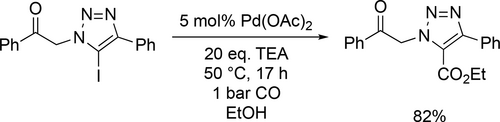
The ethoxycarbonylation of a 5-iodo-1,2,3-triazole derivative was reported by Latyshev and co-workers. Good yield (82%) was reached under mild conditions (50 °C, 1 bar CO) after 17 h (Scheme 29).[113]
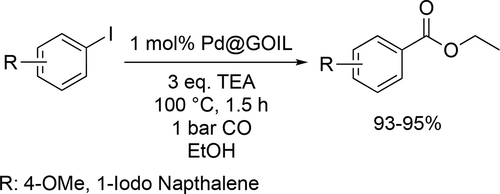
Bhanage and co-workers reported a highly efficient palladium catalyst which can be applied in ethoxycarbonylation reactions of aryl iodides. Excellent yields (93%—95%) were achieved at 100 °C under 1 bar CO after 1.5 hours (Scheme 30).[114]
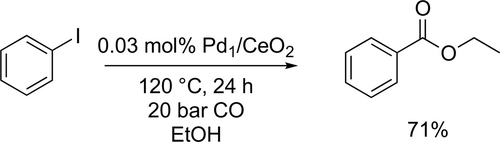
Zhang and co-workers first reported that Pd1/CeO2 single- atom catalyst showed a good activity in the alkoxycarbonylation of aryl iodides. The ethoxycarbonylation of iodobenzene was carried out with a good yield (71%) at 120 °C under 20 bar CO for 24 h (Scheme 31).[115] Uozumi and co-workers developed an aqueous continuous-flow reaction system which could be applied to the ethoxycarbonylation of iodobenzene using a mixture of water and ethanol as solvent. Full conversion and good isolated yield (76%) were reached under the optimized reaction conditions (1 : 2 H2O/EtOH, 100 °C, 5 bar).[116]
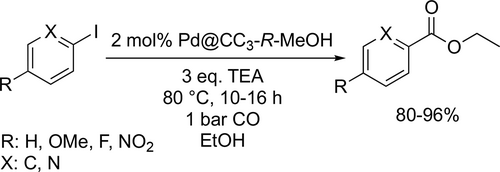
Porous organic cage stabilized palladium nanoparticles were prepared by Wang and co-workers that showed a high catalytic activity for the ethoxycarbonylation reaction of aryl iodide derivatives. Good to excellent yields (80%—96%) were achieved under mild conditions (80 °C, 1 bar CO) after 12 h in ethanol (Scheme 32).[117]
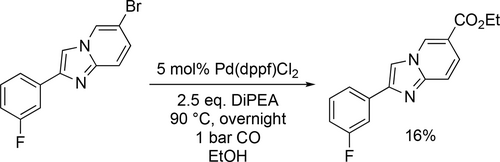
Schulte and co-workers discovered a distinct class of 2-phenylimidazo[1,2-a]pyridine-6-carboxamide H-PGDS inhibitors and part of the synthesis route was a ethoxycarbonylation reaction at 90 °C under 1 bar CO. Unfortunately, they find the yield (16%—92%) was unsatisfactory for the carbonylation step, so an alternative route was developed (Scheme 33).[118]
Taran and co-workers applied the ethoxycarbonylation in the synthetic route to obtain iminosydnone-based prodrugs. 61% yield was achieved in the carbonylation reaction at 80 °C under 1 bar CO after 5 h.[119] The same group used ethoxycarbonylation for the synthesis of iminosydnone derivatives. Good yield (71%) was achieved at 60 °C under 1 bar CO after 2.5 h (Scheme 34).[120]
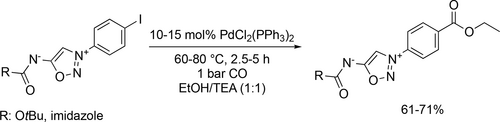
Baxendale and co-workers examined the ethoxycarbonylation in ethanol to develop a multi-step flow operation to synthesize an hydrazine intermediate used in the assembly of the neurotensin modulator SR 142948. 87% yield was achieved in the flow based system under the optimal conditions (120 °C, 10 bar). Despite the good yield, it was not used in the final set of multi-step flow sequences because of the unsatisfactory throughput (Scheme 35).[121]
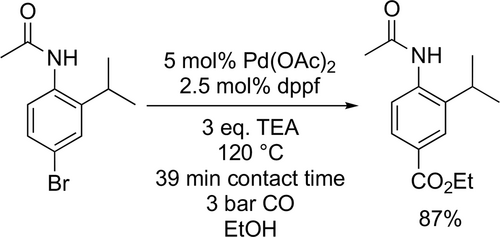
Ulrich and co-workers applied ethoxycarbonylation to synthesize various water-soluble boron-dipyrromethene derivatives, which displayed optical responses in pH range of 4—8. Good to excellent yields (64%—98%) were achieved at 60 °C and under 1 bar CO pressure (Scheme 36).[122]
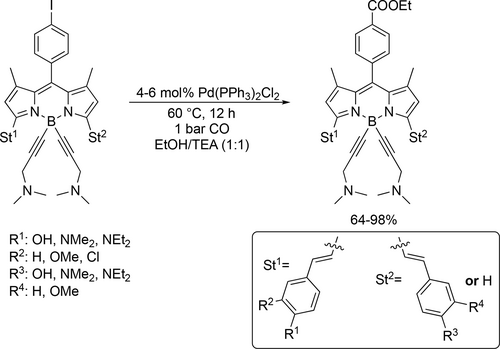
Oyarzabal and co-workers applied the ethoxycarbonylation in the synthetic route to obtain different potent HDAC inhibitors (Figure 6 a—c) that were investigated for the treatment of Alzheimer's disease. The carbonylation steps had good yields (61%— 85%) at 100 °C under 15 bar CO overnight (Scheme 37).[123-125] The same method was applied to manufacture a class 1 HDAC inhibitor containing a 3-amino-pyridine-2-urea core structure (Figure 6 d) by Ivarsson and co-workers. The carbonylation step had a good yield (84%) at 100 °C under 15 bar after 12 h (Scheme 38).[126] IC50 values of target compounds are collected in Table 2 for the synthesized components.
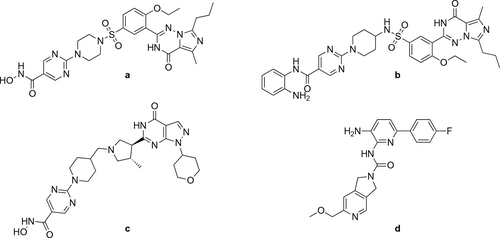
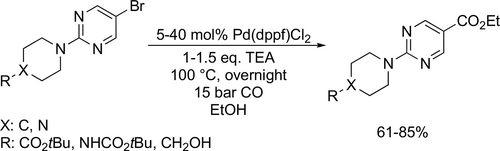
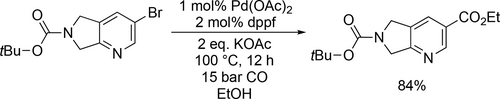
| Compoundb | PDE5 | HDAC1 | HDAC2 | HDAC6 |
|---|---|---|---|---|
| a | 0.200 | 1.00 | 12.9 | 19.1 |
| b | 24 | >20000 | >20000 | >20000 |
| c | 8450 | 90 | 516 | 495 |
| d | n.a | 120 | 280 | >30000 |
- a Figure 6. b Values in cells are IC50 (nM).
Banwell and co-workers applied the palladium-catalyzed ethoxycarbonylation in a reaction sequence that enables the synthesis of a C7N aminocyclitol derivative (Scheme 39). The carbonylation step had an excellent yield (91%) under 1 bar CO at 22 °C after 48 h.[127]
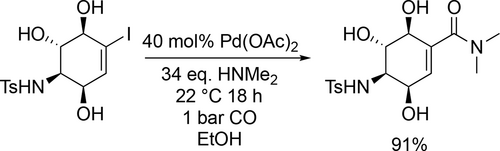
2.2.2 Glycerol
Glycerol is a readily available green solvent and can be obtained as the by-product of biodiesel and natural fatty acid derivative(s) production via transesterification of a triglyceride at industrial scale. It exhibits several advantageous solvent properties; however, the production of glycerol possessing solvent quality could require high energy consuming processes.[128, 129] Although it is considered as an O-nucleophiles, it can be used as environmentally benign reaction medium without significant formation of O-coupled by-product(s).
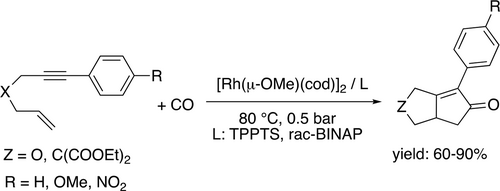
A facile protocol for Rh-catalyzed Pauson-Khand carbocyclizations of the 1,6-enynes to bicyclo[3.3.0]octenones using neat glycerol as reaction media was reported by Gómez (Scheme 40). The reaction can be performed by [Rh(μ-OMe)(cod)]2 in the absence of any ligand under atmospheric CO pressure at 80 °C. It was shown that the presence of TPPTS ligand can enhance the efficiency of the catalyst system even at low Rh/TPPTS ratios.[130] It is important to emphasize that glycerol has a key role in the catalytic cycle via stabilization of catalytically active Rh-species [Rh(CO)2(Glycerol)2]+[OR]– (R: H or Me) thanks to the coordinating ability of glycerol.
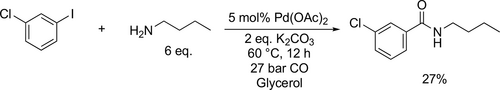
Gylcerol was also examined as the reaction media for the Pd-catalyzed aminocarbonylation of butan-1-amine with 1-chloro- 3-iodobenzene (Scheme 41) under mild condition (60 °C and 27 bar CO). The achieved yield was mediocre (27%) probably because of the high viscosity of the glycerol.[131]
2.2.3 γ-Valerolactone
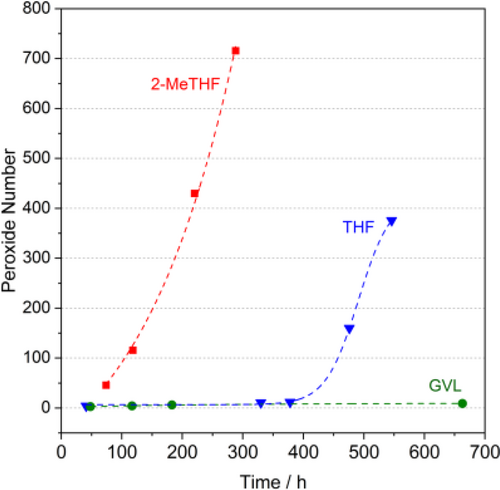
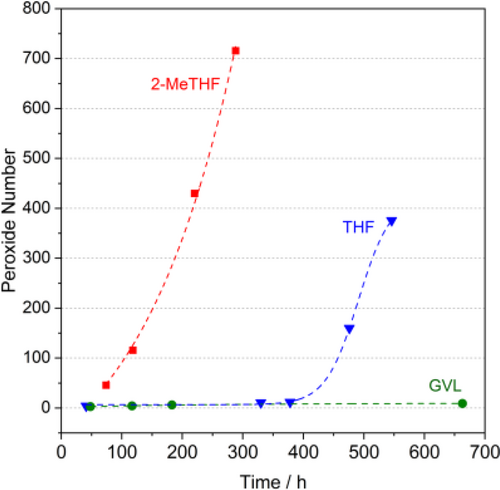
γ-Valerolactone was first proposed as a sustainable liquid for energy and carbon-based chemicals,[60] and as a potential alternative bio-based solvent in 2008 by Horváth.[132] It can easily be synthesized from lignocellulosic biomass via acid catalyzed dehydration of its cellulosic fraction to levulinic acid[133] followed by its subsequent selective hydrogenation to 4-hydroxyvaleric acid.[134] Since it is an aprotic, dipolar, non-toxic, biomass-derived molecule having low vapor pressure even at high temperature (Figure 3), it could be a particularly promising substances to replace the fossil-based and usually highly toxic common organic media for carbonylation reactions such as DMF, NMP, DMA, THF, dioxane, aliphatic- and aromatic hydrocarbons. In contrast to tetrahydrofuran and 2-MeTHF, it does not form any measurable peroxide within a test month (Figure 7, 8), which could be crucial for large scale use, storage, and even transportation.[135] It should be noted that peroxide number is one of the crucial factors. On the other hand, the temperature dependent vapor pressure and boiling point at atmospheric pressure should also be considered for selection. The latter assists the ease of separation and recovery, for example. GVL and 2-MeTHF were evaluated using environmental, health, and safety scores in detail by CHEM21 selection guide.[65d] Recently, it was successfully utilized as alternative media for catalysis.
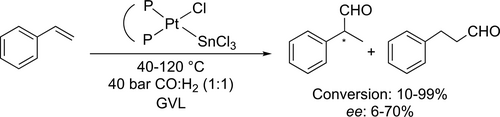
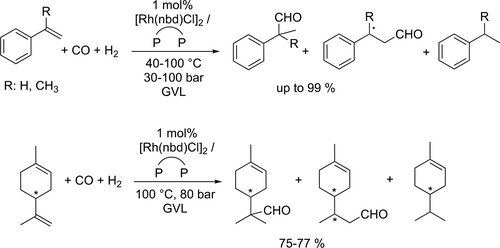
Since chiral aldehydes are potential precursors of non-steroidal anti-inflammatory drugs, such as ibuprofen or naproxen, the enantioselective hydroformylation of vinyl aromatics to corresponding 2-arylpropanals in the presence of chiral phosphine ligand-modified Pt- or Rh-based catalyst systems is of the utmost importance.[73, 136] In addition, the introduction of a non-toxic reaction medium is fundamentally important in the pharmaceutical industry, where the residual solvent traces could result in serious health issues. Following this line of thought, the application of GVL as reaction media for carbonylation was first demonstrated by the enantioselective hydroformylation of styrene in the presence of in situ generated optically active bidentate ligand-modified Pt-diphosphine-tin(II)chloride catalyst systems.[137] Various types of chirality elements such as central (BDPP), axial (BINAP, SEGPHOS) and planar/central (JOSIPHOS) were monitored to optimize enantioselectivity (Scheme 42). Generally, in comparison with toluene as reference common media, slightly higher activities and regioselectivities towards branched aldehyde (2-phenylpropanal) were obtained. However, higher chemoselectivities towards aldehydes (up to 98%) in GVL were detected at lower temperatures. The application of GVL proved to be also advantageous regarding enantioselectivity: although moderate enantioselectivities were obtained in both solvents, in most cases higher ee values were detected in GVL. Asymmetric hydroformylation of various prochiral substrates, i.e., styrene, α-methylstyrene, dimethyl itaconate, and (R)-limonene was investigated in details in GVL (Scheme 43).[138] Several chiral enantiopure bidentate ligands such as (S,S)-BDPP, (R)-BINAP, (R)-QUINAP, (R,R)-DIOP, (Rc),(Sp)-JOSIPHOS, (S)-SEGPHOS, (S)-(DM)-SEGPHOS) were tested in in situ generated Rh-diphosphine systems. Due to the coordination ability of GVL, the catalysts' activity was lower than that in commonly utilized toluene. It should be noted that remarkable chemo- (>99%) and regioselectivities (>95%) were observed in GVL under identical conditions. The BDPP-modified Rh-catalyst could be recycled for three consecutive cycles.
Iodobenzene as a model substrate and its 4-substituted derivatives were subjected to carbonylation in the presence of tert-butylamine to the corresponding amides and 2-ketocarboxamides in GVL by the use of catalyst in situ generated from Pd(OAc)2 and PPh3. The reaction exhibited good functional group tolerance. The best reaction efficiency, i.e., selectivity towards 2-ketocarboxamide was achieved under 25 bar of CO at 50 °C (Scheme 44).[139]
Ligand free Pd-catalyzed phenoxycarbonylation reactions for the synthesis of functionalized aromatic esters (Scheme 45) were developed using GVL as solvent. Twenty-four phenyl-benzoate derivatives were prepared with a yield of 39%—99+% depending on the substituent of corresponding aryl ring at 100 °C under 7 bar CO for 3 h. It should be noted that volatile Et3N was successfully replaced by K2CO3 as a base. Based on the correlation between the electronic parameters of the aromatic substituents (Hammett σ) and the reaction rates, it was proposed that the rate determining step is the oxidative addition of Ar–I to Pd0 species.[140]
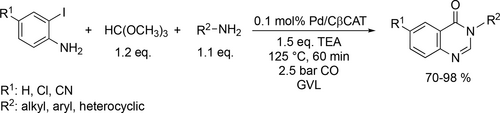
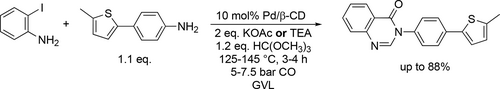
Aminocarbonylative coupling reactions for the synthesis of different 4(3H)-quinazolinones using microwave irradiation was tested in GVL.[141] The Pd-catalyzed, four-component carbonylative coupling reactions of trimethyl orthoformate, o-iodoaniline derivatives, and various amines were found to be highly efficient and selective for 4(3H)-quinazolinones under 2.5 bar CO at 125 °C (Scheme 46). The products were isolated in good yields (70%—98%). While the used Pd/β-cyclodextrin−cross-linked catalyst system showed high performance and recyclability. A MW-assisted alternative method was reported for the one-pot synthesis of an S-containing 4(3H)-quinazolinone derivative. A pressure-resistant MW reactor was used to carry out the three sequential reaction steps, the C—H arylation, the reduction and the carbonylation. Under the optimal conditions, a good yield (88%) was reached in the carbonylation step (Scheme 47).[142]
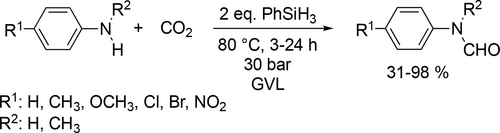
Han and co-workers demonstrated the transformation of CO2 and both primary and secondary amines to formamides in GVL. In comparison with THF, CH2Cl2, 1,4-dioxane, acetone or CHCl3, remarkable activities were detected in GVL at 50 °C or even at 80 °C exhibiting 100% conversion (Scheme 48).[143]
Despite several advantages of GVL, it should be noted that it has higher coordination ability to the catalytically active coordinative unsaturated transition metal species, which results in slightly lower reaction rates in comparison with reactions performed in common polar aprotic solvents. Nevertheless, it can be considered as a potential environmentally benign alternative even in the pharmaceutical industry.
2.2.4 2-Methyltetrahydrofuran
2-Methyltetrahydrofuran, which was listed as a renewable fuel additive and a component of the alternative P-fuels,[144] has been considered as alternative solvents for several organic - and[145] organometallic[146] reactions and catalytic transformations.[147] However, its ability to form peroxide has to be noted as a serious limiting factor. Nevertheless, a few studies were reported on its applications for carbonylations.
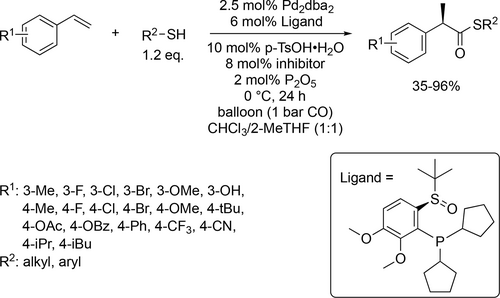
A Pd catalyzed highly enantioselective thiocarbonylation of styrenes with CO and thiol derivatives has been achieved using chloroform/2-MeTHF (V/V = 1 : 1) solvent system (Scheme 49). The reported yields of enantioenriched thioesters were good to excellent (35%—96%). The thiocarbonylation of high variety of substrates went under mild reaction conditions (1 bar and 0 °C). Key component for the enantioselectivity was the applied chiral sulfoxide-(P-dialkyl)-phosphine ligands. The generality and effectiveness of the method was proven by its application to the synthetic transformations of thioester products and the direct acylation of cysteine-containing dipeptides. A primary mechanism was investigated, and a plausible catalytic cycle was proposed.[38]
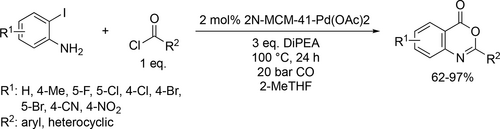
A highly efficient and green cyclocarbonylation of different 2-iodoanilines with CO and various acyl chlorides has been reported. The reaction proceeds smoothly in the biomass-based 2-methyltetrahydrofuran solvent using N,N-diisopropylethylamine as base at 100 °C and under 20 bar. A wide variety of 2-substituted 4H-3,1-benzoxazin-4-one derivatives were synthesized in good to excellent yields (62%—97%) by an 2-aminoethylamino- modified MCM-41-anchored palladium acetate complex [2N-MCM-41-Pd(OAc)2] as the heterogeneous catalyst (Scheme 50). The expensive palladium catalyst was recycled at least 8 times without any noticeable loss of catalytic efficiency.[148]
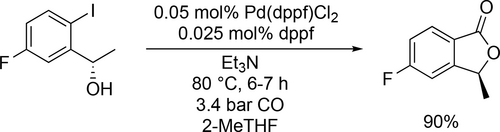
2-MeTHF was used as solvent in a Pd-catalyzed CO insertion reaction to obtain (S)-5-fluoro-3-methylisobenzofuran-1(3H)-one, a key intermediate of anti-cancer drug lorlatinib. The lactone formation proceeded smoothly using Pd(dppf)Cl2 (dppf: 1,1'-Bis(diphenylphosphino)ferrocene) as catalyst, under 3.4 bar CO and at 80 °C. The reaction reached full conversion in 6—7 h by using as low as 0.05 mol% catalyst (Scheme 51). Applying higher catalyst load resulted in a mass transfer limited condition because of the fast hydrogen uptake by catalytic cycle. Addition of small amount of dppf was preferred to prevent the formation of palladium black. Finally, no stereochemistry inversion was observed in 2-MeTHF.[149]
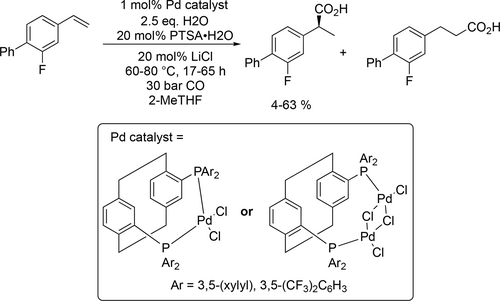
Clark and co-worker described the synthesis of flurbiprofen by enantioselective hydroxycarbonylation and the methoxycarbonylation with Pd-phanephos catalysts using 2-MeTHF as solvent.[150] In case of the hydroxycarbonylation, high conversion (91%—97%) and high yield to the branched product (69%) could be reached but only moderate enantioselectivity (42%—47%) was observed (Scheme 52). While in case of the methoxycarbonylation, most of the catalysts showed poor activity in 2-MeTHF as the observed conversion was between 0 and 15%. These results were surprising as in all previous work,[151] the hydroxycarbonylation and the methoxycarbonylation of given alkenes showed fairly similar regioselectivity and enantioselectivity.
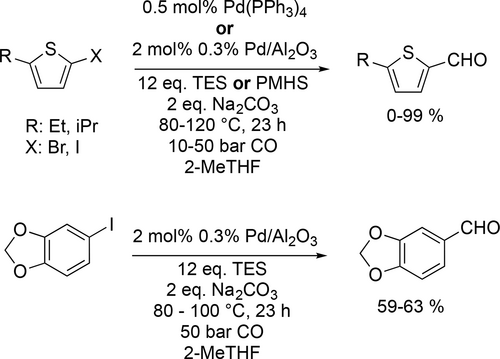
Preparation of different heteroaromatic aldehydes by reductive carbonylation of halo derivative precursors was carried out in 2-MeTHF. These heteroaromatic aldehydes can be used either as industrial fragnances/flavor, like Heliotropine® or as key intermediates for the synthesis of APIs or some fragrances, like Lioral® or Helional®. For the reductive carbonylation, different organosilanes were applied as H-donor instead of the gaseous, flammable and explosive hydrogen making the process easier and more applicable. Under optimal condition, excellent conversion (99%) and yield (98%—99%) were achieved in the reductive carbonylation of some 5-iodo-2-alkylthiophene. While the desired aldehyde did not form during the carbonylation of 2-bromo-5-ethylthiophene using the heterogeneous Pd/Al2O3, only 2-ethylthiophene was observed as main product via substitution of Br with a H. Moreover, piperonal, an important chemical in industrial scale, was synthetized using Pd/Al2O3 as catalyst and TES as H-donor, but unfortunately, the conversion (82%—90%) and yield (59%—63%) were unsatisfactory (Scheme 53).[152]
2.2.5 Other biomass-originated solvents
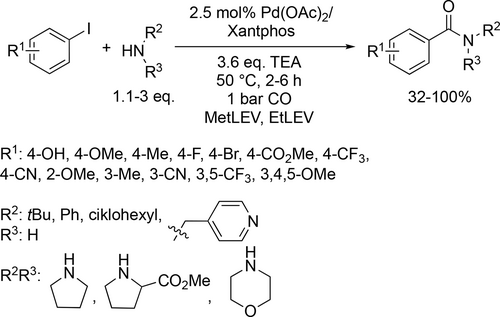
Beyond the discussed bio-originated media, some new candidates were investigated for carbonylation. Takács and co-workers first applied hemicellulose-based solvents, such as ethyl levulinate and methyl levulinate, as green alternatives in palladium-catalyzed aminocarbonylation of various iodobenzenes. It should be noted that these
esters could easily be prepared from acid-catalyzed dehydration of carbohydrates in methanol or ethanol.[153-155] The Pd(OAc)2/Xantphos catalyst system was found to be excellent as 98% conversion and 100% chemoselectivity were achieved in the coupling of iodobenzene and morpholine. Moreover, good to excellent conversion (32%—100%) was achieved with various iodobenzene derivatives and amines under the optimal reaction conditions (50 °C, 1 bar CO)(Scheme 54).[156]
Limonene, as a natural substance which can be easily obtained from waste citrus peels was recently successfully introduced into carbonylation methods.[157] Dimethyl carbonate as a preferred green solvent was also applied for carbonylation. Nowadays, industrial DMC production is based on either methanol phosgenation as an older technology or oxidative carbonylation methanol over Cu catalyst, recently.[158]
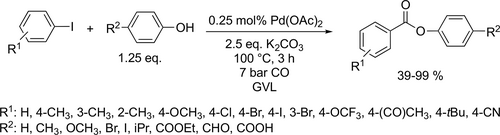
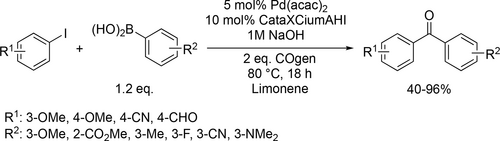
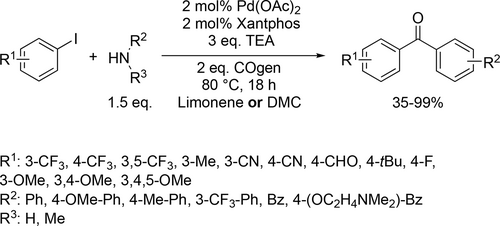
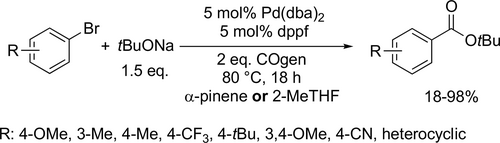
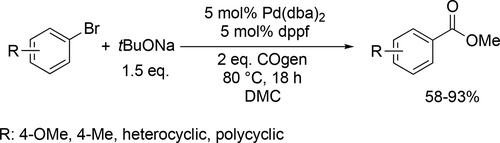
Bayer and co-workers applied different renewable solvents in the Pd-catalyzed carbonylative coupling reactions, such as carbonylative cross-couplings, aminocarbonylations, and alkoxycarbonylations. They first reported good to excellent yields (40%— 96%) using limonene in the carbonylative coupling of boronic acids and aryl bromides (Scheme 55). Good to excellent yields (35%—99%) were achieved in the aminocarbonylation of aryl bromides using DMC (dimethyl carbonate) or limonene as reaction media (Scheme 56). This method could be used for the synthesis of commercial drug Trimetozine and an analogue of Itopride. α-Pinene, which is readily available from various coniferous plants was utilized in tert-butoxycarbonylation reaction[159] and 2-MeTHF was found to be excellent reaction media for the tert-butoxycarbonylation of aryl bromides and overall good yields (18%—98%) were achieved (Scheme 57) by the same group. The formation of the methoxycarbonylated products was observed with excellent yields (58%—93%) using DMC instead of α-pinene or 2-MeTHF as reaction media (Scheme 58, 57).[68]
2.3 Ionic liquids and deep eutectic solvents
Ionic liquids are organic molten salts, which have melting point typically below 100 °C; however, most of them are liquids at or close to room temperature. While the cations are generally large organic compounds such as N,N-dialkyl-imidazolium, N-alkyl- pyridinium, N,N-alkylpyrolinium, tetraalkylphosphonium, trialkylsulfonium, etc., the anions are comparably smaller such as AlCl4–, HF2–, BF4–, PF6–, SbF6–, CF3SO3–, etc., and some of them are commercially available. One of the most attractive properties of ionic liquids is the very low vapor pressure. Furthermore, they are not flammable, easy to handle, have reasonable thermal stability and are liquids at large range of temperature. The hydrophilicity, polarity, coordinating ability, acidity or basicity of these media can easily be tuned by using the right combinations of cations and anions. For example, they can behave as a Lewis acid and serve as a solvent at the same time, or they can be a ligand and solvent simultaneously.[160] Consequently, they have received significant interest as “designer solvents” for synthesis and catalysis,[161-163] involving carbonylation reactions.
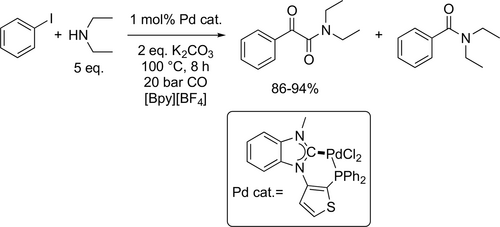
Recently, the synthesis of α-ketoamides by the Pd catalyzed amino dicarbonylation of aryl halides (Scheme 59) is highly dependent on the stereoelectronic properties of the involved ligands. The ionic diphosphine ligand can serve as a precursor of a hemilabile P, C-hybrid ligand to form stable Pd(II)-complex. The examined PdCl2(MeCN)2- ionic 3-thiophenyl-benzimidazolium-based diphosphine catalytic system maintained a good yield (94%—100%) and selectivity to the α-ketoamide (89%—95%) in [Bpy][BF4] ionic solvent through six runs. The observed leaching of Pd in the combined organic phase was minimal, only 0.37% after the sixth run.[164]
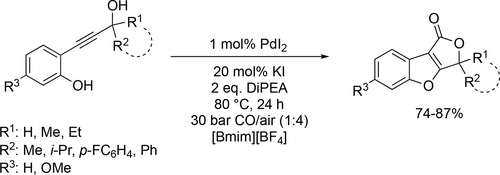
Application of different ionic liquids for the synthesis of furo[3,4-b]benzofuran-1(3H)-ones was reported by Mancuso and Gabriele. It was based on the PdI2 catalyzed carbonylative double cyclization of 2-(3-hydroxy-1-yn-1-yl)phenols, CO and oxygen in the presence of KI and diisopropylethylamine (Scheme 60) at 80 °C for 24 h under 30 bar of a 1 : 4 mixture of CO-air. The reaction in [BMIm][BF4] showed a good yield (74%—87%) and chemoselectivity toward the formation of the double cyclization compound, whereas the method was not selective in classical organic non-nucleophilic solvents (such as MeCN or DME), leading to a mixture of the benzofurofuranone derivative and the benzofuran ensuing from simple cycloisomerization. Moreover, the catalyst could be easily recycled six times without detectable loss of activity.[165]
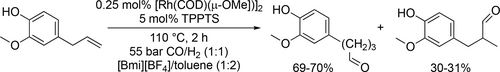
[BMIm][BF4]/toluene biphasic medium was applied in the Rh- catalyzed hydroformylation of natural olefins (eugenol, estragole and safrole) (Scheme 61). The Rh/TPPTS system showed a high activity and selectivity towards the formation of the desired aldehydes under moderate conditions (110 °C, 55 bar). Full conversion of eugenol was achieved in 2 h and the selectivity for the aldehydes was 95% (Branched aldehyde/Linear aldehyde: 1 : 3). In case of estragole, the selectivity and conversion were similar, while the safrole was much less active (full conversion after 15 h) and the selectivity was smaller (82%) as well. The catalytic phase could be recycled up to four times without an immense loss of activity (64%—99% conversion) and selectivity (87%—94%).[166]
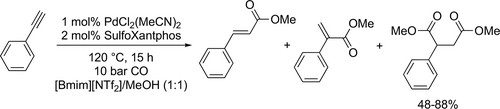
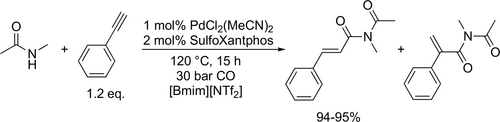
Liu and Zhang synthesized a bi-functional sulfonated Xantphos ligand, which enabled the PdCl2(MeCN)2 catalyzed tandem bis-alkoxycarbonylation of terminal alkynes (Scheme 62) to synthetize aryl-/alkyl-substituted succinate. The catalyst system could be recycled successfully for at least 3 runs without notable activity loss and detectable metal leaching using [Bmim][NTf2] ionic liquid as solvent for the bis-methoxycarbonylation of phenylacetylene. However, the selectivity to the bis-methoxycarbonylated product dropped after the 4th run from 82% to 48%.[167] The same ligand could be applied for the synthesis of α,β-unsaturated imides (Scheme 63) by the Pd-catalyzed hydroamidocarbonylation of alkynes with amides. The catalyst system could be reused for 4 runs in [Bmim][NTf2] without detectable metal leaching. The conversion slightly decreased from 91% to 89%, while the selectivity to the branched-imide remained the same after the 4th run (95%).[168]
Deep eutectic solvents (DESs) have been introduced as a new class of ionic liquid (IL) analogs because they represent many advantageous characteristics and properties with ionic liquids.[169, 170] Among these properties, the low vapor pressures and tunability to the corresponding chemical systems should be highlighted. DESs also consist of large, asymmetric ion pairs having low lattice energy and hence low melting points. They are usually formed by the complexation of a quaternary ammonium salt with a metal salt or a hydrogen bond donor species.[171, 172] One of the important building blocks of DES is choline chloride (ChCl), of which based solvents have been widely utilized in synthesis and catalysis, especially in the pharmaceutical industry.[173] For carbonylation reactions, the ChCl-based DESs were mostly investigated.
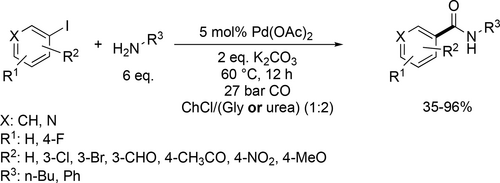
ChCl/urea (1 : 2) and ChCl/Gly (1 : 2) deep eutectic solvents were introduced in Pd-catalyzed aminocarbonylation of (hetero)aryl iodides (Scheme 64) as an environmentally benign and recyclable media under mild conditions (60 °C and 27 bar CO). In both cases, a variety of amides were synthesized with good to excellent yields (35%—96%). The Pd-catalyst and both DES could be easily recycled, and the catalyst remained active for over 5 cycles while only a slight decrease in yield was observed.[174]
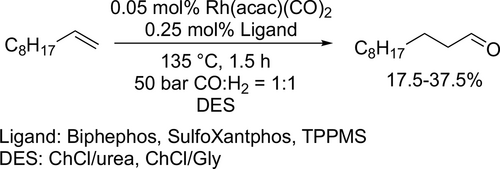
The hydroformylation of 1-decene (Scheme 65) was carried out in a gas-liquid-liquid system featuring a deep eutectic solvent to enable recycling of the rhodium catalyst. The reaction was conducted with three different phosphine-based ligand (Biphephos, Sulfoxantphos and TPPMS) and five deep eutectic solvents were tested as polar solvent. According to screening reactions, the best performing DESs were the ChCl:Urea, ChCl:Gly and ChCl:Urea using Biphenos, Sulfoxantphos and TPPMS, respectively.[175]
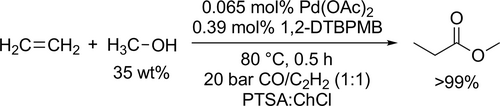
Rationally tailored acidic deep eutectic solvents (ADESs) were used as acid promoter, phase separator and extractant in the Pd-catalyzed methoxycarbonylation of ethylene (Scheme 66) to get a highly effective and easy-to-separate system. Almost full conversion (>99%) and selectivity of methyl propionate (>99%) were reached at 80 °C under 20 bar for 30 min applying PTSA: ChCl as solvent. Moreover, the catalyst system activity remained the same after 20 runs.[176]
2.4 Fluorous solvents
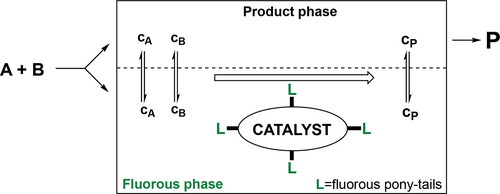
Perfluorinated substances (all hydrogen atoms are replaced with fluor atoms), e.g., alkanes, dialkylethers, and trialkylamines are very unusual because of their nonpolar nature and low intermolecular forces. Their miscibility even with common organic solvents such as perfluoro methylcyclohexane, toluene, THF, acetone, and alcohols is low at room temperature, thus these materials could form fluorous biphase systems. The term fluorous was introduced in 1994 by Horváth,[177] as the analogue to the term aqueous,[178] to emphasize the fact that one of the phases of a biphase system is richer in fluorocarbons than the other one. The concept of Fluorous biphase systems (FBS) can be efficiently applied for a wide scope of the catalytic chemical transformations via immobilization of the catalysts in the fluorous phase.[179, 180]
A fluorous catalyst system consists of a fluorous phase containing a preferentially fluorous soluble catalyst and a second product phase, which may be any organic or non-organic solvent with limited solubility in the fluorous phase.
The first FBS was reported for the fluorous ligand (P[CH2CH2(CF2)5CF3]3) modified Rh-catalyzed hydroformylation of 1-octene to nonanals in a toluene/C6F11CF3 system.[177] The reaction was performed under homogeneous conditions under 10 bar of CO : H2 = 1 : 1 at 100 °C using a catalyst generated in situ from [Rh(CO)2(acac)] and P(C2H4C6F13)3 with Rh/P=1 : 40, which gave an 85% conversion towards aldehydes with an n/i ratio of 2.9. Regarding the mechanistic aspects, it was clearly shown by high- pressure NMR that the solution structure of the fluorous soluble HRh(CO){P[CH2CH2(CF2)5CF3]3}3 in C6F11CF3 is similar to HRh(CO)(PPh3)3 in toluene and HRh(CO)[P(m-C6H4SO3Na)3]3 in water. It has also been established that the coordinatively unsaturated {HRh(CO)(PR3)2} and {HRh(CO)2(PR3)} act as catalytically active species, similarly to the organic and water-containing systems. Their reaction with olefins leads to competing catalytic cycles involving one or two phosphine ligands on the Rh. Kinetic
studies revealed that the reaction is first order in both rhodium and olefin. While the reaction is inhibited by the excess of P[CH2CH2(CF2)5CF3]3, the n/i ratio (7.84) of the aldehyde increases with increasing phosphine concentration until an optimum P : Rh ratio of approximately 100 : 1.[181]
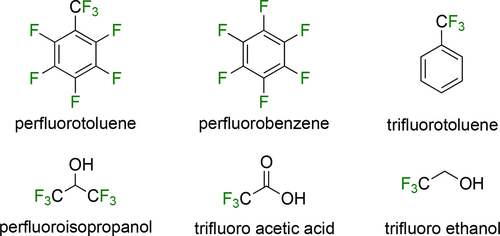
Since its discovery, several attractive fluorous systems have been developed for carbonylation reactions. These achievements are reviewed in the corresponding time frames.[182, 183] However, several recent innovative applications were published for carbonylation protocols. It should be noted that the recently used perfluorinated materials (Figure 9) as solvent do not lead to the formation of biphasic system by necessity. Their advantages can be exploited in many single-phase systems by using either well-established or innovated catalyst systems.
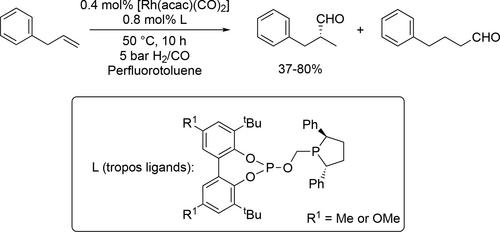
Clark and co-workers reported improved enantioselectivity (80 : 20—96 : 4 (R) e.r.) and regioselectivity (70%—86% towards branched aldehyde) for the hydroformlyation of allylbenzene (Scheme 67) using perfluorotoluene as solvent and bidentate phospholane phosphites ligand modified Rh catalyst.[184] The same group reported the selective hydroformylation of propene with a selectivity range (74%—78%) between 75 and 105 °C using perfluorotoluene as solvent and a racemic rhodium complex of a similar ligand structures, which was synthesized from a tropos-biphenol with an extended backbone exhibiting a high stability at high temperatures.[185]
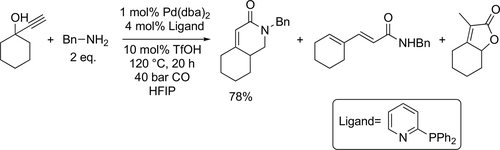
Ru-catalyzed hydroxycarbonylation of olefins applying Ru3(CO)12 precursor and PCy3 catalyst system was reported by Beller's group. It was crucial for the successful reaction to use hexafluoroisopropanol (HFIP) as solvent and the p-toluenesulfonic acid as an acid co-catalyst. Overall good yield (29%—84%) was achieved with different olefins.[186] A new Pd-catalyzed cascade carbonylation in HFIP was presented to obtain α,β-unsaturated piperidones (Scheme 68). The reaction conditions were optimized by the carbonylation of 1-ethynyl-1-cyclohexanol with benzylamine. Good to excellent yields (25%—94%) were achived at 120 °C under 40 bar after 20 h by the carbonylation of different alkynols with amines.[187]
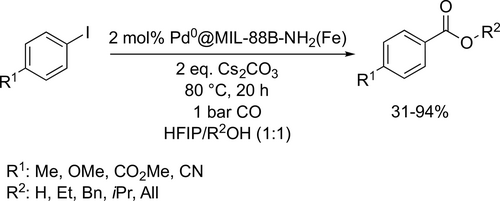
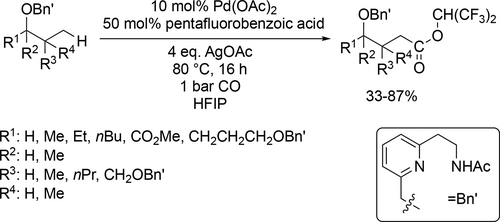
Both alkoxy- and hydroxycarbonylation were achieved with good to excellent yields (31%—94%) in the same medium at 80 °C under 1 bar CO by palladium nanoparticles (Scheme 69).[188] HFIP was found to be excellent solvent for Pd-catalyzed C(sp3)-H alkoxycarbonylation of different alcohols under mild conditions (Scheme 70). Mediocre to good yields (33%—87%) were achieved at 80 °C under atmospheric CO pressure after 16 h.[189]
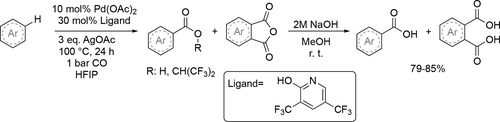
A Pd-based 2-pyridone ligand containing catalyst, which accelerates non-directed C—H functionalization of arenes was reported by Yu and co-workers. A carboxylation method was developed using HFIP as reaction media (Scheme 71, 72). The reaction products were a mixture of the mono-HFIP ester and the phthalic anhydride derivatives. A mixture of the mono-acid and the phthalic acid derivatives could be obtained after treatment with NaOH solution in methanol. Good combined yields (79%—85%) were achieved after the alkaline treatment.[190]
A mixture of HFIP (70%) and methanol (30%) was successfully used as a reaction media in the solution polymerization of CO/ethene/propene as an alternative to the typically used slurry polymerization techniques. High-molecular-weight polyketone products (240.3 Da) and high catalytic activity were achieved by the palladium-based homogeneous catalyst.[191]
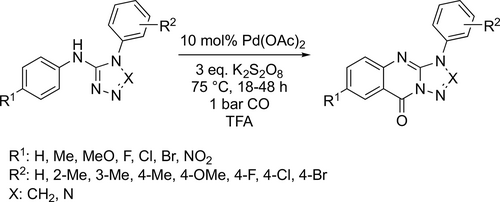
The Pd-catalyzed C—H carbonylative annulation of N,1-diaryl- 1H-tetrazol-5-amines and N,4-diaryl-4H-1,2,4-triazol-3-amines was reported, which resulted in the corresponding triazole and tetrazole fused quinazolinones (Scheme 72). Good yields (30%— 83%) were obtained at 75 °C under 1 bar CO in trifluoroacetic acid (TFA). A mechanism has been presented based on the observed kinetic isotope effects and the isolation and characterization of the catalytically active C—H activated intermediate dimeric Pd complex.[192]
Palladium-catalyzed asymmetric C—H carbonylation was carried out to synthesize isoquinoline derivatives (Scheme 73). High enantioselectivities (75%—92% ee) and good yields (20%—83%) were achieved at 60 °C after 24 h in trifluoroethanol (TFE).[193]
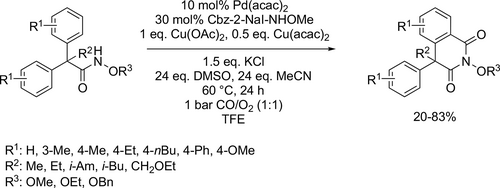
Ackermann developed an electrocatalytic C—H activation with carbon-monoxide by earth-abundant, inexpensive cobalt salts, which was effective for the electrooxidative C—H/N—H functionalizations of benzhydrazides at room temperature in the mixture of TFE and HFIP as reaction media (Scheme 74). The metallaelectrocatalysis was carried out with outstanding functional group tolerance in an undivided cell setup. In this way, biologically relevant, highly substituted cyclic imidates were possible to be synthesized with good yields (41%—89%).[194]
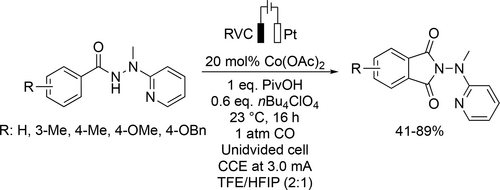
Palladium catalyst was applied in ethylene/CO and styrene/CO copolymerization and ethylene/styrene/CO terpolymerization in TFE (Scheme 75). It was found that the less electron-rich and sterically bulky catalysts had the highest catalytic activities. Moreover, the thermal properties of the polyketone products could be tuned by the styrene content.[195]
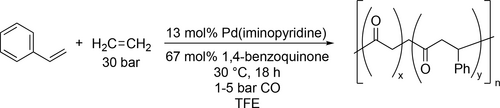
Pd catalyzed CO/vinyl arene copolymerization was carried out in TFE (Scheme 76) to examine the effect of the prochrial comonomer on the stereochemistry of the copolymerization. The result showed that the catalytic activity, the copolymer molecular weight and the stereochemistry depend both on the ligand and on the vinyl arene comonomer. High stereoselectivity (up to 85%) was possible to achieve with styrene and 4-tert-butyl styrene using racemic mixture of the ligand.[196]
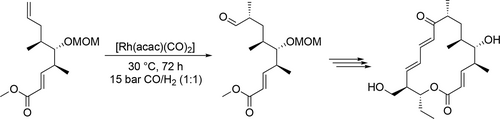
Rhodium-catalyzed hydroformylation was applied by Fürstner et al. in the total synthesis of Mycinolide IV (Scheme 77), an antibiotic species. The reaction step had a mediocre yield (60%) and a good selectivity (83%) in perfluorobenzene at 30 °C under 15 bar after 72 h.[197]
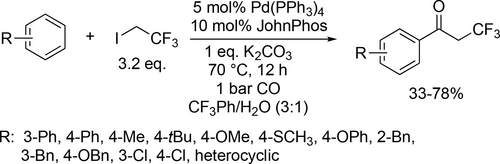
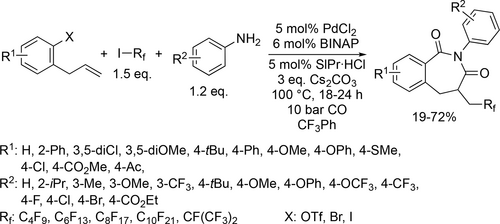
A concise and practical method was presented to synthesize α-CF3 aryl ketones through the Pd-catalyzed carbonylative cross-coupling of arylboronic acids and ICH2CF3 (Scheme 78). Overall good yields (33%—78%) were obtained using a mixture of trifluorotoluene and water solvent under mild reaction conditions (70 °C, 1 bar CO) after 12 h.[198] Pd-catalyzed aerobic oxidative alkoxycarbonylation of aryl hydrazines was developed (Scheme 79) using the same reaction environment. Good yields (55%—93%) were obtained under mild pressure (1 bar). Furthermore, no double carbonylation was detected and the normally reactive aryl- halogen bonds remained intact in this palladium-catalyzed carbonylation.[199] The same mixture was used for a regioselective cobalt catalyzed carbonylation of unactivated C(sp3)–H bonds of aliphatic amides. During the investigation of the scope of the aliphatic amides (Scheme 80), good to excellent yields (45%—95%) were achieved and phenyl, ester and Br substituents on the backbone were tolerated. Mechanistic investigation revealed that the C—H activation step is irreversible and probably the rate determining step.[200]
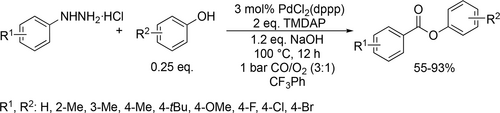
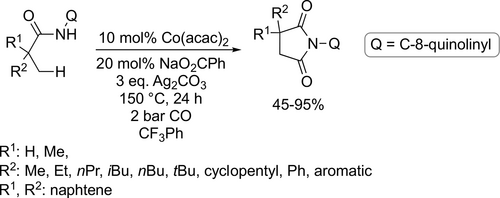
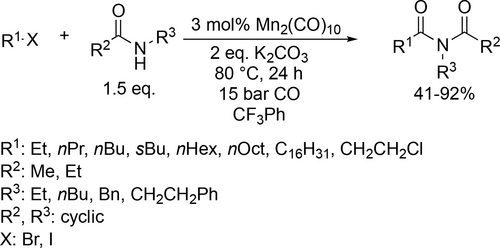
Similar trifluorotoluene-based system was applied by Wu and co-workers for a Pd-catalyzed multicomponent carbonylation of unactivated alkenes with perflouoroalkyl halides and amines. High variety of substrates were suitable for this catalyst system (Scheme 81), resulting in the corresponding β-perfluoroalkyl amides with good functional-group tolerance and moderate to high yields (10%—87%).[201] Trifluorotoluene was found to be appropriate environment for a selective Mn-catalyzed carbonylative coupling of alkyl halides and amides (Scheme 82). A wide range of imides were synthesized with moderate to good yields (41%—92%).[202] A Pd-catalyzed perfluoroalkylative carbonylation of 2-allylaryl trifluoromethanesulfonates with perfluoroalkyl halides and amines was developed by the same group to obtain the corresponding β-perfluoroalkyl amides (Scheme 83). Moderate to good yields (19%—72%) were obtained with good functional group tolerance and high chemoselectivity. The site of the multicomponent reaction could be controlled to obtain three different types of polyfluoroalkyl-substituted amides by the applied base.[203]
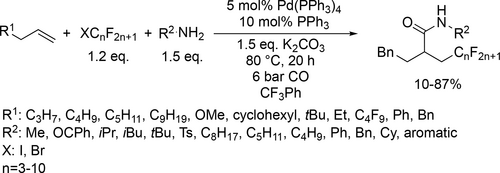
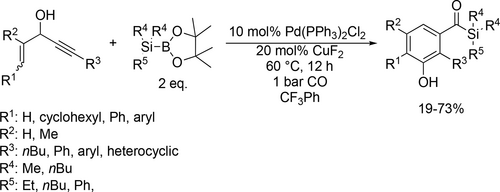
A new and general Pd/Cu-cocatalyzed dicarbonyalitve annulation was developed to obtain 3-hydroxyarylacylsilanes from 3-acetoxy-1,4-enynes, CO and silylboranes (Scheme 84). Moderate to good yields (19%—73%) were achieved in trifluorotoluene with excellent regio-, chemoselectivity and functional group tolerance. According to the mechanism investigation, the formation of the silyl-Cu intermediate from CuF2 and silylboranes, and the silyl-Pd intermediate via transmetallation were the key factors for the successful reaction and selectivity.[204]
A visible-light-induced and copper-catalyzed aminocarbonylation was developed to obtain cyanoalkylated amides from cycloketone oxime esters, amines and CO gas. Overall good yields were achieved in trifluorotoluene with various cycloketone oxime esters and alkyl/aryl amines (Scheme 85). A CuI/CuII/CuIII-based catalytic cycle was proposed as well according to the preliminary mechanistic studies.[205]
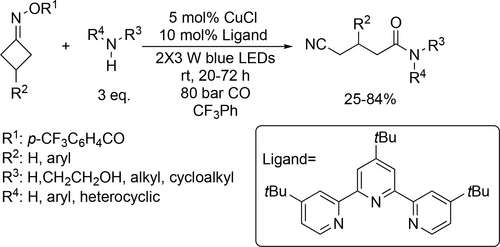
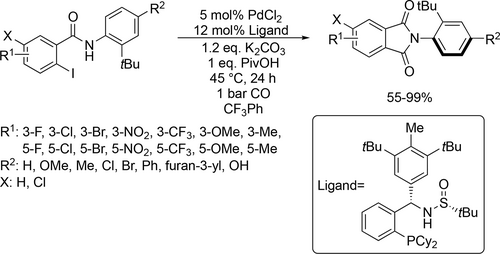
A palladium-catalyzed asymmetric carbonylation of aryl iodide was reported to synthesize cyclic and acyclic amides (Scheme 86) using trifluorotoluene as solvent. A wide range of 2-arylisoindoline-1,3-diones could be obtained with good to excellent yields (55%—99%) and high enantioselectivities (89%—97% ee). The stereochemical configuration of the product could be controlled by the position of the substituents present on the substrate.[206]
3 Conclusions and Perspectives
In this review, the recent applications of alternative reaction media, i.e., water, biomass-based alcohols, γ-valerolactone (GVL), 2-methyltetrahydrofuran (2-MeTHF), ionic liquids (ILs) and deep eutectic solvents (DES), fluorous media for transition metal catalyzed carbonylation reactions were overviewed. Since its discovery, the plethora of carbonylation protocols have been developed and many of them are utilized routinely from petrochemical to pharmaceutical industries. Most of them proceed by use of the conventional, fossil-based, and usually toxic reaction environments. To eliminate these hazardous materials and improve reaction efficiency, the investigations of novel alternative solvents have still received significant interest and resulted in several innovations in carbonylation methods.
It can be concluded that water and ethanol have still significant importance in carbonylation as they can act as both solvent and an O-nucleophile. Water was mainly applied under biphasic conditions, preferably in water/toluene system, the fossil-base compounds could not be completely eliminated from the process; but successful catalyst separation and reuse were demonstrated in several cases. By application of water, the fate of generated waste water containing any trace of catalyst species has to be considered.
The biomass-based liquids such as γ-valerolactone have gained importance; however, with maintaining the excellent functional group tolerance and high selectivity, the activities of the well-known catalytic systems, e.g., various mono- and bidentate phosphine-modified palladium systems, were found to be slightly lower compared when they were operated in common media under identical conditions.
Ionic liquids and deep eutectic solvents have been further tested mainly for the catalyst immobilization and recycling goals.
While several fluorous solvents were applied for carbonylations reactions, no novel fluorous biphasic systems were developed and investigated.
It is important to note that most of the overviewed studies reported GC yields. However, for better evaluations, isolated yields have to be provided even in the light of the fact that the product isolation and subsequent purification has to be an intrinsic part of the development of a viable and robust catalytic system. The latter could be critical when scaling-up. The isolation is also crucial for waste generation. By calculating Environmental factor (E-factor, defined by Sheldon)[207, 208] for carbonylation in overviewed media, it can be seen that the average E-factors are between 5—80 depending on the substrates’ concentration and catalyst loading, which corresponds to the fine chemical industry. Although additional auxiliary materials are taken into account, these values could be higher by one or even two orders of magnitude. These calculations should be applied as a practical green metric.
When designing an environmentally benign carbonylation protocol, it is very hard to identify “universal” green alternative solvent(s). However, the optimization of catalytic systems, including fine-tune on ligand structures, could help to increase the efficiency of the systems involving bioliquids that recently usually showed lower process performance.
Acknowledgement
This work was funded by National Research, Development and Innovation Office – NKFIH under project number of FK 143197 and 2019-1.3.1-KK-2019-00004. TKP2021-EGA-02, implemented with the support provided by the Ministry for Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021 funding scheme.
Biographies

Csaba Árvai is currently a PhD student at the Department of Chemical and Environmental Process Engineering, Budapest University of Technology and Economics, under the supervision of Prof. László T. Mika. He received both BSc and MSc in chemical engineering at the same institution. His research interests mainly focus on the kinetic studies of homogeneously catalyzed hydrogenation and cross-coupling reactions in alternative solvents. He received distinguished award of the Hungarian Chemical Industry Association in 2023.
László T. Mika is a full professor with tenure at the Budapest University of Technology and Economics and Head of the Laboratory of Catalysis and the Department of Chemical and Environmental Process Engineering. He received his PhD in organic- and organometallic chemistry at Eötvös University Budapest, Hungary, under the direction of Prof. István T. Horváth in 2010. His research activity, documented by about 60 scientific papers, covers different areas of green chemistry, including the application of novel reaction media, homogeneous catalysis, biomass conversion, applications of alternative reaction media, and the design of new catalytic systems. He received several prizes and awards, such as Bolyai Plaquette of the Hungarian Academy of Sciences, Best Teacher Award of the Budapest University of Technology and Economics




