Cobalt-Catalyzed Difunctionalization of Styrenes via Ligand Relay Catalysis†
Bingcheng Wang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorYufeng Sun
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorCorresponding Author
Zhan Lu
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorBingcheng Wang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorYufeng Sun
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorCorresponding Author
Zhan Lu
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Here, we report a cobalt-catalyzed sequential dehydrogenative Heck silylation/hydroamination of styrenes with hydrosilane and diazo compound to access 1-amino-2-silyl compounds with excellent regioselectivity. This difunctionalization reaction could undergo smoothly using 1 mol% catalyst loading with good functional group tolerance. Not only di- and tri-substituted hydrosilanes, but also alkoxysilane is suitable, which does explore the scope of the family of 1-amino-2-silyl compounds. The ligand relay phenomenon between neutral tridentate NNN ligand and anionic NNN ligand is observed for the first time via absorption spectral analysis in this one-pot, two-step transformations. The primary mechanism has been proposed based on the control experiments.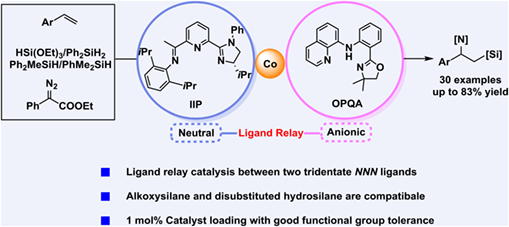
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300471-sup-0001-supinfo.pdfPDF document, 7.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Langkopf, E.; Schinzer, D. Uses of Silicon-Containing Compounds in the Synthesis of Natural Products. Chem. Rev. 1995, 95, 1375–1408; (b) Chen, J.; Cao, Y. Silole-Containing Polymers: Chemistry and Optoelectronic Properties. Macromol. Rapid Commun. 2007, 28, 1714–1742; (c) Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L. M.; Pagliaro, M. The Sol–Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620; (d) Franz, A. K.; Wilson, S. O. Organosilicon Molecules with Medicinal Applications. J. Med. Chem. 2013, 56, 388–405.
- 2(a) Meanwell, N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591; (b) Barraza, S. J.; Denmark, S. E. Synthesis, Reactivity, Functionalization, and ADMET Properties of Silicon-Containing Nitrogen Heterocycles. J. Am. Chem. Soc. 2018, 140, 6668–6684; (c) Ramesh, R.; Reddy, D. S. Quest for Novel Chemical Entities through Incorporation of Silicon in Drug Scaffolds. J. Med. Chem. 2018, 61, 3779–3798.
- 3(a) Min, G. K.; Skrydstrup, T. Regioselective Rh(I)-Catalyzed Sequential Hydrosilylation toward the Assembly of Silicon-Based Peptidomimetic Analogues. J. Org. Chem. 2012, 77, 5894–5906; (b) Zhu, S.; Buchwald, S. L. Enantioselective CuH-Catalyzed Anti-Markovnikov Hydroamination of 1,1-Disubstituted Alkenes. J. Am. Chem. Soc. 2014, 136, 15913–15916; (c) Liu, Y.-J.; Liu, Y.-H.; Zhang, Z.-Z.; Yan, S.-Y.; Chen, K.; Shi, B.-F. Divergent and Stereoselective Synthesis of β-Silyl-α-Amino Acids through Palladium-Catalyzed Intermolecular Silylation of Unactivated Primary and Secondary C−H Bonds. Angew. Chem. Int. Ed. 2016, 55, 13859–13862; (d) Lardy, S. W.; Schmidt, V. A. Intermolecular Radical Mediated Anti-Markovnikov Alkene Hydroamination Using N-Hydroxyphthalimide. J. Am. Chem. Soc. 2018, 140, 12318–12322; (e) Su, B.; Lee, T.; Hartwig, J. F. Iridium-Catalyzed, β-Selective C(sp3)–H Silylation of Aliphatic Amines to Form Silapyrrolidines and 1,2-Amino Alcohols. J. Am. Chem. Soc. 2018, 140, 18032–18038; (f) Takeda, Y.; Shibuta, K.; Aoki, S.; Tohnai, N.; Minakata, S. Catalyst-controlled regiodivergent ring-opening C(sp3)–Si bond-forming reactions of 2-arylaziridines with silylborane enabled by synergistic palladium/copper dual catalysis. Chem. Sci. 2019, 10, 8642–8647; (g) Yi, H.; Oestreich, M. Regiodivergent and Stereospecific Aziridine Opening by Copper-Catalyzed Addition of Silicon Grignard Reagents. Chem. Eur. J. 2019, 25, 6505–6507; (h) Zeng, Y.; Liu, X.-D.; Guo, X.-Q.; Gu, Q.-S.; Li, Z.-L.; Chang, X.-Y.; Liu, X.-Y. Cu/chiral phosphoric acid-catalyzed radical-initiated asymmetric aminosilylation of alkene with hydrosilane. Sci. China Chem. 2019, 62, 1529–1536.
- 4 Yang, Y.; Song, R.-J.; Ouyang, X.-H.; Wang, C.-Y.; Li, J.-H.; Luo, S. Iron-Catalyzed Intermolecular 1,2-Difunctionalization of Styrenes and Conjugated Alkenes with Silanes and Nucleophiles. Angew. Chem. Int. Ed. 2017, 56, 7916–7919.
- 5 Plöger, S.; Studer, A. Visible-Light-Mediated Radical Silyl-Oximation of Activated Alkenes Using tert-Butyl Nitrite and Silanes. Org. Lett. 2022, 24, 8568–8572.
- 6 Cheng, B.; Lu, P.; Zhao, J.; Lu, Z. Cobalt-Catalyzed Dehydrogenative Silylation of Vinylarenes. Chin. J. Org. Chem. 2019, 39, 1704–1710.
- 7(a) Sun, Y.; Guo, J.; Shen, X.; Lu, Z. Ligand relay catalysis for cobalt-catalyzed sequential hydrosilylation and hydrohydrazidation of terminal alkynes. Nat. Commun. 2022, 13, 650; (b) Sun, Y.; Wang, B.; Lu, Z. Ligand relay catalysis: a newly emerged synthetic strategy. Org. Chem. Front. 2023, 10, 4146–4160; (c) Banerjee, A.; Sarkar, S.; Shah, J. A.; Frederiks, N. C.; Bazan-Bergamino, E. A.; Johnson, C. J.; Ngai, M.-Y. Excited-State Copper Catalysis for the Synthesis of Heterocycles. Angew. Chem. Int. Ed. 2022, 61, e202113841; (d) Chen, C.; Peters, J. C.; Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 2021, 596, 250–256; (e) Chen, M. S.; Narayanasamy, P.; Labenz, N. A.; White, M. C. Serial Ligand Catalysis: A Highly Selective Allylic C−H Oxidation. J. Am. Chem. Soc. 2005, 127, 6970–6971; (f) Chen, M. S.; White, M. C. A Sulfoxide-Promoted, Catalytic Method for the Regioselective Synthesis of Allylic Acetates from Monosubstituted Olefins via C−H Oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347; (g) Corpas, J.; Gomez-Mendoza, M.; Ramírez-Cárdenas, J.; de la Peña O'Shea, V. A.; Mauleón, P.; Gómez Arrayás, R.; Carretero, J. C. One-Metal/Two-Ligand for Dual Activation Tandem Catalysis: Photoinduced Cu-Catalyzed Anti-hydroboration of Alkynes. J. Am. Chem. Soc. 2022, 144, 13006–13017; (h) Fors, B. P.; Buchwald, S. L. A Multiligand Based Pd Catalyst for C−N Cross-Coupling Reactions. J. Am. Chem. Soc. 2010, 132, 15914–15917; (i) Fraunhoffer, K. J.; Narayanasamy, P.; Sirois, L. E.; White, M. C. Macrolactonization via Hydrocarbon Oxidation. J. Am. Chem. Soc. 2006, 128, 9032–9033; (j) Hamby, T. B.; LaLama, M. J.; Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 2022, 376, 410–416; (k) He, Y.; Ma, J.; Song, H.; Zhang, Y.; Liang, Y.; Wang, Y.; Zhu, S. Regio- and enantioselective remote hydroarylation using a ligand-relay strategy. Nat. Commun. 2022, 13, 2471; (l) Ji, X.; Shen, C.; Tian, X.; Zhang, H.; Ren, X.; Dong, K. Asymmetric Double Hydroxycarbonylation of Alkynes to Chiral Succinic Acids Enabled by Palladium Relay Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202204156; (m) Jiang, X.; Sheng, F.-T.; Zhang, Y.; Deng, G.; Zhu, S. Ligand Relay Catalysis Enables Asymmetric Migratory Reductive Acylation of Olefins or Alkyl Halides. J. Am. Chem. Soc. 2022, 144, 21448–21456; (n) Kim-Lee, S.-H.; Mauleón, P.; Gómez Arrayás, R.; Carretero, J. C. Dynamic multiligand catalysis: A polar to radical crossover strategy expands alkyne carboboration to unactivated secondary alkyl halides. Chem 2021, 7, 2212–2226; (o) Li, H.; Ma, B.; Liu, Q.-S.; Wang, M.-L.; Wang, Z.-Y.; Xu, H.; Li, L.-J.; Wang, X.; Dai, H.-X. Transformations of Aryl Ketones via Ligand-Promoted C−C Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 14388–14393; (p) Semba, K.; Ohta, N.; Paulus, F.; Ohata, M.; Nakao, Y. Merging Pd0/PdII Redox and PdII/PdII Non-redox Catalytic Cycles for the Allylarylation of Electron-Deficient Alkenes. Chem. Eur. J. 2021, 27, 5035–5040; (q) Tosaki, S.-y.; Tsuji, R.; Ohshima, T.; Shibasaki, M. Dynamic Ligand Exchange of the Lanthanide Complex Leading to Structural and Functional Transformation: One-Pot Sequential Catalytic Asymmetric Epoxidation-Regioselective Epoxide-Opening Process. J. Am. Chem. Soc. 2005, 127, 2147–2155; (r) Wang, G.-Y.; Ge, Z.; Ding, K.; Wang, X. Cooperative Bimetallic Catalysis via One-Metal/ Two-Ligands: Mechanistic Insights of Polyfluoroarylation-Allylation of Diazo Compounds. Angew. Chem. Int. Ed. 2023, 62, e202307973; (s) Young, A. J.; White, M. C. Allylic C-H Alkylation of Unactivated α-Olefins: Serial Ligand Catalysis Resumed. Angew. Chem. Int. Ed. 2011, 50, 6824–6827; (t) Zhang, Y.; Ma, J.; Chen, J.; Meng, L.; Liang, Y. Zhu, S.; A relay catalysis strategy for enantioselective nickel-catalyzed migratory hydroarylation forming chiral α-aryl alkylboronates. Chem 2021, 7, 3171–3188.
- 8(a) Shen, X.; Chen, X.; Chen, J.; Sun, Y.; Cheng, Z.; Lu, Z. Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes. Nat. Commun. 2020, 11, 783; (b) Atienza, C. C. H.; Diao, T.; Weller, K. J.; Nye, S. A.; Lewis, K. M.; Delis, J. G. P.; Boyer, J. L.; Roy, A. K.; Chirik, P. J. Bis(imino)pyridine Cobalt-Catalyzed Dehydrogenative Silylation of Alkenes: Scope, Mechanism, and Origins of Selective Allylsilane Formation. J. Am. Chem. Soc. 2014, 136, 12108–12118.




