Photo-Modulation of Gene-Editing Enzymes CRISPR/Cas9 with Bifunctional Small-Molecule Ligands†
Yidan Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
‡These authors contributed equally to this work.
Search for more papers by this authorYixin Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
‡These authors contributed equally to this work.
Search for more papers by this authorLili Han
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
‡These authors contributed equally to this work.
Search for more papers by this authorQiaoling Che
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
Search for more papers by this authorJiawei Tan
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPeng Zou
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Yiyun Chen
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorYidan Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
‡These authors contributed equally to this work.
Search for more papers by this authorYixin Zhang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
‡These authors contributed equally to this work.
Search for more papers by this authorLili Han
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
‡These authors contributed equally to this work.
Search for more papers by this authorQiaoling Che
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
Search for more papers by this authorJiawei Tan
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPeng Zou
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Yiyun Chen
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
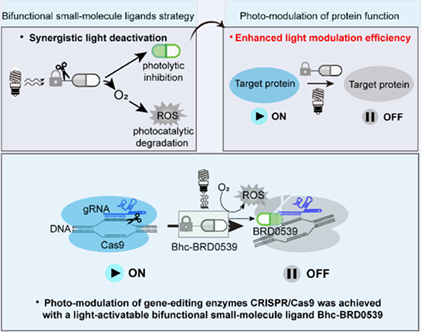
The control of protein functions with light is valuable for spatiotemporal probing of biological systems. Current small-molecule photo- modulation methods include the light-induced uncaging of inhibitors and chromophore-assisted light inactivation with reactive oxygen species (ROS). However, the constant target protein expression results in inadequate photo-modulation efficiency, particularly for less potent inhibitors and chromophores. Herein, we report a novel bifunctional small-molecule ligands strategy to photo-modulate gene-editing enzymes CRISPR/Cas9. A coumarin-derived small-molecule ligand Bhc-BRD0539 is developed to uncage the active inhibitor upon light irradiation and to generate ROS in the Cas9 proximity for the dual inhibition of Cas9 activity. Our results highlight the synergistic photo-modulation with bifunctional small-molecule ligands, which offers a valuable addition to current CRISPR/Cas9 photo-modulation technologies and may extend to other protein classes.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300452-sup-0001-Supinfo.pdfPDF document, 1.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Fehrentz, T.; Schönberger, M.; Trauner, D. Optochemical Genetics. Angew. Chem. Int. Ed. 2011, 50, 12156–12182.
- 2 Ankenbruck, N.; Courtney, T.; Naro, Y.; Deiters, A. Optochemical Control of Biological Processes in Cells and Animals. Angew. Chem. Int. Ed. 2018, 57, 2768–2798.
- 3 Wang, H.; Li, W. G.; Zeng, K.; Wu, Y. J.; Zhang, Y.; Xu, T. L.; Chen, Y. Photocatalysis Enables Visible-Light Uncaging of Bioactive Molecules in Live Cells. Angew. Chem. Int. Ed. 2019, 58, 561–565.
- 4 Yang, L.; Ozawa, T.; Dong, H.; Zhang, X. Optogenetic Control of Phosphatidylinositol (3,4,5)-Triphosphate Production by Light-Sensitive Cryptochrome Proteins on the Plasma Membrane. Chin. J. Chem. 2021, 39, 1240–1246.
- 5 Stockwell, B. R. Exploring biology with small organic molecules. Nature 2004, 432, 846–854.
- 6 Rakhit, R.; Navarro, R.; Wandless, T. J. Chemical biology strategies for posttranslational control of protein function. Chem. Biol. 2014, 21, 1238–1252.
- 7 Zeng, K.; Han, L.; Chen, Y. Endogenous Proteins Modulation in Live Cells with Small Molecules and Light. ChemBioChem 2022, 23, e202200244.
- 8 Silva, J. M.; Silva, E.; Reis, R. L. Light-triggered release of photocaged therapeutics-Where are we now? J. Control. Release 2019, 298, 154–176.
- 9 Wang, H.; Zhang, Y.; Zeng, K.; Qiang, J.; Cao, Y.; Li, Y.; Fang, Y.; Zhang, Y.; Chen, Y. Selective Mitochondrial Protein Labeling Enabled by Biocompatible Photocatalytic Reactions inside Live Cells. JACS Au 2021, 1, 1066–1075.
- 10 Zhang, Y.; Han, L.; Tian, X.; Peng, C.; Chen, Y. Ligand-Directed Caging Enables the Control of Endogenous DNA Alkyltransferase Activity with Light inside Live Cells. Angew. Chem. Int. Ed. 2022, 61, e202115472.
- 11 Cheng, B.; Wan, Y.; Tang, Q.; Du, Y.; Xu, F.; Huang, Z.; Qin, W.; Chen, X. A Photocaged Azidosugar for Light-Controlled Metabolic Labeling of Cell-Surface Sialoglycans. Chin. J. Chem. 2022, 40, 806–812.
- 12 Ellis-Davies, G. C. R. Reverse Engineering Caged Compounds: Design Principles for their Application in Biology. Angew. Chem. Int. Ed. 2023, 62, e202206083.
- 13 Jacobson, K.; Rajfur, Z.; Vitriol, E.; Hahn, K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008, 18, 443–450.
- 14 Jay, D. G. Selective destruction of protein function by chromophore- assisted laser inactivation. Proc. Natl. Acad. Sci. U. S. A. 1988, 85, 5454–5458.
- 15 Davies, M. J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770.
- 16 Lee, J.; Udugamasooriya, D. G.; Lim, H.-S.; Kodadek, T. Potent and selective photo-inactivation of proteins with peptoid-ruthenium conjugates. Nat. Chem. Biol. 2010, 6, 258–260.
- 17 Guerrero, T.; Vázquez-Ortega, F.; Lagunes, I.; Ortiz-Blanco, E.; Sosa-Ortiz, G.; Tovar-Miranda, R.; Medina, M. E.; Trigos, Á. Antagonistic activity of hydroxycoumarin-based antioxidants as possible singlet oxygen precursor photosensitizers. Dyes Pigm. 2021, 192, 109447.
- 18 Vellakkaran, M.; Hong, S. Visible-light-induced Reactions Driven by Photochemical Activity of Quinolinone and Coumarin Scaffolds. Asian J. Org. Chem. 2021, 10, 1012–1023.
- 19 Klan, P.; Solomek, T.; Bochet, C. G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191.
- 20 Wang, J. Y.; Doudna, J. A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643.
- 21 Hsu, P. D.; Lander, E. S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278.
- 22 Komor, A. C.; Badran, A. H.; Liu, D. R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 2017, 168, 20–36.
- 23 Gangopadhyay, S. A.; Cox, K. J.; Manna, D.; Lim, D.; Maji, B.; Zhou, Q.; Choudhary, A. Precision Control of CRISPR-Cas9 Using Small Molecules and Light. Biochemistry 2019, 58, 234–244.
- 24 Truong, D. J.; Kuhner, K.; Kuhn, R.; Werfel, S.; Engelhardt, S.; Wurst, W.; Ortiz, O. Development of an intein-mediated split-Cas9 system for gene therapy. Nucleic Acids Res. 2015, 43, 6450–6458.
- 25 Hemphill, J.; Borchardt, E. K.; Brown, K.; Asokan, A.; Deiters, A. Optical Control of CRISPR/Cas9 Gene Editing. J. Am. Chem. Soc. 2015, 137, 5642–5645.
- 26 Zhou, W.; Brown, W.; Bardhan, A.; Delaney, M.; Ilk, A. S.; Rauen, R. R.; Kahn, S. I.; Tsang, M.; Deiters, A. Spatiotemporal Control of CRISPR/ Cas9 Function in Cells and Zebrafish using Light-Activated Guide RNA. Angew. Chem. Int. Ed. 2020, 59, 8998–9003.
- 27 Jain, P. K.; Ramanan, V.; Schepers, A. G.; Dalvie, N. S.; Panda, A.; Fleming, H. E.; Bhatia, S. N. Development of Light-Activated CRISPR Using Guide RNAs with Photocleavable Protectors. Angew. Chem. Int. Ed. 2016, 55, 12440–12444.
- 28 Wang, S. R.; Wei, L.; Wang, J. Q.; Ji, H. M.; Xiong, W.; Liu, J.; Yin, P.; Tian, T.; Zhou, X. Light-Driven Activation of RNA-Guided Nucleic Acid Cleavage. ACS Chem. Biol. 2020, 15, 1455–1463.
- 29 Zou, R. S.; Liu, Y.; Wu, B.; Ha, T. Cas9 deactivation with photocleavable guide RNAs. Mol. Cell 2021, 81, 1553–1565.
- 30 Carlson-Stevermer, J.; Kelso, R.; Kadina, A.; Joshi, S.; Rossi, N.; Walker, J.; Stoner, R.; Maures, T. CRISPRoff enables spatio-temporal control of CRISPR editing. Nat. Commun. 2020, 11, 5041.
- 31 Maji, B.; Gangopadhyay, S. A.; Lee, M.; Shi, M.; Wu, P.; Heler, R.; Mok, B.; Lim, D.; Siriwardena, S. U.; Paul, B.; Dancik, V.; Vetere, A.; Mesleh, M. F.; Marraffini, L. A.; Liu, D. R.; Clemons, P. A.; Wagner, B. K.; Choudhary, A. A High-Throughput Platform to Identify Small-Molecule Inhibitors of CRISPR-Cas9. Cell 2019, 177, 1067–1079.
- 32 Lindig, B. A.; Rodgers, M. A. J.; Schaap, A. P. Determination of the lifetime of singlet oxygen in water-d2 using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980, 102, 5590–5593.
- 33BRD0539 displays dose-dependent Cas9 inhibition (apparent IC50 = 22 μmol/L) and completely inhibits Cas9 protein-gRNA complex (5 nmol/L) at 30 μmol/L, see ref [31]. Our initial tests with BRD0539 at varying concentrations revealed the significant inhibition of Cas9 (0.15 μmol/L) by 0.45 mmol/L of BRD0539, however, no inhibition was observed at 0.30 mmol/L (Figure 3a and Figure S9). Therefore, we assessed Bhc-BRD0539's inhibitory potential at 0.45 mmol/L and explored lower-dose Bhc-BRD0539 modulation at 0.30 mmol/L.
- 34Photolysis of Bhc-BRD0539 was observed upon exposure to higher- intensity UV dot light irradiation (365 nm, 650 mW·cm–2) (Figure S5) .
- 35 Lim, D.; Zhou, Q.; Cox, K. J.; Law, B. K.; Lee, M.; Kokkonda, P.; Sreekanth, V.; Pergu, R.; Chaudhary, S. K.; Gangopadhyay, S. A.; Maji, B.; Lai, S.; Amako, Y.; Thompson, D. B.; Subramanian, H. K. K.; Mesleh, M. F.; Dančík, V.; Clemons, P. A.; Wagner, B. K.; Woo, C. M.; Church, G. M.; Choudhary, A. A general approach to identify cell-permeable and synthetic anti-CRISPR small molecules. Nat. Cell Biol. 2022, 24, 1766–1775.
- 36 Sinha, R. P.; Richter, P.; Faddoul, J.; Braun, M.; Häder, D.-P. Effects of UV and visible light on cyanobacteria at the cellular level. Photochem. Photobiol. Sci. 2002, 1, 553–559.
- 37 Lemke, E. A.; Summerer, D.; Geierstanger, B. H.; Brittain, S. M.; Schultz, P. G. Control of protein phosphorylation with a genetically encoded photocaged amino acid. Nat. Chem. Biol. 2007, 3, 769–772.




