Identification of in situ Generated Iron-Vacancy Induced Oxygen Evolution Reaction Kinetics on Cobalt Iron Oxyhydroxide†
Na Yao
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Na Yao and Juan Zhu contribute equally to this work.
Search for more papers by this authorJuan Zhu
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Na Yao and Juan Zhu contribute equally to this work.
Search for more papers by this authorHongnan Jia
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorHengjiang Cong
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei Luo
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorNa Yao
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Na Yao and Juan Zhu contribute equally to this work.
Search for more papers by this authorJuan Zhu
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Na Yao and Juan Zhu contribute equally to this work.
Search for more papers by this authorHongnan Jia
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorHengjiang Cong
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei Luo
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorDedicated to the 130th Anniversary of Wuhan University.
Comprehensive Summary
Developing highly efficient and low-cost electrocatalysts towards oxygen evolution reaction (OER) is essential for practical application in water electrolyzers and rechargeable metal-air batteries. Although Fe-based oxyhydroxides are regarded as state-of-the-art non-noble OER electrocatalysts, the origin of performance enhancement derived from Fe doping remains a hot topic of considerable discussion. Herein, we demonstrate that in situ generated Fe vacancies in the pristine CoFeOOH catalyst through a pre-conversion process during alkaline OER result from dynamic Fe dissolution, identifying the origin of Fe-vacancy-induced enhanced OER kinetics. Density functional theory (DFT) calculations and experimental results including X-ray absorption fine-structure spectroscopy, in situ UV-Vis spectroscopy, and in situ Raman spectroscopy reveal that the Fe vacancies could significantly promote the d-band center and valence states of adjacent Co sites, alter the active site from Fe atom to Co atom, accelerate the formation of high-valent active Co4+ species, and reduce the energy barrier of the potential-determining step, thereby contribute to the significantly enhanced OER performance.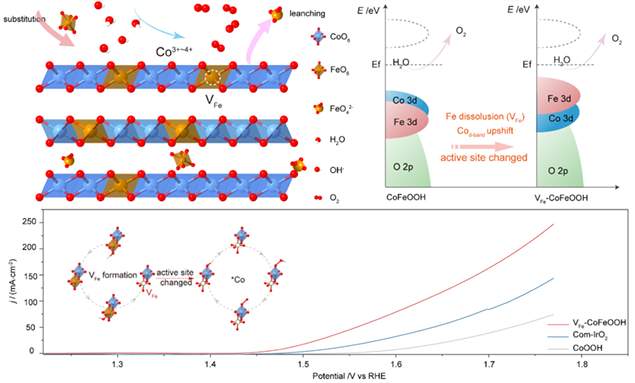
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300388-sup-0001-supinfo.pdfPDF document, 2.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Tang, L.; Rao, Y.; Wei, L.; Zheng, H.; Liu, H.; Zhang, W.; Tang, K. A-site cation defects (Ba0.5Sr0.5)1-xCo0.8Fe0.2O3-δ perovskites as active oxygen evolution reaction catalyst in alkaline electrolyte. Chin. J. Chem. 2021, 39, 2692–2698.
- 2 Zhong, W. X.; Zhao, X. R.; Qin, J. Y.; Yang, J. An active hybrid electrocatalyst with synergized pyridinic nitrogen-cobalt and oxygen vacancies for bifunctional oxygen reduction and evolution. Chin. J. Chem. 2021, 39, 655–660.
- 3 Cheng, W.; Zhao, X.; Su, H.; Tang, F.; Che, W.; Zhang, H.; Liu, Q. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy 2019, 4, 115–122.
- 4 Yang, Y.; Su, J.; Jiang, P.; Chen, J.; Hu, L.; Chen, Q. Mofs-derived n-doped carbon-encapsulated metal/alloy electrocatalysts to tune the electronic structure and reactivity of carbon active sites. Chin. J. Chem. 2021, 39, 2626–2637.
- 5 Sun, W.; Wei, Z.; Qi, J.; Kang, L.; Li, J.; Xie, J.; Tang, B.; Xie, Y. Rapid and scalable synthesis of prussian blue analogue nanocubes for electrocatalytic water oxidation. Chin. J. Chem. 2021, 39, 2347–2353.
- 6 Liu, H.; Luo, Q.; Hu, J.; Wei, L.; Zhang, W.; Zheng, H.; Wu, S.; Tang, K. Iridium-doped 10h-phase perovskite BaCo0.8Fe0.15Ir0.05O3–δ as an efficient oxygen evolution reaction catalyst. Chin. J. Chem. 2022, 40, 2276–2284.
- 7 An, Q.; Pan, B.; Li, L.; Peng, X.; Zeng, M. H. Exploring the pyrolysis mechanism towards OER performance optimization of salophen-ligated binuclear cobalt complex. Chin. J. Chem. 2021, 39, 2529–2535.
- 8 Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021, 33, e2004243.
- 9 Lee, W. H.; Han, M. H.; Ko, Y. J.; Min, B. K.; Chae, K. H.; Oh, H. S. Electrode reconstruction strategy for oxygen evolution reaction: Maintaining Fe-CoOOH phase with intermediate-spin state during electrolysis. Nat. Commun. 2022, 13, 605.
- 10 Haase, F. T.; Rabe, A.; Schmidt, F. P.; Herzog, A.; Jeon, H. S.; Frandsen, W.; Narangoda, P. V.; Spanos, I.; Friedel Ortega, K.; Timoshenko, J.; Lunkenbein, T.; Behrens, M.; Bergmann, A.; Schlogl, R.; Roldan Cuenya, B. Role of nanoscale inhomogeneities in Co2FeO4 catalysts during the oxygen evolution reaction. J. Am. Chem. Soc. 2022, 144, 12007–12019.
- 11 Zhang, S.; Tan, C.; Yan, R.; Zou, X.; Hu, F. L.; Mi, Y.; Yan, C.; Zhao, S. Constructing built-in electric field in heterogeneous nanowire arrays for efficient overall water electrolysis. Angew. Chem. Int. Ed. 2023, 62, e202302795.
- 12 Zhao, S.; Tan, C.; He, C.-T.; An, P.; Xie, F.; Jiang, S.; Zhu, Y.; Wu, K.-H.; Zhang, B.; Li, H.; Zhang, J.; Chen, Y.; Liu, S.; Dong, J.; Tang, Z. Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 2020, 5, 881–890.
- 13 Pei, Z.; Tan, H.; Gu, J.; Lu, L.; Zeng, X.; Zhang, T.; Wang, C.; Ding, L.; Cullen, P. J.; Chen, Z.; Zhao, S. A polymeric hydrogel electrocatalyst for direct water oxidation. Nat. Commun. 2023, 14, 818.
- 14 Liu, Y.; Li, C.; Tan, C.; Pei, Z.; Yang, T.; Zhang, S.; Huang, Q.; Wang, Y.; Zhou, Z.; Liao, X.; Dong, J.; Tan, H.; Yan, W.; Yin, H.; Liu, Z.-Q.; Huang, J.; Zhao, S. Electrosynthesis of chlorine from seawater-like solution through single-atom catalysts. Nat. Commun. 2023, 14, 247.
- 15 Stevens, M. B.; Trang, C. D. M.; Enman, L. J.; Deng, J.; Boettcher, S. W. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity. J. Am. Chem. Soc. 2017, 139, 11361–11364.
- 16 Hunter, B. M.; Blakemore, J. D.; Deimund, M.; Gray, H. B.; Winkler, J. R.; Muller, A. M. Highly active mixed-metal nanosheet water oxidation catalysts made by pulsed-laser ablation in liquids. J. Am. Chem. Soc. 2014, 136, 13118–13121.
- 17 Ahn, H. S.; Bard, A. J. Surface interrogation scanning electrochemical microscopy of Ni1-xFexOOH (0 < x < 0.27) oxygen evolving catalyst: Kinetics of the "fast" iron sites. J. Am. Chem. Soc. 2016, 138, 313–318.
- 18 Ye, S. H.; Shi, Z. X.; Feng, J. X.; Tong, Y. X.; Li, G. R. Activating CoOOH porous nanosheet arrays by partial iron substitution for efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 2018, 57, 2672–2676.
- 19 Zhou, J.; Wang, Y.; Su, X.; Gu, S.; Liu, R.; Huang, Y.; Yan, S.; Li, J.; Zhang, S. Electrochemically accessing ultrathin Co (oxy)-hydroxide nanosheets and operando identifying their active phase for the oxygen evolution reaction. Energy Environ. Sci. 2019, 12, 739–746.
- 20 Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; Arquer, F. P. G. D.; Dinh, C. T.; Fan, F.; Yuan, M.; Yassitepe, E.; Chen, N.; Regier, T.; Liu, P.; Li, Y.; Luna, P. D.; Janmohamed, A.; Xin, H. L.; Yang, H.; Vojvodic, A.; Sargent, E. H. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337.
- 21 Zhao, Y.; Dongfang, N.; Triana, C. A.; Huang, C.; Erni, R.; Wan, W.; Li, J.; Stoian, D.; Pan, L.; Zhang, P.; Lan, J.; Iannuzzi, M.; Patzke, G. R. Dynamics and control of active sites in hierarchically nanostructured cobalt phosphide/chalcogenide-based electrocatalysts for water splitting. Energy Environ. Sci. 2022, 15, 727–739.
- 22 Chen, J.; Zheng, F.; Zhang, S.-J.; Fisher, A.; Zhou, Y.; Wang, Z.; Li, Y.; Xu, B. B.; Li, J. T.; Sun, S. G. Interfacial interaction between FeOOH and Ni–Fe LDH to modulate the local electronic structure for enhanced oer electrocatalysis. ACS Catal. 2018, 8, 11342–11351.
- 23 Gorlin, M.; Chernev, P.; Ferreira de Araujo, J.; Reier, T; Dresp, S.; Paul, B.; Krahnert, R.; Dau, H.; Strasser, P. Oxygen evolution reaction dynamics, faradaic charge efficiency, and the active metal redox states of Ni-Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 2016, 138, 5603–5614.
- 24 Louie, M. W.; Bell, A. T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337.
- 25 Li, N.; Bediako, D. K.; Hadt, R. G.; Hayes, D.; Kempa, T. J.; von Cube, F.; Bell, D. C.; Chen, L. X.; Nocera, D. G. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 1486–1491.
- 26 Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793.
- 27 Lee, S.; Moysiadou, A.; Chu, Y. C.; Chen, H. M.; Hu, X. Tracking high-valent surface iron species in the oxygen evolution reaction on cobalt iron (oxy)hydroxides. Energy Environ. Sci. 2022, 15, 206–214.
- 28 Gao, J.; Tao, H.; Liu, B. Progress of nonprecious-metal-based electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 2021, 33, e2003786.
- 29 Burke, M. S.; Kast, M. G.; Trotochaud, L.; Smith, A. M.; Boettcher, S. W. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648.
- 30 Gao, L.; Li, X.; Yao, Z.; Bai, H.; Lu, Y.; Ma, C.; Lu, S.; Peng, Z.; Yang, J.; Pan, A.; Huang, H. Unconventional p-d hybridization interaction in PtGa ultrathin nanowires boosts oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2019, 141, 18083–18090.
- 31 Hammer, B.; Nørskov, J. K. Theoretical surface science and catalysis—calculations and concepts. Adv. Catal. 2019, 141, 18083–18090.
- 32 Hammer, B.; Morikawa, Y.; Nørskov, J. K. Co chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 1996, 76, 2141.
- 33 Cheng, J.; Yang, J.; Kitano, S.; Juhasz, G.; Higashi, M.; Sadakiyo, M.; Kato, K.; Yoshioka, S.; Sugiyama, T.; Yamauchi, M.; Nakashima, N. Impact of Ir-valence control and surface nanostructure on oxygen evolution reaction over a highly efficient Ir-TiO2 nano-rod catalyst. ACS Catal. 2019, 9, 6974–6986.
- 34 Mom, R. V.; Cheng, J.; Koper, M. T. M.; Sprik, M. Modeling the oxygen evolution reaction on metal oxides: The influence of unrestricted DFT calculations. J. Phys. Chem. C 2014, 118, 4095–4102.
- 35 Pei, Z.; Ding, L.; Wang, C.; Meng, Q.; Yuan, Z.; Zhou, Z.; Zhao, S.; Chen, Y. Make it stereoscopic: Interfacial design for full-temperature adaptive flexible Zinc–air batteries. Energy Environ. Sci. 2021, 14, 4926–4935.
- 36 Chen, J.; Li, H.; Chen, S.; Fei, J.; Liu, C.; Yu, Z.; Shin, K.; Liu, Z.; Song, L.; Henkelman, G.; Wei, L.; Chen, Y. Co–Fe–Cr (oxy)hydroxides as efficient oxygen evolution reaction catalysts. Adv. Energy Mater. 2021, 11, 2003412.
- 37
Rotella, H.; Mazel, Y.; Brochen, S.; Valla, A.; Pautrat, A.; Licitra, C.; Rochat, N.; Sabbione, C.; Rodriguez, G.; Nolot, E. Role of vacancy defects in Al doped ZnO thin films for optoelectronic devices. J. Phys. D 2017, 50, 485106.
10.1088/1361-6463/aa920b Google Scholar
- 38 Majumder, S.; Dey, S.; Bagani, K.; Dey, S. K.; Banerjee, S.; Kumar, S. A comparative study on the structural, optical and magnetic properties of Fe3O4 and Fe3O4@SiO2 core-shell microspheres along with an assessment of their potentiality as electrochemical double layer capacitors. Dalton Trans. 2015, 44, 7190–7202.
- 39 Gong, X.; Tang, L.; Zou, J.; Guo, Z.; Li, Y.; Lei, J.; Liu, H.; Liu, M.; Zhou, L.; Huang, P.; Ruan, H.; Lu, Y.; Zhu, W.; He, R. Introduction of cation vacancies and iron doping into TiO2 enabling efficient uranium photoreduction. J. Hazard. Mater. 2022, 423, 126935.
- 40 Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 5867–5871.
- 41 Guo, X.; Song, E.; Zhao, W.; Xu, S.; Zhao, W.; Lei, Y.; Fang, Y.; Liu, J.; Huang, F. Charge self-regulation in 1T-MoS2 structure with rich S vacancies for enhanced hydrogen evolution activity. Nat. Commun. 2022, 13, 5954.
- 42 Liang, S.; Zou, L. C.; Zheng, L. J.; Li, F.; Wang, X. X.; Song, L. N.; Xu, J. J. Highly stable Co single atom confined in hierarchical carbon molecular sieve as efficient electrocatalysts in metal–air batteries. Adv. Energy Mater. 2022, 12, 2103097.
- 43 Enman, L. J.; Stevens, M. B.; Dahan, M. H.; Nellist, M. R.; Toroker, M. C.; Boettcher, S. W. Operando x-ray absorption spectroscopy shows iron oxidation is concurrent with oxygen evolution in cobalt-iron (oxy)hydroxide electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 12840–12844.
- 44 Zhang, Z.; Feng, C.; Wang, D.; Zhou, S.; Wang, R.; Hu, S.; Li, H.; Zuo, M.; Kong, Y.; Bao, .J; Zeng, J. Selectively anchoring single atoms on specific sites of supports for improved oxygen evolution. Nat. Commun. 2022, 13, 2473.
- 45 Li, P.; Wang, M.; Duan, X.; Zheng, L.; Cheng, X.; Zhang, Y.; Kuang, Y.; Li, Y.; Ma, Q.; Feng, Z.; Liu, W.; Sun, X. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 2019, 10, 1711.
- 46 Koo, B.; Xiong, H.; Slater, M. D.; Prakapenka, V. B.; Balasubramanian, M.; Podsiadlo, P.; Johnson, C. S.; Rajh, T.; Shevchenko, E. V. Hollow iron oxide nanoparticles for application in lithium ion batteries. Nano Lett. 2012, 12, 2429–2435.
- 47 Gao, P.; Chen, Z.; Gong, Y.; Zhang, R.; Liu, H.; Tang, P.; Chen, X.; Passerini, S.; Liu, J. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals. Adv. Energy Mater. 2020, 10, 1903780.
- 48 Ma, L.; Zhou, H.; Xu, M.; Hao, P.; Kong, X.; Duan, H. Integrating hydrogen production with anodic selective oxidation of sulfides over a CoFe layered double hydroxide electrode. Chem. Sci. 2020, 12, 938–945.
- 49 Yang, C.; Zhong, W.; Shen, K.; Zhang, Q.; Zhao, R.; Xiang, H.; Wu, J.; Li, X.; Yang, N. Electrochemically reconstructed Cu-FeOOH/Fe3O4 catalyst for efficient hydrogen evolution in alkaline media. Adv. Energy Mater. 2022, 12, 2200077.
- 50 Wu, Q.; Xiao, M.; Wang, W.; Cui, C. In situ coordination environment tuning of cobalt sites for efficient water oxidation. ACS Catal. 2019, 9, 11734–11742.
- 51 Li, Y.; Wu, Y.; Yuan, M.; Hao, H.; Lv, Z.; Xu, L.; Wei, B. Operando spectroscopies unveil interfacial FeOOH induced highly reactive β-NiFeOOH for efficient oxygen evolution. Appl. Catal. B 2022, 318, 121825.
- 52 Francas, L.; Corby, S.; Selim, S.; Lee, D.; Mesa, C. A.; Godin, R.; Pastor, E.; Stephens, I. E. L.; Choi, K. S.; Durrant, J. R. Spectroelectrochemical study of water oxidation on nickel and iron oxyhydroxide electrocatalysts. Nat. Commun. 2019, 10, 5208.
- 53 Goldsmith, Z. K.; Harshan, A. K.; Gerken, J. B.; Voros, M.; Galli, G.; Stahl, S. S.; Hammes-Schiffer, S. Characterization of NiFe oxyhydroxide electrocatalysts by integrated electronic structure calculations and spectroelectrochemistry. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3050–3055.
- 54 Hu, Y.; Hu, C.; Du, A.; Xiao, T.; Yu, L.; Yang, C.; Xie, W. Interfacial evolution on co-based oxygen evolution reaction electrocatalysts probed by using in situ surface-enhanced Raman spectroscopy. Anal. Chem. 2023, 95, 1703–1709.
- 55 Pauporté, T.; Mendoza, L.; Cassir, M.; Bernard, M. C.; Chivot, J. Direct low-temperature deposition of crystallized CoOOH films by potentiostatic electrolysis. J. Electrochem. Soc. 2005, 152, C49–C53.




