Iron-Catalyzed Alkenylzincation of Internal Alkynes†
Wei-Na Wang
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQiang Huang
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYe Jin
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQi-Lin Zhou
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Shou-Fei Zhu
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]Search for more papers by this authorWei-Na Wang
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQiang Huang
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYe Jin
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQi-Lin Zhou
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Shou-Fei Zhu
Frontiers Science Center for New Organic Matter, State Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
The alkenylzincation of internal alkynes is an effective method for the synthesis of multi-substituted conjugated dienes; however, the current catalytic systems for this reaction are limited in terms of substrate scope and selectivity control, which restricts its practical applications. Herein, we report the first iron-catalyzed alkenylzincation of internal alkynes, which features mild conditions, simple operation, broad substrate scope (including aryl/alkyl, diaryl, and dialkyl acetylenes), excellent functional group tolerance (tolerating highly active functional groups such as ester, methylthio, amide, sulfonyl, cyano, etc.), and high activity (with a turnover number of up to 11500, the highest record for carbometallation reactions). Notably, the catalytic system described in this article also realized the highly selective vinylzincation of unfunctionalized internal alkynes as well as the alkenylzincation of unsymmetrical diarylacetylenes and dialkyl acetylenes, which have not been achieved with other catalytic systems reported in the literatures. The current study provides a highly selective access to synthetically important multi-substituted conjugated dienes.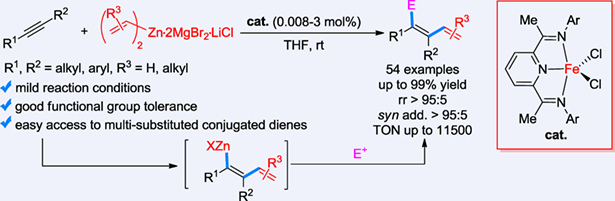
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300356-sup-0001-supinfo.pdfPDF document, 7.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698;
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar(b) Tripoteau, F.; Verdelet, T.; Hercouet, A.; Carreaux, F.; Carboni, B. Boron- and silicon-substituted [3]-1-heterodendralenes as versatile building blocks for the rapid construction of polycyclic architectures. Chem. - Eur. J. 2011, 17, 13670–13675.
- 2(a) Boukouvalas, J.; Cheng, Y.-X.; Robichaud, J. Total synthesis of (+)-dysidiolide. J. Org. Chem. 1998, 63, 228−229; (b) Pyziak, J.; Walkowiak, J.; Marciniec, B. Recent advances in boron-substituted 1,3-dienes chemistry: synthesis and application. Chem. - Eur. J. 2017, 23, 3502–3541; (c) Gao, X.-H.; Wang, X.-Y.; Zhou, J.-S.; Zhang, Y.; Liu, H.-C.; Zhou, B.; Yue, J.-M. Rearranged Dichapetalin-type triterpenoids with cytotoxic activity from Dichapetalum Gelonioides. Chin. J. Chem. 2022, 40, 2531–2538.
- 3 Nguyen, V. T.; Dang, H. T.; Pham, H. H.; Nguyen, V. D.; Flores-Hansen, C.; Arman, H. D.; Larionov, O. V. Highly regio- and stereoselective catalytic synthesis of conjugated dienes and polyenes. J. Am. Chem. Soc. 2018, 140, 8434−8438.
- 4(a) Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857; (b) Koizumi, T.; Kameda, T.; Saito, H.; Sato, A.; Hayashi, S. Facile synthesis of ortho-phenylene-based conjugated polymers through transformation of cross-conjugated poly(2,3-diaryl[2]dendralene)s and their optical properties. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 827–832.
- 5(a) Shimizu, M.; Nakamaki, C.; Shimono, K.; Schelper, M.; Kurahashi, T.; Hiyama, T. Stereoselective cross-coupling reaction of 1,1-diboryl- 1-alkenes with electrophiles: A highly stereocontrolled approach to 1,1,2-triaryl-1-alkenes. J. Am. Chem. Soc. 2005, 127, 12506–12507; (b) Dumonteil, G.; Sabine, B. R. Synthesis of conjugated dienes in natural compounds. Catalysts 2022, 12, 86–112.
- 6(a) Kwong, C. K.; Fu, M. Y.; Lam, C. S.; Toy, P. H. The phosphine-catalyzed alkyne to 1,3-diene isomerization reaction. Synthesis 2008, 15, 2307–2317; (b) Liu, Y.-S.; Wang, L.-J.; Deng, L. Selective double carbomagnesiation of internal alkynes catalyzed by iron-N-heterocyclic carbene complexes: a convenient method to highly substituted 1,3-dienyl magnesium reagents. J. Am. Chem. Soc. 2016, 138, 112–115; (c) Hubert, P.; Seibel, E.; Beemelmanns, C.; Campagne, J.-M.; Figueiredo, R. M. Stereoselective construction of (E,Z)-1,3-dienes and its application in natural product synthesis. Adv. Synth. Catal. 2020, 362, 5532–5575; (d) Soengas, R. G.; Rodríguez, H. Modern synthetic methods for the stereoselective construction of 1,3-dienes. Molecules 2021, 26, 249–289; (e) Li, Y.; Hao, M.; Chang, Y.-C.; Liu, Y.; Wang, W.-F.; Sun, N.; Zhu, W.-Q.; Gao, Z.-W. Synthesis of 4-trifluoromethylated 1,3-butadienes via palladium catalyzed Heck reaction. Chin. J. Chem. 2021, 39, 2962–2966.
- 7(a) Williams, J. M. J. Preparation of Alkenes: A Practical Approach, Oxford University Press, Oxford, U.K., 1996;
10.1093/oso/9780198557951.001.0001 Google Scholar(b) Vasilèv, A. A.; Serebryakov, E. P. Synthetic methodologies for carbo-substituted conjugated dienes. Russ. Chem. Rev. 2001, 70, 735–776; (c) Oger, C.; Balas, L.; Durand, T.; Galano, J.-M. Are alkyne reductions chemo-, regio-, and stereoselective enough to provide pure (Z)-olefins in polyfunctionalized bioactive molecules? Chem. Rev. 2013, 113, 1313–1350.
- 8(a) Huang, Q.; Su, Y.-X.; Sun, W.; Hu, M.-Y.; Wang, W.-N.; Zhu, S.-F. Iron-catalyzed vinylzincation of terminal alkynes. J. Am. Chem. Soc. 2022, 144, 515–526; For other carbozincation of terminal alkynes, see: (b) Huang, Q.; Wang, W.-N.; Zhu, S.-F. Iron-catalyzed alkylzincation of terminal alkynes. ACS Catal. 2022, 12, 2581–2588; (c) Cheung, C. W.; Zhurkin, F. E.; Hu, X.-L. Z-Selective olefin synthesis via iron-catalyzed reductive coupling of alkyl halides with terminal arylalkynes. J. Am. Chem. Soc. 2015, 137, 4932–4935; (d) Corpet, M.; Gosmini, C. Cobalt-catalysed synthesis of highly substituted styrene derivatives via arylzincation of alkynes. Chem. Commun. 2012, 48, 11561–11563.
- 9 Gourdet, B.; Lam, H. W. Stereoselective synthesis of multisubstituted enamides via rhodium-catalyzed carbozincation of ynamides. J. Am. Chem. Soc. 2009, 131, 3802–3803.
- 10 Wu, J.-L.; Yoshikai, N. Cobalt-catalyzed alkenylzincation of unfunctionalized alkynes. Angew. Chem. Int. Ed. 2016, 55, 336–340.
- 11 Tucker, C. E.; Rao, S. A.; Knochel, P. The olefination of functionalized alkylidenemalonates by 1,1-dimetalloalkanes: a new chemo- and stereoselective preparation of functionalized olefins. J. Org. Chem. 1990, 55, 5446–5448.
- 12(a) Hu, M.-Y.; He, Q.; Fan, S.-J.; Wang, Z.-C.; Liu, L.-Y.; Mu, Y.-J.; Peng, Q.; Zhu, S.-F. Ligands with 1,10-phenanthroline scaffold for highly regioselective iron-catalyzed alkene hydrosilylation. Nat. Commun. 2018, 9, 221–232; (b) Hu, M.-Y.; Lian, J.; Sun, W.; Qiao, T.-Z.; Zhu, S.-F. Iron-catalyzed di-hydrosilylation of alkynes: efficient access to geminal bis(silanes). J. Am. Chem. Soc. 2019, 141, 4579–4583; (c) Hu, M.-Y.; He, P.; Qiao, T.-Z.; Sun, W.; Li, W.-T.; Lian, J.; Li, J.-H.; Zhu, S.-F. Iron-catalyzed regiodivergent alkyne hydrosilylation. J. Am. Chem. Soc. 2020, 142, 16894–16902; (d) Sun, W.; Li, M.-P.; Li, L.-J.; Huang, Q.; Hu, M.-Y.; Zhu, S.-F. Phenanthroline-imine ligands for iron-catalyzed alkene hydrosilylation. Chem. Sci. 2022, 13, 2721–2728; (e) Li, W.-T.; Hu, M.-Y.; Xiong, J.-W.; Zhang, X.-Y.; Zhu, S.-F. Iron-catalyzed hydroalumination of internal alkynes. Chem. Sci. 2022, 13, 7873–7879; For a recent account, see: (f) Zhu, S.-F. Catalytic hydrogen transfer reactions. Chin. J. Chem. 2021, 39, 3211–3218.
- 13(a) Stiidemann, T.; Ibrahim-Ouali, M.; Knochel, P. A nickel-catalyzed carbozincation of aryl-substituted alkynes. Tetrahedron 1998, 54, 1299–1316; (b) Yasui, H.; Nishikawa, T.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed allylzincations of internal alkynes. Bull. Chem. Soc. Jpn. 2006, 79, 1271–1274; (c) Murakami, K.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed arylzincation of alkynes. Org. Lett. 2009, 11, 2373–2375; (d) Murakami, K.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed benzylzincation of alkynes. Chem. - Eur. J. 2010, 16, 7688–7691; (e) Li, S.-H.; Ma, S.-M. Highly selective nickel-catalyzed methyl-carboxylation of homopropargylic alcohols for α-alkylidene-γ-butyrolactones. Org. Lett. 2011, 13, 6046–6049; (f) Kadikova, R. N.; Ramazanov, I. R.; Gabdullin, A. M.; Mozgovoi, O. S.; Dzhemilev, U. M. Carbozincation of substituted 2-alkynylamines, 1-alkynylphosphines, 1-alkynylphosphine sulfides with Et2Zn in the presence of catalytic system of Ti(O-iPr)4 and EtMgBr. Catalysts 2019, 9, 1022–1033.
- 14(a) Huang, L.-B.; Ackerman, L. K. G.; Kang, K.; Parsons, A. M.; Weix, D. J. LiCl-accelerated multimetallic cross-coupling of aryl chlorides with aryl triflates. J. Am. Chem. Soc. 2019, 141, 10978−10983; (b) Koszinowski, K.; Böhrer, P. Formation of organozincate anions in LiCl- mediated zinc insertion reactions. Organometallics 2009, 28, 771–779.
- 15(a) Marek, I.; Minko, Y. Carbometallation Reactions in Metal-Catalyzed Cross-Coupling Reactions and More, Eds.: A. Meijere; S. Bräse; M. Oestreich, Wiley-VCH, 2014; (b) Murakami, K.; Yorimitsu, H. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein J. Org. Chem. 2013, 9, 278–302; (c) Muller, D. S.; Marek, I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 2016, 45, 4552—4566.
- 16(a) Flynn, A. B.; Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 2007, 107, 4698–4745; (b) Guo, P.; Zhou, Y.; Zhao, J. Z:E selective preparation of disubstituted internal alkenes and trisubstituted alkenes. Chin. J. Org. Chem. 2023, 43, 855–872.




